A Brief Review of the EEG Literature on Mindfulness and Fear Extinction and its Potential Implications for Posttraumatic Stress Symptoms (PTSS)
Abstract
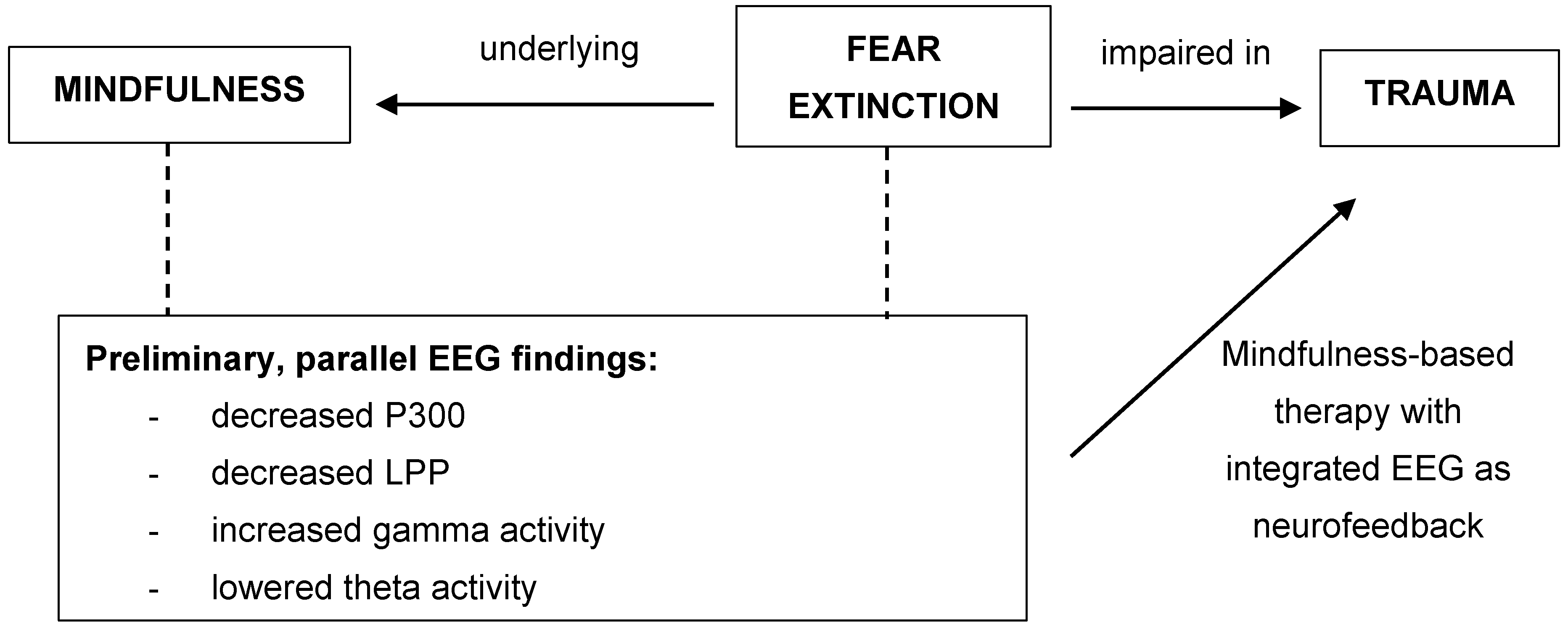
1. Introduction
1.1. Mindfulness
1.2. Fear Extinction
1.2.1. Neural Correlates of Fear Extinction
1.2.2. Neurophysiological Literature on Fear Extinction using EEG
Event-Related Potentials
Source Localization
1.3. Link between Fear Extinction and Mindfulness
1.3.1. Neurophysiological Literature on Mindfulness Using EEG
Event-Related Potentials
Spectral Power and Coherence
1.4. Implications for Trauma
1.4.1. Mindfulness-Based Exposure Therapy
1.4.2. Neurophysiological Literature on PTSD Using EEG
1.5. Future Studies
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chiesa, A.; Serretti, A. Mindfulness-Based Stress Reduction for Stress Management in Healthy People: A Review and Meta-Analysis. J. Altern. Complement. Med. 2009, 15, 593–600. [Google Scholar] [CrossRef]
- Eberth, J.; Sedlmeier, P. The effects of mindfulness meditation: A meta-analysis. Mindfulness 2012, 3, 174–189. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Sawyer, A.T.; Witt, A.A.; Oh, D. The effect of mindfulness- based therapy on anxiety and depression: A meta-analytic review. J. Consult. Clin. Psychol. 2010, 78, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Khoury, B.; Lecomte, T.; Fortin, G.; Masse, M.; Therien, P.; Bouchard, V.; Chapleau, M.-A.; Paquin, K.; Hofmann, S.G. Mindfulness-Based Therapy: A Comprehensive Meta-Analysis. Clin. Psychol. Rev. 2013, 33, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Howard, M.O. Mindfulness-Oriented Recovery Enhancement Reduces Pain Attentional Bias in Chronic Pain Patients. Psychother. Psychosom. 2013, 82, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J.; Lipworth, L.; Burney, R. The Clinical Use of Mindfulness Meditation for the Self-Regulation of Chronic Pain. J. Behav. Med. 1985, 8, 163–190. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.R.; Kreitzer, M.J.; Reilly-Spong, M.; Wall, M.; Winbush, N.Y.; Patterson, R.; Mahowald, M.; Cramer-Bornemann, M. Mindfulness-Based Stress Reduction versus Pharmacotherapy for Chronic Primary Insomnia: A Randomized Controlled Clinical Trial. Explore J. Sci. Heal. 2011, 7, 76–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ong, J.C.; Manber, R.; Segal, Z.; Xia, Y.; Shapiro, S.; Wyatt, J.K. A Randomized Controlled Trial of Mindfulness Meditation for Chronic Insomnia. Sleep 2014, 37, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Bowen, S.; Witkiewitz, K.; Clifasefi, S.L.; Grow, J.; Chawla, N.; Hsu, S.H.; Carroll, H.A.; Harrop, E.; Collins, S.E.; Lustyk, M.K. Relative Efficacy of Mindfulness-Based Relapse Prevention, Standard Relapse Prevention, and Treatment as Usual for Substance Use Disorders: A Randomized Clinical Trial. JAMA Psychiatry 2014, 71, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Witkiewitz, K.; Bowen, S.; Douglas, H.; Hsu, S.H. Mindfulness-Based Relapse Prevention for Substance Craving. Addict. Behav. 2013, 38, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, N.T.; van Vugt, M.K.; Vago, D.R.; Schmalzl, L.; Saron, C.D.; Olendzki, A.; Meissner, T.; Lazar, S.W.; Kerr, C.E.; Gorchov, J. Mind the Hype: A Critical Evaluation and Prescriptive Agenda for Research on Mindfulness and Meditation. Perspect. Psychol. Sci. 2018, 13, 36–61. [Google Scholar] [CrossRef] [PubMed]
- Baer, R.A. Mindfulness Training as a Clinical Intervention: A Conceptual and Empirical Review. Clin. Psychol. Sci. Pract. 2003, 10, 125–143. [Google Scholar] [CrossRef]
- Baer, R.A.; Smith, G.T.; Hopkins, J.; Krietemeyer, J.; Toney, L. Using Self-Report Assessment Methods to Explore Facets of Mindfulness. Assessment 2006, 13, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.W.; Ryan, R.M.; Creswell, J.D. Mindfulness: Theoretical Foundations and Evidence for Its Salutary Effects. Psychol. Inq. 2007, 18, 211–237. [Google Scholar] [CrossRef]
- Coffey, K.A.; Hartman, M.; Fredrickson, B.L. Deconstructing Mindfulness and Constructing Mental Health: Understanding Mindfulness and Its Mechanisms of Action. Mindfulness 2010, 1, 235–253. [Google Scholar] [CrossRef]
