Sample Size for Oxidative Stress and Inflammation When Treating Multiple Sclerosis with Interferon-β1a and Coenzyme Q10
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Laboratory Analyses
2.3. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tur, C.; Moccia, M.; Barkhof, F.; Chataway, J.; Sastre-Garriga, J.; Thompson, A.J.; Ciccarelli, O. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat. Rev. Neurol. 2018, 14, 75–93. [Google Scholar] [CrossRef]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Zaratin, P.; Comi, G.; Coetzee, T.; Ramsey, K.; Smith, K.; Thompson, A.; Panzara, M. Progressive MS Alliance Industry Forum: Maximizing Collective Impact to Enable Drug Development. Trends Pharmacol. Sci. 2016, 37, 808–810. [Google Scholar] [CrossRef]
- Haider, L.; Zrzavy, T.; Hametner, S.; Höftberger, R.; Bagnato, F.; Grabner, G.; Trattnig, S.; Pfeifenbring, S.; Brück, W.; Lassmann, H. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 2016, 139, 807–815. [Google Scholar] [CrossRef]
- Friese, M.A.; Schattling, B.; Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2014, 10, 225–238. [Google Scholar] [CrossRef]
- Moccia, M.; Capacchione, A.; Lanzillo, R.; Carbone, F.; Micillo, T.; Perna, F.; De Rosa, A.; Carotenuto, A.; Albero, R.; Matarese, G.; et al. Coenzyme Q10 supplementation reduces peripheral oxidative stress and inflammation in Interferon-Beta1a treated multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Sedgwick, P. What is a crossover trial? BMJ 2014, 348, 9–10. [Google Scholar] [CrossRef]
- Altmann, D.R.; Jasperse, B.; Barkhof, F.; Beckmann, K.; Filippi, M.; Kappos, L.D.; Molyneux, P.; Polman, C.H.; Pozzilli, C.; Thompson, A.J.; et al. Sample sizes for brain atrophy outcomes in trials for secondary progressive multiple sclerosis. Neurology 2009, 72, 595–601. [Google Scholar] [CrossRef]
- Moccia, M.; Prados, F.; Filippi, M.; Rocca, M.A.; Valsasina, P.; Brownlee, W.J.; Zecca, C.; Gallo, A.; Rovira, A.; Gass, A.; et al. Longitudinal spinal cord atrophy in multiple sclerosis using the generalised boundary shift integral. Ann. Neurol. 2019. [Google Scholar] [CrossRef]
- Li, G.; Taljaard, M.; Van den Heuvel, E.R.; Levine, M.A.; Cook, D.J.; Wells, G.A.; Devereaux, P.J.; Thabane, L. An introduction to multiplicity issues in clinical trials: The what, why, when and how. Int. J. Epidemiol. 2017, 46, 746–755. [Google Scholar] [CrossRef]
- Pocock, S. Group sequential methods in the design and analysis of clinical trials. Biometrika 1977, 64, 191–199. [Google Scholar] [CrossRef]
- Fox, R.; Chataway, J. Advancing Trial Design in Progressive Multiple Sclerosis. Mult. Scler. 2017, 23, 1573–1578. [Google Scholar] [CrossRef]
