Feasibility of a Community-Based Boxing Program with Tailored Balance Training in Parkinson’s Disease: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
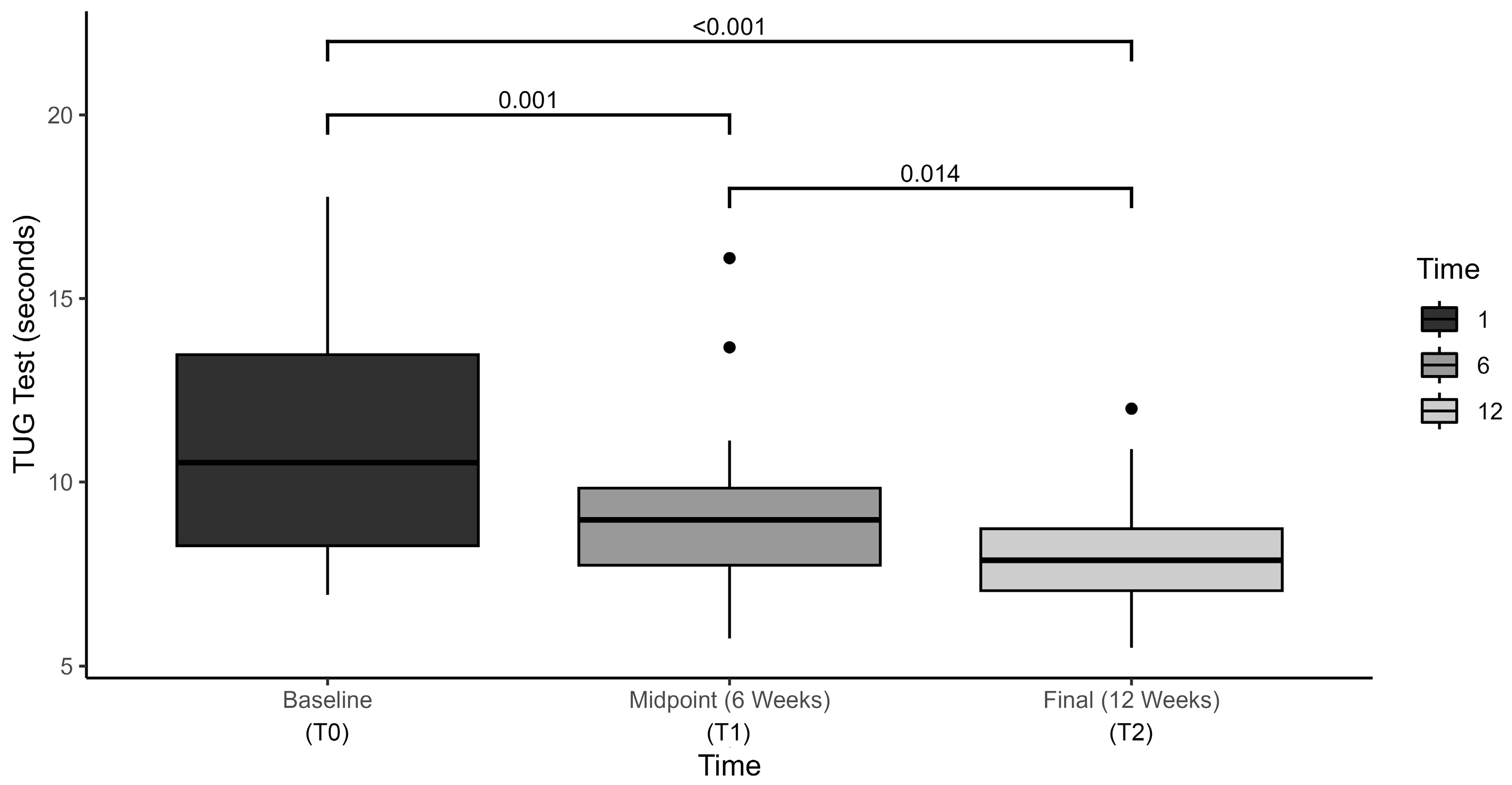
3.1. Functional Mobility Outcomes
3.2. Balance Outcomes
3.3. Gait Parameters
3.4. Falls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| CBP | Community-based boxing programs |
| BMI | Body Mass Index |
| UPDRS | Unified Parkinson Disease Rating Scale |
| CTSIB-M | Modified Clinical Test of Sensory Interaction in Balance |
| TUG | Timed Up and Go |
| FRT | Functional Reach Test |
| 5-STS | Five Times Sit-to-Stand |
| BBS | Berg Balance Scale |
| ABC | Activities-specific Balance Confidence Scale |
References
- Pelicioni, P.H.S.; Menant, J.C.; Latt, M.D.; Lord, S.R. Falls in Parkinson’s Disease Subtypes: Risk Factors, Locations and Circumstances. Int. J. Environ. Res. Public Health 2019, 16, 2216. [Google Scholar] [CrossRef]
- Contreras, A.; Grandas, F. Risk of falls in Parkinson’s disease: A cross-sectional study of 160 patients. Park. Dis. 2012, 2012, 362572. [Google Scholar] [CrossRef]
- Allen, N.E.; Schwarzel, A.K.; Canning, C.G. Recurrent falls in Parkinson’s disease: A systematic review. Park. Dis. 2013, 2013, 906274. [Google Scholar] [CrossRef]
- Murueta-Goyena, A.; Muiño, O.; Gómez-Esteban, J.C. Prognostic factors for falls in Parkinson’s disease: A systematic review. Acta Neurol. Belg. 2024, 124, 395–406. [Google Scholar] [CrossRef]
- Paul, S.S.; Sherrington, C.; Canning, C.G.; Fung, V.S.; Close, J.C.; Lord, S.R. The relative contribution of physical and cognitive fall risk factors in people with Parkinson’s disease: A large prospective cohort study. Neurorehabilit. Neural Repair. 2014, 28, 282–290. [Google Scholar] [CrossRef]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Dibble, L.E.; Addison, O.; Papa, E. The effects of exercise on balance in persons with Parkinson’s disease: A systematic review across the disability spectrum. J. Neurol. Phys. Ther. 2009, 33, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Yee, E.; Willis, B.W.; Prost, E.L.; Gray, A.D.; Mann, J.B. A Community-based Boxing Program is Associated with Improved Balance in Individuals with Parkinson’s Disease. Int. J. Exerc. Sci. 2021, 14, 876–884. [Google Scholar]
- Shen, X.; Wong-Yu, I.S.; Mak, M.K. Effects of Exercise on Falls, Balance, and Gait Ability in Parkinson’s Disease: A Meta-analysis. Neurorehabilit. Neural Repair. 2016, 30, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, M.R.; Schmidt, P.N.; Luo, S.T.; Li, K.; Marras, C.; Davis, T.L.; Guttman, M.; Cubillos, F.; Simuni, T. Regular Exercise, Quality of Life, and Mobility in Parkinson’s Disease: A Longitudinal Analysis of National Parkinson Foundation Quality Improvement Initiative Data. J. Park. Dis. 2017, 7, 193–202. [Google Scholar] [CrossRef]
- Ellis, T.D.; Colón-Semenza, C.; DeAngelis, T.R.; Thomas, C.A.; Hilaire, M.S.; Earhart, G.M.; Dibble, L.E. Evidence for Early and Regular Physical Therapy and Exercise in Parkinson’s Disease. Semin. Neurol. 2021, 41, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Domingos, J.; Radder, D.; Riggare, S.; Godinho, C.; Dean, J.; Graziano, M.; de Vries, N.M.; Ferreira, J.; Bloem, B.R. Implementation of a Community-Based Exercise Program for Parkinson Patients: Using Boxing as an Example. J. Park. Dis. 2019, 9, 615–623. [Google Scholar] [CrossRef]