- Hölzel, B.K.; Lazar, S.W.; Gard, T.; Schuman-Olivier, Z.; Vago, D.R.; Ott, U. How Does Mindfulness Meditation Work? Proposing Mechanisms of Action from a Conceptual and Neural Perspective. Perspect. Psychol. Sci. 2011, 6, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.L.; Carlson, L.E.; Astin, J.A.; Freedman, B. Mechanisms of Mindfulness. J. Clin. Psychol. 2006, 62, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.S.; Arnkoff, D.B.; Glass, C.R. The Neuroscience of Mindfulness: How Mindfulness Alters the Brain and Facilitates Emotion Regulation. Mindfulness 2017, 8, 1471–1487. [Google Scholar] [CrossRef]
- Chiesa, A.; Calati, R.; Serretti, A. Does Mindfulness Training Improve Cognitive Abilities? A Systematic Review of Neuropsychological Findings. Clin. Psychol. Rev. 2011, 31, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Y.; Hölzel, B.K.; Posner, M.I. The Neuroscience of Mindfulness Meditation. Nat. Rev. Neurosci. 2015, 16, 213. [Google Scholar] [CrossRef] [PubMed]
- Vago, D.R.; Silbersweig, D.A. Self-Awareness, Self-Regulation, and Self-Transcendence (S-ART): A Framework for Understanding the Neurobiological Mechanisms of Mindfulness. Front. Hum. Neurosci. 2012, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Kummar, A.S. Mindfulness and fear extinction: A brief review of its current neuropsychological literature and possible implications for posttraumatic stress disorder. Psychol. Rep. 2017, 121, 792–814. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Murphy, F. Claustrophobia in Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. Radiography 2015, 21, e59–e63. [Google Scholar] [CrossRef]
- Fisher, S.F.; Lanius, R.A.; Frewen, P.A. EEG Neurofeedback as Adjunct to Psychotherapy for Complex Developmental Trauma-Related Disorders: Case Study and Treatment Rationale. Traumatology 2016, 22, 255–260. [Google Scholar] [CrossRef]
- Gil, M.N.; Marco, C.E.; Montero-Marín, J.; Zafra, J.M.; Shonin, E.; Campayo, J.G. Efficacy of Neurofeedback on the Increase of Mindfulness-Related Capacities in Healthy Individuals: A Controlled Trial. Mindfulness 2018, 9, 303–311. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life; Hyperion: New York, NY, USA, 1994. [Google Scholar]
- La Forge, R. Aligning Mind and Body: Exploring the Disciplines of Mindful Exercise. ACSM Health Fit. J. 2005, 9, 7–14. [Google Scholar] [CrossRef]
- Siegel, D.J. The Mindful Brain: Reflection and Attunement in the Cultivation of Well-Being; WW Norton & Company: New York, NY, USA, 2007. [Google Scholar]
- Davis, D.M.; Hayes, J.A. What are the benefits of mindfulness? A practice review of psychotherapy-related research. Psychotherapy 2011, 48, 198–208. [Google Scholar] [CrossRef]
- Lutz, A.; Slagter, H.A.; Dunne, J.D.; Davidson, R.J. Attention Regulation and Monitoring in Meditation. Trends Cogn. Sci. 2008, 12, 163–169. [Google Scholar] [CrossRef]
- Lippelt, D.P.; Hommel, B.; Colzato, L.S. Focused Attention, Open Monitoring and Loving Kindness Meditation: Effects on Attention, Conflict Monitoring, and Creativity—A Review. Front. Psychol. 2014, 5, 1083. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. An Outpatient Program in Behavioral Medicine for Chronic Pain Patients Based on the Practice of Mindfulness Meditation: Theoretical Considerations and Preliminary Results. Gen. Hosp. Psychiatry 1982, 4, 33–47. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. Full Catastrophe Living: The Program of the Stress Reduction Clinic at the University of Massachusetts Medical Center; Delta: New York, NY, USA, 1990. [Google Scholar]
- Segal, Z.V.; Williams, J.M.G.; Teasdale, J.D. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse; Guildford Press: New York, NY, USA, 2002. [Google Scholar]
- Segal, Z.V.; Teasdale, J.D.; Williams, J.M.G. Mindfulness-Based Cognitive Therapy: Theoretical Rationale and Empirical Status. In Mindfulness and Acceptance: Expanding the Cognitive-Behavioral Tradition; Hayes, S.C., Follette, V.M., Linehan, M.M., Eds.; Guildford Press: New York, NY, USA, 2004; pp. 45–65. [Google Scholar]
- Cullen, M. Mindfulness-Based Interventions: An Emerging Phenomenon. Mindfulness 2011, 2, 186–193. [Google Scholar] [CrossRef]