- Moccia, M.; Palladino, R.; Carotenuto, A.; Saccà, F.; Russo, C.V.; Lanzillo, R.; Brescia Morra, V. A 8-year retrospective cohort study comparing Interferon-β formulations for relapsing-remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 19, 50–54. [Google Scholar] [CrossRef]
- Sanoobar, M.; Eghtesadi, S.; Azimi, A.; Khalili, M.; Khodadadi, B.; Jazayeri, S.; Gohari, M.R.; Aryaeian, N. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: A double blind, placebo, controlled randomized clinical trial. Nutr. Neurosci. 2015, 18, 169–176. [Google Scholar] [CrossRef]
- Sanoobar, M.; Eghtesadi, S.; Azimi, A.; Khalili, M.; Jazayeri, S.; Gohari, M.R. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Int. J. Neurosci. 2013, 123, 776–782. [Google Scholar] [CrossRef]
- Sanoobar, M.; Dehghan, P.; Khalil, M.; Azimi, A.; Seifar, F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: A double blind randomized clinical trial. Nutr. Neurosci. 2016, 19, 138–143. [Google Scholar] [CrossRef]
- Göbel, K.; Ruck, T.; Meuth, S.G. Cytokine signaling in multiple sclerosis: Lost in translation. Mult. Scler. J. 2018, 24, 432–439. [Google Scholar] [CrossRef]
- Lee, P.W.; Xin, M.K.; Pei, W.; Yang, Y.; Lovett-Racke, A.E. IL-3 Is a Marker of Encephalitogenic T Cells, but Not Essential for CNS Autoimmunity. Front. Immunol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Lin, C.-C.; Edelson, B.T. New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef]
- Bassi, M.S.; Iezzi, E.; Landi, D.; Monteleone, F.; Gilio, L.; Simonelli, I.; Musella, A.; Mandolesi, G.; De Vito, F.; Furlan, R.; et al. Delayed treatment of MS is associated with high CSF levels of IL-6 and IL-8 and worse future disease course. J. Neurol. 2018, 265, 2540–2547. [Google Scholar] [CrossRef]
- Tavakolpour, S. Interleukin 7 receptor polymorphisms and the risk of multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2016, 8, 66–73. [Google Scholar] [CrossRef]
- Guglielmetti, C.; Le Blon, D.; Santermans, E.; Salas-Perdomo, A.; Daans, J.; De Vocht, N.; Shah, D.; Hoornaert, C.; Praet, J.; Peerlings, J.; et al. Interleukin-13 immune gene therapy prevents CNS inflammation and demyelination via alternative activation of microglia and macrophages. Glia 2016, 64, 2181–2200. [Google Scholar] [CrossRef]
- Hu, W.T.; Howell, J.C.; Ozturk, T.; Gangishetti, U.; Kollhoff, A.L.; Hatcher-Martin, J.M.; Anderson, A.M.; Tyor, W.R. CSF Cytokines in Aging, Multiple Sclerosis, and Dementia. Front. Immunol. 2019, 10, 480. [Google Scholar] [CrossRef]