- Sharp, K.; Hewitt, J. Dance as an intervention for people with Parkinson’s disease: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2014, 47, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Z.; Harmer, P.; Fitzgerald, K.; Eckstrom, E.; Stock, R.; Galver, J.; Maddalozzo, G.; Batya, S.S. Tai Chi and Postural Stability in Patients with Parkinson’s Disease. N. Engl. J. Med. 2012, 366, 511–519. [Google Scholar] [CrossRef]
- Combs, S.A.; Diehl, M.D.; Chrzastowski, C.; Didrick, N.; McCoin, B.; Mox, N.; Staples, W.H.; Wayman, J. Community-based group exercise for persons with Parkinson disease: A randomized controlled trial. NeuroRehabilitation 2013, 32, 117–124. [Google Scholar] [CrossRef]
- Larson, D.; Bega, D.; Johnson, E.; Slowey, L. Effects of Rock Steady Boxing on Activities of Daily Living and Motor Symptoms of Parkinson’s Disease (P5.075). Neurology 2018, 90, P5.075. [Google Scholar] [CrossRef]
- Combs, S.A.; Diehl, M.D.; Staples, W.H.; Conn, L.; Davis, K.; Lewis, N.; Schaneman, K. Boxing training for patients with Parkinson disease: A case series. Phys. Ther. 2011, 91, 132–142. [Google Scholar] [CrossRef]
- Larson, D.; Yeh, C.; Rafferty, M.; Bega, D. High satisfaction and improved quality of life with Rock Steady Boxing in Parkinson’s disease: Results of a large-scale survey. Disabil. Rehabil. 2022, 44, 6034–6041. [Google Scholar] [CrossRef]
- Morris, M.E.; Ellis, T.D.; Jazayeri, D.; Heng, H.; Thomson, A.; Balasundaram, A.P.; Slade, S.C. Boxing for Parkinson’s Disease: Has Implementation Accelerated Beyond Current Evidence? Front. Neurol. 2019, 10, 1222. [Google Scholar] [CrossRef]
- Domingos, J.; de Lima, A.L.S.; Steenbakkers-van der Pol, T.; Godinho, C.; Bloem, B.R.; de Vries, N.M. Boxing with and without Kicking Techniques for People with Parkinson’s Disease: An Explorative Pilot Randomized Controlled Trial. J. Park. Dis. 2022, 12, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Sangarapillai, K.; Norman, B.M.; Almeida, Q.J. Boxing vs Sensory Exercise for Parkinson’s Disease: A Double-Blinded Randomized Controlled Trial. Neurorehabilit. Neural Repair. 2021, 35, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Nocera, J.R.; Stegemoller, E.L.; Malaty, I.A.; Okun, M.S.; Marsiske, M.; Hass, C.J. Using the Timed Up & Go test in a clinical setting to predict falling in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2013, 94, 1300–1305. [Google Scholar]
- Freeman, L.; Gera, G.; Horak, F.B.; Blackinton, M.T.; Besch, M.; King, L. Instrumented Test of Sensory Integration for Balance: A Validation Study. J. Geriatr. Phys. Ther. 2018, 41, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.; Seney, M. Test-Retest Reliability and Minimal Detectable Change on Balance and Ambulation Tests, the 36-Item Short-Form Health Survey, and the Unified Parkinson Disease Rating Scale in People With Parkinsonism. Phys. Ther. 2008, 88, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Hsieh, C.L.; Wu, R.M.; Tai, C.H.; Lin, C.H.; Lu, W.S. Minimal detectable change of the timed “up & go” test and the dynamic gait index in people with Parkinson disease. Phys. Ther. 2011, 91, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Morris, M.E.; Iansek, R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys. Ther. 2001, 81, 810–818. [Google Scholar] [CrossRef]