- Shonin, E.; Van Gordon, W.; Griffiths, M. Mindfulness-Based Interventions: Towards Mindful Clinical Integration. Front. Psychol. 2013, 4, 194. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.M.; Davis, M. Mechanisms of Fear Extinction. Mol. Psychiatry 2007, 12, 120–150. [Google Scholar] [CrossRef] [PubMed]
- Bouton, M.E. Context and Behavioral Processes in Extinction. Learn. Mem. 2004, 11, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Haubrich, J.; Crestani, A.P.; Cassini, L.F.; Santana, F.; Sierra, R.O.; de O Alvares, L.; Quillfeldt, J.A. Reconsolidation Allows Fear Memory to Be Updated to a Less Aversive Level through the Incorporation of Appetitive Information. Neuropsychopharmacology 2015, 40, 315. [Google Scholar] [CrossRef] [PubMed]
- Schiller, D.; Monfils, M.-H.; Raio, C.M.; Johnson, D.C.; LeDoux, J.E.; Phelps, E.A. Preventing the Return of Fear in Humans Using Reconsolidation Update Mechanisms. Nature 2010, 463, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Gershman, S.J.; Jones, C.E.; Norman, K.A.; Monfils, M.-H.; Niv, Y. Gradual Extinction Prevents the Return of Fear: Implications for the Discovery of State. Front. Behav. Neurosci. 2013, 7, 164. [Google Scholar] [CrossRef]
- Maren, S.; Phan, K.L.; Liberzon, I. The Contextual Brain: Implications for Fear Conditioning, Extinction and Psychopathology. Nat. Rev. Neurosci. 2013, 14, 417–428. [Google Scholar] [CrossRef]
- Milad, M.R.; Quirk, G.J. Fear Extinction as a Model for Translational Neuroscience: Ten Years of Progress. Annu. Rev. Psychol. 2012, 63, 129–151. [Google Scholar] [CrossRef]
- Davidson, R.J.; Jackson, D.C.; Kalin, N.H. Emotion, Plasticity, Context, and Regulation: Perspectives from Affective Neuroscience. Psychol. Bull. 2000, 126, 890–909. [Google Scholar] [CrossRef]
- Milad, M.R.; Wright, C.I.; Orr, S.P.; Pitman, R.K.; Quirk, G.J.; Rauch, S.L. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol. Psychiatry 2007, 62, 446–454. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; John Wiley and Sons, Inc.: New York, NY, USA, 1962. [Google Scholar]
- Johansen, J.P.; Cain, C.K.; Ostroff, L.E.; LeDoux, J.E. Molecular Mechanisms of Fear Learning and Memory. Cell 2011, 147, 509–524. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Michel, C.M.; Lehmann, D. Low Resolution Electromagnetic Tomography: A New Method for Localizing Electrical Activity in the Brain. Int. J. Psychophysiol. 1994, 18, 49–65. [Google Scholar] [CrossRef]
- Miltner, W.H.; Trippe, R.H.; Krieschel, S.; Gutberlet, I.; Hecht, H.; Weiss, T. Event-Related Brain Potentials and Affective Responses to Threat in Spider/Snake-Phobic and Non-Phobic Subjects. Int. J. Psychophysiol. 2005, 57, 43–52. [Google Scholar] [CrossRef]
- Schienle, A.; Schäfer, A.; Naumann, E. Event-Related Brain Potentials of Spider Phobics to Disorder-Relevant, Generally Disgust-and Fear-Inducing Pictures. J. Psychophysiol. 2008, 22, 5–13. [Google Scholar] [CrossRef]
- Olofsson, J.K.; Nordin, S.; Sequeira, H.; Polich, J. Affective Picture Processing: An Integrative Review of ERP Findings. Biol. Psychol. 2008, 77, 247–265. [Google Scholar] [CrossRef]
- Wessa, M.; Flor, H. Failure of Extinction of Fear Responses in Posttraumatic Stress Disorder: Evidence from Second-Order Conditioning. Am. J. Psychiatry 2007, 164, 1684–1692. [Google Scholar] [CrossRef]
- Linden, D.E. The P300: Where in the Brain Is It Produced and What Does It Tell Us? Neuroscientist 2005, 11, 563–576. [Google Scholar] [CrossRef]
- Polich, J.; Kok, A. Cognitive and Biological Determinants of P300: An Integrative Review. Biol. Psychol. 1995, 41, 103–146. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An Integrative Theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Cuthbert, B.N.; Schupp, H.T.; Bradley, M.M.; Birbaumer, N.; Lang, P.J. Brain Potentials in Affective Picture Processing: Covariation with Autonomic Arousal and Affective Report. Biol. Psychol. 2000, 52, 95–111. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. Motivated Attention: Affect, Activation, and Action. In Attention and Orienting: Sensory and Motivational Processes; Lang, P.J., Simmons, R.F., Balaban., M., Eds.; Erlbaum: Hillsdale, NJ, USA, 1997; pp. 97–135. [Google Scholar]