- Moccia, M.; Lanzillo, R.; Palladino, R.; Russo, C.; Carotenuto, A.; Massarelli, M.; Vacca, G.; Vacchiano, V.; Nardone, A.; Triassi, M.; et al. Uric acid: A potential biomarker of multiple sclerosis and of its disability. Clin. Chem. Lab. Med. 2015, 53, 753–759. [Google Scholar] [CrossRef]
- Moccia, M.; Lanzillo, R.; Costabile, T.; Russo, C.; Carotenuto, A.; Sasso, G.; Postiglione, E.; De Luca Picione, C.; Vastola, M.; Maniscalco, G.T.; et al. Uric acid in relapsing-remitting multiple sclerosis: A 2-year longitudinal study. J. Neurol. 2015, 262, 961–967. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.; Nicholas, R.; Cruciani, C.; Castellaro, M.; Romualdi, C.; Rossi, S.; Pittieri, M.; Benedetti, M.; Gajofatto, A.; et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann. Neurol. 2018, 83, 739–755. [Google Scholar] [CrossRef]
- Lanzillo, R.; Carbone, F.; Quarantelli, M.; Bruzzese, D.; Carotenuto, A.; De Rosa, V.; Colamatteo, A.; Micillo, T.; De Luca Picione, C.; Saccà, F.; et al. Immunometabolic profiling of patients with multiple sclerosis identifies new biomarkers to predict disease activity during treatment with interferon Interferon beta-1a. Clin. Immunol. 2017, 183, 249–253. [Google Scholar] [CrossRef]
- Ziliotto, N.; Bernardi, F.; Jakimovski, D.; Baroni, M.; Bergsland, N.; Ramasamy, D.P.; Weinstock-Guttman, B.; Zamboni, P.; Marchetti, G.; Zivadinov, R.; et al. Increased CCL18 plasma levels are associated with neurodegenerative MRI outcomes in multiple sclerosis patients. Mult. Scler. Relat. Disord. 2018, 25, 37–42. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Cignarella, F.; Bollman, B.; Cross, A.H.; Salter, A.; Galimberti, D.; Cella, M.; Piccio, L. T cells producing GM-CSF and IL-13 are enriched in the cerebrospinal fluid of relapsing MS patients. Mult. Scler. 2019, 1352458519852092. [Google Scholar] [CrossRef]
- Gibellini, L.; De Biasi, S.; Bianchini, E.; Bartolomeo, R.; Fabiano, A.; Manfredini, M.; Ferrari, F.; Albertini, G.; Trenti, T.; Nasi, M.; et al. Anti-TNF-α drugs differently affect the TNFα-sTNFR system and monocyte subsets in patients with psoriasis. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Ellrichmann, G.; Bolz, J.; Peschke, M.; Duscha, A.; Hellwig, K.; Lee, D.H.; Linker, R.A.; Gold, R.; Haghikia, A. Peripheral CD19 + B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. J. Neurol. 2019, 266, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Moccia, M.; de Stefano, N.; Barkhof, F. Imaging outcomes measures for progressive multiple sclerosis trials. Mult. Scler. 2017, 23, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Cawley, N.; Tur, C.; Prados, F.; Plantone, D.; Kearney, H.; Abdel-Aziz, K.; Ourselin, S.; Wheeler-Kingshott, C.A.M.G.; Miller, D.H.; Thompson, A.J.; et al. Spinal cord atrophy as a primary outcome measure in phase II trials of progressive multiple sclerosis. Mult. Scler. 2018, 24, 932–941. [Google Scholar] [CrossRef] [PubMed]