- Brusse, K.J.; Zimdars, S.; Zalewski, K.R.; Steffen, T.M. Testing functional performance in people with Parkinson disease. Phys. Ther. 2005, 85, 134–141. [Google Scholar] [CrossRef]
- Dal Bello-Haas, V.; Klassen, L.; Sheppard, M.S.; Metcalfe, A. Psychometric Properties of Activity, Self-Efficacy, and Quality-of-Life Measures in Individuals with Parkinson Disease. Physiother. Can. 2011, 63, 47–57. [Google Scholar] [CrossRef]
- Schenkman, M.; Cutson, T.M.; Kuchibhatla, M.; Chandler, J.; Pieper, C. Reliability of impairment and physical performance measures for persons with Parkinson’s disease. Phys. Ther. 1997, 77, 19–27. [Google Scholar] [CrossRef]
- Lim, L.I.; van Wegen, E.E.; de Goede, C.J.; Jones, D.; Rochester, L.; Hetherington, V.; Nieuwboer, A.; Willems, A.M.; Kwakkel, G. Measuring gait and gait-related activities in Parkinson’s patients own home environment: A reliability, responsiveness and feasibility study. Park. Relat. Disord. 2005, 11, 19–24. [Google Scholar] [CrossRef]
- Dibble, L.E.; Lange, M. Predicting Falls In Individuals with Parkinson Disease: A Reconsideration of Clinical Balance Measures. J. Neurol. Phys. Ther. 2006, 30, 60–67. [Google Scholar] [CrossRef]
- Behrman, A.L.; Light, K.E.; Flynn, S.M.; Thigpen, M.T. Is the functional reach test useful for identifying falls risk among individuals with Parkinson’s disease? Arch. Phys. Med. Rehabil. 2002, 83, 538–542. [Google Scholar] [CrossRef]
- Smithson, F.; Morris, M.E.; Iansek, R. Performance on clinical tests of balance in Parkinson’s disease. Phys. Ther. 1998, 78, 577–592. [Google Scholar] [CrossRef][Green Version]
- Leddy, A.L.; Crowner, B.E.; Earhart, G.M. Functional gait assessment and balance evaluation system test: Reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys. Ther. 2011, 91, 102–113. [Google Scholar] [CrossRef]
- Duncan, R.P.; Leddy, A.L.; Earhart, G.M. Five times sit-to-stand test performance in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2011, 92, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Lohnes, C.A.; Earhart, G.M. External validation of abbreviated versions of the activities-specific balance confidence scale in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Dodge, K.; Lynch, R.; Tippett, S.R. Reliability of real time motion analysis system VirtuBalance as compared to traditional measurement methods of functional reach and postural Sway. J. Stud. Phys. Ther. Res. 2015, 8, 141–149. [Google Scholar]
- Dinglasan, V.; Landers, M.R. Reliability and concurrent validity of a portable infrared camera to assess gait. In Proceedings of the American Physical Therapy Association Combined Sections Meeting, Washington, DC, USA, 23–26 January 2019. [Google Scholar]