- Ritter, W.; Ruchkin, D.S. A Review of Event-Related Potential Components Discovered in the Context of Studying P3a. Ann. N. Y. Acad. Sci. 1992, 658, 1–32. [Google Scholar] [CrossRef]
- Schupp, H.T.; Cuthbert, B.N.; Bradley, M.M.; Cacioppo, J.T.; Ito, T.; Lang, P.J. Affective Picture Processing: The Late Positive Potential Is Modulated by Motivational Relevance. Psychophysiology 2000, 37, 257–261. [Google Scholar] [CrossRef]
- Schupp, H.T.; Öhman, A.; Junghöfer, M.; Weike, A.I.; Stockburger, J.; Hamm, A.O. The Facilitated Processing of Threatening Faces: An ERP Analysis. Emotion 2004, 4, 189–200. [Google Scholar] [CrossRef]
- Mahjoory, K.; Nikulin, V.V.; Botrel, L.; Linkenkaer-Hansen, K.; Fato, M.M.; Haufe, S. Consistency of EEG Source Localization and Connectivity Estimates. Neuroimage 2017, 152, 590–601. [Google Scholar] [CrossRef]
- Moezzi, B.; Goldsworthy, M.R. Commentary: Consistency of EEG source localization and connectivity estimates. Front. Neurosci. 2018, 12, 590–601. [Google Scholar] [CrossRef]
- Fenton, G.E.; Pollard, A.K.; Halliday, D.M.; Mason, R.; Bredy, T.W.; Stevenson, C.W. Persistent Prelimbic Cortex Activity Contributes to Enhanced Learned Fear Expression in Females. Learn. Mem. 2014, 21, 55–60. [Google Scholar] [CrossRef]
- Fenton, G.E.; Halliday, D.M.; Mason, R.; Bredy, T.W.; Stevenson, C.W. Sex Differences in Learned Fear Expression and Extinction Involve Altered Gamma Oscillations in Medial Prefrontal Cortex. Neurobiol. Learn. Mem. 2016, 135, 66–72. [Google Scholar] [CrossRef]
- Mueller, E.M.; Panitz, C.; Hermann, C.; Pizzagalli, D.A. Prefrontal Oscillations during Recall of Conditioned and Extinguished Fear in Humans. J. Neurosci. 2014, 34, 7059–7066. [Google Scholar] [CrossRef]
- Paré, D.; Collins, D.R.; Pelletier, J.G. Amygdala Oscillations and the Consolidation of Emotional Memories. Trends Cogn. Sci. 2002, 6, 306–314. [Google Scholar] [CrossRef]
- Stujenske, J.M.; Likhtik, E.; Topiwala, M.A.; Gordon, J.A. Fear and Safety Engage Competing Patterns of Theta-Gamma Coupling in the Basolateral Amygdala. Neuron 2014, 83, 919–933. [Google Scholar] [CrossRef]
- Lesting, J.; Narayanan, R.T.; Kluge, C.; Sangha, S.; Seidenbecher, T.; Pape, H.-C. Patterns of Coupled Theta Activity in Amygdala-Hippocampal-Prefrontal Cortical Circuits during Fear Extinction. PLoS ONE 2011, 6, e21714. [Google Scholar] [CrossRef]
- Lesting, J.; Daldrup, T.; Narayanan, V.; Himpe, C.; Seidenbecher, T.; Pape, H.-C. Directional Theta Coherence in Prefrontal Cortical to Amygdalo-Hippocampal Pathways Signals Fear Extinction. PLoS ONE 2013, 8, e77707. [Google Scholar] [CrossRef]
- Hayes, S.C.; Wilson, K.G.; Gifford, E.V.; Follette, V.M.; Strosahl, K. Experiential Avoidance and Behavioral Disorders: A Functional Dimensional Approach to Diagnosis and Treatment. J. Consult. Clin. Psychol. 1996, 64, 1152–1168. [Google Scholar] [CrossRef]
- Amihai, I.; Kozhevnikov, M. Arousal vs. Relaxation: A Comparison of the Neurophysiological and Cognitive Correlates of Vajrayana and Theravada Meditative Practices. PLoS ONE 2014, 9, e102990. [Google Scholar] [CrossRef]
- Atchley, R.; Klee, D.; Memmott, T.; Goodrich, E.; Wahbeh, H.; Oken, B. Event-Related Potential Correlates of Mindfulness Meditation Competence. Neuroscience 2016, 320, 83–92. [Google Scholar] [CrossRef]
- Berkovich-Ohana, A.; Glicksohn, J.; Goldstein, A. Mindfulness-Induced Changes in Gamma Band Activity–Implications for the Default Mode Network, Self-Reference and Attention. Clin. Neurophysiol. 2012, 123, 700–710. [Google Scholar] [CrossRef]
- Berkovich-Ohana, A.; Glicksohn, J.; Goldstein, A. Studying the Default Mode and Its Mindfulness-Induced Changes Using EEG Functional Connectivity. Soc. Cogn. Affect. Neurosci. 2013, 9, 1616–1624. [Google Scholar] [CrossRef]
- Braboszcz, C.; Cahn, B.R.; Levy, J.; Fernandez, M.; Delorme, A. Increased Gamma Brainwave Amplitude Compared to Control in Three Different Meditation Traditions. PLoS ONE 2017, 12, e0170647. [Google Scholar] [CrossRef]