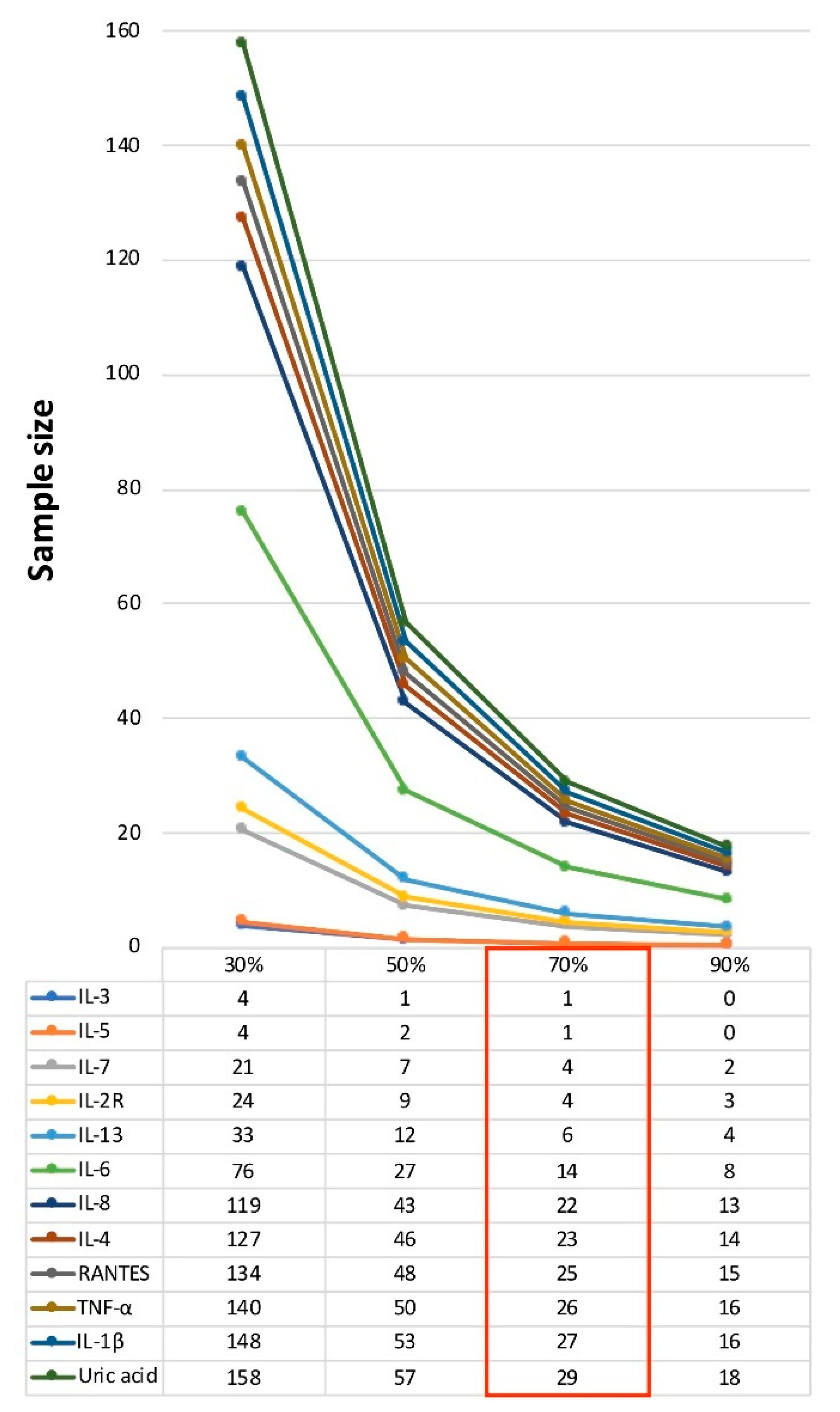
| Baseline | Adj. Coeff. (3-Month Variation) | SD (3-Month Variation) | Sample Size (70% Treatment Effect) | ||||
|---|---|---|---|---|---|---|---|
| One Primary Outcome | Two Primary Outcomes | Interim Analyses (Pocock Method) | |||||
| One Interim | Two Interim | ||||||
| 5% alpha | 2.5% alpha | 2.94% alpha | 2.21% alpha | ||||
| Markers of scavenging activity | |||||||
| Uric acid (mg/dL) | 4.670 ± 0.566 | 0.123 * | 0.117 | 29 | 37 | 15 | 11 |
| Bilirubin (mg/dL) | 1.466 ± 0.268 | 0.066 | 0.190 | 265 | 323 | 134 | 98 |
| Markers of oxidative damage | |||||||
| CellROX cells (%) | 76.405 ± 9.348 | −9.925 * | 11.25 | 41 | 52 | 21 | 15 |
| CellROX cells (MFI) | 2605.320 ± 828.707 | −523.308 * | 1124.538 | 148 | 181 | 75 | 55 |
| Protein carbonyls (nmol/mg) | 2.976 ± 1.402 | −0.266 | 1.393 | 878 | 1066 | 444 | 326 |
| 8-OHdG (ng/mL) | 6.379 ± 1.140 | −0.630 * | 0.708 | 40 | 51 | 20 | 15 |
| Markers of inflammation | |||||||
| EGF (pg/mL) | 6.597 ± 12.877 | −3.637 | 8.513 | 175 | 214 | 89 | 65 |
| Eotaxin (pg/mL) | 116.432 ± 46.800 | −18.669 * | 31.968 | 94 | 116 | 47 | 35 |
| Basic-FGF (pg/mL) | 53.218 ± 282.165 | −2.736 | 4.863 | 101 | 124 | 51 | 38 |
| G-CSF (pg/mL) | 80.445 ± 48.370 | −4.692 | 61.503 | 5498 | 6667 | 2783 | 2041 |
| GM-CSF (pg/mL) | 5.791 ± 4.953 | −1.751 * | 2.524 | 66 | 82 | 34 | 25 |
| HGF (pg/mL) | 64.959 ± 77.650 | −26.397 * | 33.925 | 53 | 66 | 27 | 20 |
| IFN-α (pg/mL) | 80.869 ± 469.445 | 1.780 | 11.498 | 1335 | 1618 | 676 | 496 |
| IFN-γ (pg/mL) | 2.311 ± 1.952 | −1.526 * | 1.937 | 52 | 64 | 26 | 19 |
| IL-1α (pg/mL) | 4.427 ± 6.477 | −2.460 * | 2.526 | 34 | 43 | 17 | 13 |
| IL-1β (pg/mL) | 1.694 ± 7.274 | −1.188 | 1.096 | 27 | 35 | 14 | 10 |
| IL-1RA (pg/mL) | 33.085 ± 39.824 | −10.464 | 18.329 | 98 | 121 | 50 | 36 |
| IL-2 (pg/mL) | 20.979 ± 107.943 | 5.099 | 14.090 | 244 | 298 | 124 | 91 |
| IL-2R (pg/mL) | 105.950 ± 61.462 | −29.971 * | 11.182 | 4 | 8 | 2 | 2 |
| IL-3 (pg/mL) | 202.849 ± 1283.170 | 28.661 | 4.276 | 1 | 3 | 0 | 0 |
| IL-4 (pg/mL) | 4.421 ± 11.691 | 3.883 * | 3.317 | 23 | 30 | 12 | 9 |
| IL-5 (pg/mL) | 8.177 ± 33.861 | −12.890 | 2.069 | 1 | 3 | 0 | 0 |
| IL-6 (pg/mL) | 62.945 ± 363.131 | 5.559 | 3.671 | 14 | 19 | 7 | 5 |
| IL-7 (pg/mL) | 12.871 ± 40.625 | −16.428 | 5.639 | 4 | 7 | 2 | 1 |