- Strubhar, A.J.; Tan, P.; Storage, L.; Peterson, M. Concurrent Validity of the VirtuSense® Gait Analysis System for the Quantification of Spatial and Temporal Parameters of Gait. Int. J. Exerc. Sci. 2018, 11, 934–940. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice, 3rd ed.; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Permezel, F.; Alty, J.; Harding, I.H.; Thyagarajan, D. Brain Networks Involved in Sensory Perception in Parkinson’s Disease: A Scoping Review. Brain Sci. 2023, 13, 1552. [Google Scholar] [CrossRef]
- Lidsky, T.I.; Manetto, C.; Schneider, J.S. A consideration of sensory factors involved in motor functions of the basal ganglia. Brain Res. 1985, 356, 133–146. [Google Scholar] [CrossRef]
- Li, K.-y.; Pickett, K.A.; Fu, H.-w.; Chen, R.-s. Proprioceptive and olfactory deficits in individuals with Parkinson disease and mild cognitive impairment. Acta Neurol. Belg. 2024, 124, 419–430. [Google Scholar] [CrossRef]
- Patel, N.; Jankovic, J.; Hallett, M. Sensory aspects of movement disorders. Lancet Neurol. 2014, 13, 100–112. [Google Scholar] [CrossRef]
- Roytman, S.; Paalanen, N.; Carli, G.; Marusic, U.; Kanel, P.; van Laar, T.; Bohnen, N. Multisensory mechanisms of gait and balance in Parkinson’s disease: An integrative review. Neural Regen. Res. 2024, 20, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Vaugoyeau, M.; Viel, S.; Assaiante, C.; Amblard, B.; Azulay, J.P. Impaired vertical postural control and proprioceptive integration deficits in Parkinson’s disease. Neuroscience 2007, 146, 852–863. [Google Scholar] [CrossRef] [PubMed]
- McVey, M.A.; Amundsen, S.; Barnds, A.; Lyons, K.E.; Pahwa, R.; Mahnken, J.D.; Luchies, C.W. The effect of moderate Parkinson’s disease on compensatory backwards stepping. Gait Posture 2013, 38, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Hubble, R.P.; Naughton, G.A.; Silburn, P.A.; Cole, M.H. Trunk muscle exercises as a means of improving postural stability in people with Parkinson’s disease: A protocol for a randomised controlled trial. BMJ Open 2014, 4, e006095. [Google Scholar] [CrossRef]
- Jöbges, M.; Heuschkel, G.; Pretzel, C.; Illhardt, C.; Renner, C.; Hummelsheim, H. Repetitive training of compensatory steps: A therapeutic approach for postural instability in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1682–1687. [Google Scholar] [CrossRef]
- Allen, N.E.; Sherrington, C.; Paul, S.S.; Canning, C.G. Balance and falls in Parkinson’s disease: A meta-analysis of the effect of exercise and motor training. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 1605–1615. [Google Scholar] [CrossRef]
- Canning, C.G.; Sherrington, C.; Lord, S.R.; Close, J.C.; Heritier, S.; Heller, G.Z.; Howard, K.; Allen, N.E.; Latt, M.D.; Murray, S.M.; et al. Exercise for falls prevention in Parkinson disease: A randomized controlled trial. Neurology 2015, 84, 304–312. [Google Scholar] [CrossRef]