- Brown, K.W.; Goodman, R.J.; Inzlicht, M. Dispositional Mindfulness and the Attenuation of Neural Responses to Emotional Stimuli. Soc. Cogn. Affect. Neurosci. 2012, 8, 93–99. [Google Scholar] [CrossRef]
- Cahn, B.R.; Delorme, A.; Polich, J. Occipital Gamma Activation during Vipassana Meditation. Cogn. Process. 2010, 11, 39–56. [Google Scholar] [CrossRef]
- Cahn, B.R.; Delorme, A.; Polich, J. Event-Related Delta, Theta, Alpha and Gamma Correlates to Auditory Oddball Processing during Vipassana Meditation. Soc. Cogn. Affect. Neurosci. 2012, 8, 100–111. [Google Scholar] [CrossRef]
- Delgado-Pastor, L.C.; Perakakis, P.; Subramanya, P.; Telles, S.; Vila, J. Mindfulness (Vipassana) Meditation: Effects on P3b Event-Related Potential and Heart Rate Variability. Int. J. Psychophysiol. 2013, 90, 207–214. [Google Scholar] [CrossRef]
- Eddy, M.D.; Brunyé, T.T.; Tower-Richardi, S.; Mahoney, C.R.; Taylor, H.A. The Effect of a Brief Mindfulness Induction on Processing of Emotional Images: An ERP Study. Front. Psychol. 2015, 6. [Google Scholar] [CrossRef]
- Egan, R.P.; Hill, K.E.; Foti, D. Differential Effects of State and Trait Mindfulness on the Late Positive Potential. Emotion 2018, 18, 1128. [Google Scholar] [CrossRef]
- Hauswald, A.; Übelacker, T.; Leske, S.; Weisz, N. What It Means to Be Zen: Marked Modulations of Local and Interareal Synchronization during Open Monitoring Meditation. NeuroImage 2015, 108, 265–273. [Google Scholar] [CrossRef]
- Lakey, C.E.; Berry, D.R.; Sellers, E.W. Manipulating Attention via Mindfulness Induction Improves P300-Based Brain–Computer Interface Performance. J. Neural Eng. 2011, 8, 025019. [Google Scholar] [CrossRef]
- Lehmann, D.; Faber, P.L.; Tei, S.; Pascual-Marqui, R.D.; Milz, P.; Kochi, K. Reduced Functional Connectivity between Cortical Sources in Five Meditation Traditions Detected with Lagged Coherence Using EEG Tomography. Neuroimage 2012, 60, 1574–1586. [Google Scholar] [CrossRef]
- Lutz, A.; Greischar, L.L.; Rawlings, N.B.; Ricard, M.; Davidson, R.J. Long-Term Meditators Self-Induce High-Amplitude Gamma Synchrony during Mental Practice. Proc. Natl. Acad. Sci. USA 2004, 101, 16369–16373. [Google Scholar] [CrossRef]
- Milz, P.; Faber, P.L.; Lehmann, D.; Kochi, K.; Pascual-Marqui, R.D. SLORETA Intracortical Lagged Coherence during Breath Counting in Meditation-Naïve Participants. Front. Hum. Neurosci. 2014, 8, 303. [Google Scholar] [CrossRef]
- Slagter, H.A.; Lutz, A.; Greischar, L.L.; Francis, A.D.; Nieuwenhuis, S.; Davis, J.M.; Davidson, R.J. Mental Training Affects Distribution of Limited Brain Resources. PLoS Biol. 2007, 5, e138. [Google Scholar] [CrossRef]
- Sobolewski, A.; Holt, E.; Kublik, E.; Wróbel, A. Impact of Meditation on Emotional Processing—A Visual ERP Study. Neurosci. Res. 2011, 71, 44–48. [Google Scholar] [CrossRef]
- Van Leeuwen, S.; Singer, W.; Melloni, L. Meditation Increases the Depth of Information Processing and Improves the Allocation of Attention in Space. Front. Hum. Neurosci. 2012, 6, 133. [Google Scholar] [CrossRef]
- Wong, K.F.; Teng, J.; Chee, M.W.; Doshi, K.; Lim, J. Positive Effects of Mindfulness-Based Training on Energy Maintenance and the EEG Correlates of Sustained Attention in a Cohort of Nurses. Front. Hum. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef]
- Lomas, T.; Ivtzan, I.; Fu, C.H. A Systematic Review of the Neurophysiology of Mindfulness on EEG Oscillations. Neurosci. Biobehav. Rev. 2015, 57, 401–410. [Google Scholar] [CrossRef]
- Fell, J.; Axmacher, N.; Haupt, S. From Alpha to Gamma: Electrophysiological Correlates of Meditation-Related States of Consciousness. Med. Hypotheses 2010, 75, 218–224. [Google Scholar] [CrossRef]
- Lee, D.J.; Kulubya, E.; Goldin, P.; Goodarzi, A.; Girgis, F. Review of the Neural Oscillations Underlying Meditation. Front. Neurosci. 2018, 12, 178. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. The Spherical Spline Laplacian Does Not Produce Artifactually High Coherences: Comments on Two Articles by Biggins et al. Electroencephalogr. Clin. Neurophysiol. 1993, 87, 62–66. [Google Scholar] [CrossRef]
- Fani, N.; Tone, E.B.; Phifer, J.; Norrholm, S.D.; Bradley, B.; Ressler, K.J.; Kamkwalala, A.; Jovanovic, T. Attention Bias toward Threat Is Associated with Exaggerated Fear Expression and Impaired Extinction in PTSD. Psychol. Med. 2012, 42, 533–543. [Google Scholar] [CrossRef]