| IL-8 (pg/mL) | 12.095 ± 7.422 | −11.418 | 9.425 | 22 | 28 | 11 | 8 |
| IL-9 (pg/mL) | 2.248 ± 4.814 | −3.749 * | 4.212 | 40 | 51 | 20 | 15 |
| IL-10 (pg/mL) | 1079.590 ± 6456.040 | 1615.546 | 2417.951 | 72 | 89 | 36 | 27 |
| IL-12 (pg/mL) | 58.932 ± 110.51 | 2.498 | 14.365 | 1058 | 1284 | 536 | 393 |
| IL-13 (pg/mL) | 1.714 ± 3.341 | 3.732 * | 1.628 | 6 | 9 | 3 | 2 |
| IL-15 (pg/mL) | 117.149 ± 673.398 | 21.693 | 21.658 | 32 | 40 | 16 | 12 |
| IL-17A (pg/mL) | 1.460 ± 2.265 | −0.453 | 0.941 | 138 | 169 | 70 | 51 |
| IL-17F (pg/mL) | 35.954 ± 86.735 | −68.854 * | 72.039 | 35 | 44 | 18 | 13 |
| IL-22 (pg/mL) | 250.425 ± 642.791 | −8.406 | 40.134 | 729 | 886 | 369 | 271 |
| IP-10 (pg/mL) | 26.279 ± 16.844 | 5.699 | 30.460 | 914 | 1110 | 463 | 339 |
| MCP-1 (pg/mL) | 232.083 ± 79.633 | 39.540 | 96.247 | 190 | 232 | 96 | 70 |
| MIG (pg/mL) | 32.386 ± 13.580 | −5.409 | 13.555 | 201 | 245 | 102 | 75 |
| MIP-1α (pg/mL) | 7.830 ± 11.718 | −5.327 * | 5.338 | 32 | 41 | 16 | 12 |
| MIP-1β (pg/mL) | 182.476 ± 1024.490 | 17.125 | 17.060 | 32 | 40 | 16 | 12 |
| RANTES (pg/mL) | 1739.970 ± 1475.350 | −2331.281 * | 2041.081 | 25 | 32 | 12 | 9 |
| TNF-α (pg/mL) | 2.725 ± 4.310 | −1.795 * | 1.608 | 26 | 33 | 13 | 10 |
| VEGF (pg/mL) | 0.619 ± 0.777 | −0.398 * | 0.519 | 54 | 68 | 28 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moccia, M.; Capacchione, A.; Lanzillo, R.; Carbone, F.; Micillo, T.; Matarese, G.; Palladino, R.; Brescia Morra, V. Sample Size for Oxidative Stress and Inflammation When Treating Multiple Sclerosis with Interferon-β1a and Coenzyme Q10. Brain Sci. 2019, 9, 259. https://doi.org/10.3390/brainsci9100259
Moccia M, Capacchione A, Lanzillo R, Carbone F, Micillo T, Matarese G, Palladino R, Brescia Morra V. Sample Size for Oxidative Stress and Inflammation When Treating Multiple Sclerosis with Interferon-β1a and Coenzyme Q10. Brain Sciences. 2019; 9(10):259. https://doi.org/10.3390/brainsci9100259
Chicago/Turabian StyleMoccia, Marcello, Antonio Capacchione, Roberta Lanzillo, Fortunata Carbone, Teresa Micillo, Giuseppe Matarese, Raffaele Palladino, and Vincenzo Brescia Morra. 2019. "Sample Size for Oxidative Stress and Inflammation When Treating Multiple Sclerosis with Interferon-β1a and Coenzyme Q10" Brain Sciences 9, no. 10: 259. https://doi.org/10.3390/brainsci9100259
APA StyleMoccia, M., Capacchione, A., Lanzillo, R., Carbone, F., Micillo, T., Matarese, G., Palladino, R., & Brescia Morra, V. (2019). Sample Size for Oxidative Stress and Inflammation When Treating Multiple Sclerosis with Interferon-β1a and Coenzyme Q10. Brain Sciences, 9(10), 259. https://doi.org/10.3390/brainsci9100259