- Milad, M.R.; Orr, S.P.; Lasko, N.B.; Chang, Y.; Rauch, S.L.; Pitman, R.K. Presence and Acquired Origin of Reduced Recall for Fear Extinction in PTSD: Results of a Twin Study. J. Psychiatr. Res. 2008, 42, 515–520. [Google Scholar] [CrossRef]
- Milad, M.R.; Pitman, R.K.; Ellis, C.B.; Gold, A.L.; Shin, L.M.; Lasko, N.B.; Zeidan, M.A.; Handwerger, K.; Orr, S.P.; Rauch, S.L. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol. Psychiatry 2009, 66, 1075–1082. [Google Scholar] [CrossRef]
- Norrholm, S.D.; Jovanovic, T.; Olin, I.W.; Sands, L.A.; Bradley, B.; Ressler, K.J. Fear Extinction in Traumatized Civilians with Posttraumatic Stress Disorder: Relation to Symptom Severity. Biol. Psychiatry 2011, 69, 556–563. [Google Scholar] [CrossRef]
- VanElzakker, M.B.; Dahlgren, M.K.; Davis, F.C.; Dubois, S.; Shin, L.M. From Pavlov to PTSD: The Extinction of Conditioned Fear in Rodents, Humans, and Anxiety Disorders. Neurobiol. Learn. Mem. 2014, 113, 3–18. [Google Scholar] [CrossRef]
- Cusack, K.; Jonas, D.E.; Forneris, C.A.; Wines, C.; Sonis, J.; Middleton, J.C.; Feltner, C.; Brownley, K.A.; Olmsted, K.R.; Greenblatt, A. Psychological Treatments for Adults with Posttraumatic Stress Disorder: A Systematic Review and Meta-Analysis. Clin. Psychol. Rev. 2016, 43, 128–141. [Google Scholar] [CrossRef]
- Steenkamp, M.M.; Litz, B.T.; Hoge, C.W.; Marmar, C.R. Psychotherapy for Military-Related PTSD: A Review of Randomized Clinical Trials. JAMA 2015, 314, 489–500. [Google Scholar] [CrossRef]
- Watkins, L.E.; Sprang, K.R.; Rothbaum, B. Treating PTSD: A Review of Evidence-Based Psychotherapy Interventions. Front. Behav. Neurosci. 2018, 12, 258. [Google Scholar] [CrossRef]
- Watts, B.V.; Schnurr, P.P.; Mayo, L.; Young-Xu, Y.; Weeks, W.B.; Friedman, M.J. Meta-Analysis of the Efficacy of Treatments for Posttraumatic Stress Disorder. J. Clin. Psychiatry 2013, 74, e541–e550. [Google Scholar] [CrossRef]
- Imel, Z.E.; Laska, K.; Jakupcak, M.; Simpson, T.L. Meta-Analysis of Dropout in Treatments for Posttraumatic Stress Disorder. J. Consult. Clin. Psychol. 2013, 81, 394–404. [Google Scholar] [CrossRef]
- King, A.P.; Favorite, T.K. Mindfulness-Based Cognitive Therapy. In Innovative Applications; Eisendrath, S.J., Ed.; Springer: Berlin, Germany, 2016; pp. 163–189. [Google Scholar]
- Follette, V.M.; Palm, K.M.; Hall, M.L.R. Acceptance, Mindfulness, and Trauma. In Mindfulness and Acceptance: Expanding the Cognitive–Behavioral Tradition; Hayes, S.C., Follette, V., Linehan, M.M., Eds.; Guildford: New York, NY, USA, 2004; pp. 192–208. [Google Scholar]
- King, A.P.; Block, S.R.; Sripada, R.K.; Rauch, S.; Giardino, N.; Favorite, T.; Angstadt, M.; Kessler, D.; Welsh, R.; Liberzon, I. Altered Default Mode Network (DMN) Resting State Functional Connectivity Following a Mindfulness-Based Exposure Therapy for Posttraumatic Stress Disorder (PTSD) in Combat Veterans of Afghanistan and Iraq. Depress. Anxiety 2016, 33, 289–299. [Google Scholar] [CrossRef]
- Seligowski, A.V.; Lee, D.J.; Bardeen, J.R.; Orcutt, H.K. Emotion Regulation and Posttraumatic Stress Symptoms: A Meta-Analysis. Cogn. Behav. Ther. 2015, 44, 87–102. [Google Scholar] [CrossRef]
- Call, D.; Pitcock, J.; Pyne, J. Longitudinal Evaluation of the Relationship between Mindfulness, General Distress, Anxiety, and PTSD in a Recently Deployed National Guard Sample. Mindfulness 2015, 6, 1303–1312. [Google Scholar] [CrossRef]
- Follette, V.; Palm, K.M.; Pearson, A.N. Mindfulness and Trauma: Implications for Treatment. J. Ration. Emot. Cogn. Behav. Ther. 2006, 24, 45–61. [Google Scholar] [CrossRef]
- Garland, E.L.; Roberts-Lewis, A. Differential Roles of Thought Suppression and Dispositional Mindfulness in Posttraumatic Stress Symptoms and Craving. Addict. Behav. 2013, 38, 1555–1562. [Google Scholar] [CrossRef]
- Kearney, D.J.; McDermott, K.; Malte, C.; Martinez, M.; Simpson, T.L. Association of Participation in a Mindfulness Program with Measures of PTSD, Depression and Quality of Life in a Veteran Sample. J. Clin. Psychol. 2012, 68, 101–116. [Google Scholar] [CrossRef]
- Kearney, D.J.; McDermott, K.; Malte, C.; Martinez, M.; Simpson, T.L. Effects of Participation in a Mindfulness Program for Veterans with Posttraumatic Stress Disorder: A Randomized Controlled Pilot Study. J. Clin. Psychol. 2013, 69, 14–27. [Google Scholar] [CrossRef]
- King, A.P.; Erickson, T.M.; Giardino, N.D.; Favorite, T.; Rauch, S.A.; Robinson, E.; Kulkarni, M.; Liberzon, I. A Pilot Study of Group Mindfulness-Based Cognitive Therapy (MBCT) for Combat Veterans with Posttraumatic Stress Disorder (PTSD). Depress. Anxiety 2013, 30, 638–645. [Google Scholar] [CrossRef]
- Owens, G.P.; Walter, K.H.; Chard, K.M.; Davis, P.A. Changes in Mindfulness Skills and Treatment Response among Veterans in Residential PTSD Treatment. Psychol. Trauma 2012, 4, 221. [Google Scholar] [CrossRef]
- Polusny, M.A.; Erbes, C.R.; Thuras, P.; Moran, A.; Lamberty, G.J.; Collins, R.C.; Rodman, J.L.; Lim, K.O. Mindfulness-Based Stress Reduction for Posttraumatic Stress Disorder among Veterans: A Randomized Clinical Trial. JAMA 2015, 314, 456–465. [Google Scholar] [CrossRef]
- Smith, B.W.; Ortiz, J.A.; Steffen, L.E.; Tooley, E.M.; Wiggins, K.T.; Yeater, E.A.; Montoya, J.D.; Bernard, M.L. Mindfulness Is Associated with Fewer PTSD Symptoms, Depressive Symptoms, Physical Symptoms, and Alcohol Problems in Urban Firefighters. J. Consult. Clin. Psychol. 2011, 79, 613–617. [Google Scholar] [CrossRef]
- Thompson, B.L.; Waltz, J. Mindfulness and Experiential Avoidance as Predictors of Posttraumatic Stress Disorder Avoidance Symptom Severity. J. Anxiety Disord. 2010, 24, 409–415. [Google Scholar] [CrossRef]
- Kuyken, W.; Crane, W.; Williams, J.M. Mindfulness-Based Cognitive Therapy (MBCT) Implementation Resources; Oxford University; University of Exeter; Bangor University: Oxford, UK, 2012. [Google Scholar]
- Linehan, M.M. Dialectical Behavior Therapy for Borderline Personality Disorder: Theory and Method. Bull. Menn. Clin. 1987, 51, 261. [Google Scholar]
- King, A.P.; Block, S.R.; Sripada, R.K.; Rauch, S.A.; Porter, K.E.; Favorite, T.K.; Giardino, N.; Liberzon, I. A Pilot Study of Mindfulness-Based Exposure Therapy in OEF/OIF Combat Veterans with PTSD: Altered Medial Frontal Cortex and Amygdala Responses in Social–Emotional Processing. Front. Psychiatry 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Blake, D.D.; Weathers, F.W.; Nagy, L.M.; Kaloupek, D.G.; Gusman, F.D.; Charney, D.S.; Keane, T.M. The Development of a Clinician-Administered PTSD Scale. J. Trauma. Stress 1995, 8, 75–90. [Google Scholar] [CrossRef]
- Lobo, I.; Portugal, L.C.; Figueira, I.; Volchan, E.; David, I.; Pereira, M.G.; de Oliveira, L. EEG Correlates of the Severity of Posttraumatic Stress Symptoms: A Systematic Review of the Dimensional PTSD Literature. J. Affect. Disord. 2015, 183, 210–220. [Google Scholar] [CrossRef]
- Javanbakht, A.; Liberzon, I.; Amirsadri, A.; Gjini, K.; Boutros, N.N. Event-Related Potential Studies of Post-Traumatic Stress Disorder: A Critical Review and Synthesis. Biol. Mood Anxiety Disord. 2011, 1, 5. [Google Scholar] [CrossRef]
- Johnson, J.D.; Allana, T.N.; Medlin, M.D.; Harris, E.W.; Karl, A. Meta-Analytic Review of P3 Components in Posttraumatic Stress Disorder and Their Clinical Utility. Clin. EEG Neurosci. 2013, 44, 112–134. [Google Scholar] [CrossRef]
- Karl, A.; Malta, L.S.; Maercker, A. Meta-Analytic Review of Event-Related Potential Studies in Post-Traumatic Stress Disorder. Biol. Psychol. 2006, 71, 123–147. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yoon, S.; Kim, J.-I.; Jin, S.-H.; Chung, C.K. Functional Connectivity of Resting State EEG and Symptom Severity in Patients with Post-Traumatic Stress Disorder. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2014, 51, 51–57. [Google Scholar] [CrossRef]
- Graham, B.M.; Milad, M.R. The Study of Fear Extinction: Implications for Anxiety Disorders. Am. J. Psychiatry 2011, 168, 1255–1265. [Google Scholar] [CrossRef]
| Study | Sample (Meditators/Control) | Form of Mindfulness/Meditation | EEG Analyses | Findings |
|---|---|---|---|---|
| Amihai and Kozhevnikov (2014) [72] | 10 long-term Theravada meditators (average 8 years of practice)/9 long-term Vajrayana meditators (average 7.4 years of practice) | Theraveda: Vipassana (OM), Kasina (FA); Vajrayana: Deity (OM), Rig-pa (FA) | Spectral power; Coherence |
|
| Atchley et al. (2016) [73] | 13 non-meditators/15 novice meditators/14 experienced meditators | Mindfulness-based breath counting during tone task | ERP |
|
| Berkovich-Ohana et al. (2012) [74] | 36 mindfulness meditators/12 novice controls | Mindfulness meditation (state mindfulness); Resting state (trait mindfulness) | Spectral power |
|
| Berkovich-Ohana et al. (2013) [75] | 36 mindfulness meditators/12 novice controls | Mindfulness meditation (state mindfulness); Resting state (trait mindfulness) | Mean Phase Coherence (MPC) |
|
| Braboszcz et al. (2017) [76] | 20 Vipassana/20 Himalayan Yoga/27 Isha Shoonya/32 Control | Vipassana, Himalayan Yoga, Isha Shoonya | Spectral power |
|
| Brown et al. (2012) [77] | 46 psychology undergraduates (within-subjects design) | Dispositional mindfulness | ERP |
|
| Cahn et al. (2010) [78] | 16 long-term meditators (within-subjects design) | Vipassana meditation | Spectral power |
|
| Cahn et al. (2012) [79] | 16 long-term meditators (within-subjects design) | Vipassana (mindfulness) meditation | Spectral power; Coherence |
|
| Delgado-Pastor et al. (2013) [80] | 10 experienced meditators (within-subjects design) | Vipassana (mindfulness) meditation | ERP |
|
| Eddy et al. (2015) [81] | 24 participants (within-subjects design) | Induced mindfulness (through focused breathing) | ERP |
|
| Egan et al. (2017) [82] | 118 adult sample (within-subjects design) | Brief mindfulness instructions | ERP |
|
| Hauswald et al. (2015) [83] | 11 meditators (within-subjects design) | Zen | Spectral power |
|
| Lakey et al. (2011) [84] | 9 naïve meditators/9 control | Short, 6 minutes mindfulness induction | ERP |
|
| Lehmann et al. (2012) [85] | 13 Tibetan Buddhists/15 QiGong practitioners/14 Sahaja Yoga practitioners/14 Ananda Marga Yoga practitioners/15 Zen practitioners | Various (including Zen) | Spectral power; Lagged intracortical coherence; Head-surface conventional coherence |
|
| Lutz et al. (2004) [86] | 8 long-term Buddhist practitioners/10 healthy student volunteers | Loving kindness and compassion-focused meditation | Spectral power; Coherence |
|
| Miltz et al. (2014) [87] | 23 naïve meditators (within-subjects design) | Breath counting (indicative of a meditative state) | Spectral power; Lagged intracortical coherence; Head-surface conventional coherence |
|
| Slagter et al. (2007) [88] | 17 participants/23 control (mixed design) | 3-month meditation (Vipassana) retreat | ERP |
|
| Sobolewski et al. (2011) [89] | 13 meditators/13 control | Mindfulness meditation | ERP |
|
| van Leeuwen et al. (2012) [90] | 8 Buddhist monks and nuns/8 control 8 experienced FA meditators/6 controls (mixed design) | Zen (FA and OM meditation practices) 4-day OM meditation retreat | ERP ERP |
|
| Wong et al. (2018) [91] | 36 nurses (longitudinal design) | 8-week MBT (based on MBSR) | Spectral power; ERP |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kummar, A.S.; Correia, H.; Fujiyama, H. A Brief Review of the EEG Literature on Mindfulness and Fear Extinction and its Potential Implications for Posttraumatic Stress Symptoms (PTSS). Brain Sci. 2019, 9, 258. https://doi.org/10.3390/brainsci9100258
Kummar AS, Correia H, Fujiyama H. A Brief Review of the EEG Literature on Mindfulness and Fear Extinction and its Potential Implications for Posttraumatic Stress Symptoms (PTSS). Brain Sciences. 2019; 9(10):258. https://doi.org/10.3390/brainsci9100258
Chicago/Turabian StyleKummar, Auretta S., Helen Correia, and Hakuei Fujiyama. 2019. "A Brief Review of the EEG Literature on Mindfulness and Fear Extinction and its Potential Implications for Posttraumatic Stress Symptoms (PTSS)" Brain Sciences 9, no. 10: 258. https://doi.org/10.3390/brainsci9100258
APA StyleKummar, A. S., Correia, H., & Fujiyama, H. (2019). A Brief Review of the EEG Literature on Mindfulness and Fear Extinction and its Potential Implications for Posttraumatic Stress Symptoms (PTSS). Brain Sciences, 9(10), 258. https://doi.org/10.3390/brainsci9100258