- Mollà-Casanova, S.; Pedrero-Sánchez, J.; Inglés, M.; López-Pascual, J.; Muñoz-Gómez, E.; Aguilar-Rodríguez, M.; Sempere-Rubio, N.; Serra-Añó, P. Impact of Parkinson’s Disease on Functional Mobility at Different Stages. Front. Aging Neurosci. 2022, 14, 935841. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.M.; Roschel, H.; Tricoli, V.; de Souza, E.O.; Wilson, J.M.; Laurentino, G.C.; Aihara, A.Y.; de Souza Leão, A.R.; Ugrinowitsch, C. Changes in exercises are more effective than in loading schemes to improve muscle strength. J. Strength Cond. Res./Natl. Strength Cond. Assoc. 2014, 28, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Peel, N. Validating recall of falls by older people. Accid. Anal. Prev. 2000, 32, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.A.; Dias, J.M.; Silva, S.L.; Dias, R.C. Prospective monitoring and self-report of previous falls among older women at high risk of falls and fractures: A study of comparison and agreement. Braz. J. Phys. Ther. 2015, 19, 218–226. [Google Scholar] [CrossRef]




| Participant | Sex | Age | BMI | Hoehn & Yahr | UPDRS Motor | Disease Duration | Fall Status |
|---|---|---|---|---|---|---|---|
| PD01 | M | 67 | 26.5 | 1.0 | 16 | 0.6 | NF |
| PD02 | F | 71 | 37.2 | 1.0 | 12 | 17 | NF |
| PD03 | M | 63 | 32.6 | 2.0 | 14 | 0.8 | NF |
| PD04 | M | 77 | 23.6 | 1.5 | 18 | 0.8 | F |
| PD05 | M | 59 | 25.0 | 3.0 | 19 | 1.5 | F |
| PD06 | M | 60 | 25.5 | 1.0 | 17 | 3 | NF |
| PD07 | M | 84 | 19.2 | 3.0 | 22 | 1 | F |
| PD08 | M | 73 | 24.3 | 1.0 | 13 | 4 | NF |
| PD09 | F | 73 | 30.0 | 2.5 | 12 | 1 | F |
| PD10 | M | 66 | 26.6 | 2.0 | 20 | 2 | NF |
| PD11 | F | 73 | 27.5 | 2.0 | 15 | 17 | NF |
| PD12 | M | 65 | 26.2 | 2.0 | 19 | 9 | F |
| PD13 | M | 71 | 23.8 | 2.5 | 12 | 6 | F |
| PD14 | M | 74 | 24.5 | 2.0 | 18 | 0.2 | NF |
| PD15 | M | 79 | 35.0 | 3.0 | 29 | 8 | F |
| PD16 | M | 71 | 26.6 | 2.0 | 16 | 7 | NF |
| PD17 | M | 74 | 34.8 | 3.0 | 28 | 11 | F |
| PD18 | F | 64 | 33.7 | 1.0 | 13 | 1.5 | NF |
| PD19 | F | 79 | 23.0 | 2.0 | 13 | 9 | NF |
| PD20 | F | 73 | 30.0 | 2.5 | 12 | 6 | NF |
| MEAN (SD) | 70.8 (6.4) | 27.8 (4.6) | 2.0 (0.7) | 16.9 (4.9) | 5.32 (5.2) |
| CTSIB-M Balance Input | Actions | Progressions |
|---|---|---|
| Visual | Head turns—standing with both feet on the ground slowly turn the head to the right and left. Eye tracking—holding an object in the hand, move the hand so the eyes track the object. Saccades—standing with both feet on the ground, keep the head stable and only move the eyes, all the way to the left, then quickly move the eyes to the right. |
|
| Vestibular | Adaptation (gaze stability) exercises—participants were asked to move their heads in a yaw rotation while focusing on a stationary, hand-held target, called “X1 viewing,” and to progress to “X2 viewing,” in which the target and the head rotated in equal and opposite yaw directions. Substitution exercises—participants were instructed to make smooth pursuit eye movements towards a target before the head moves. Habituation exercises—designed to mildly provoke individual symptoms, such as walking while turning the head sideways. |
|
| Somatosensory | The intention was to perform challenging balance exercises with focused attention on the somatosensory input for maintaining balance control. Specific activities included, heel-to-toe walking, balancing on a trampoline, balancing while seated on a ball, standing on foam or air-filled cushions, etc. |
|
| Measurement Tool | Construct | Evidence for Reliability | Evidence for Validity | Evidence for Responsiveness |
|---|---|---|---|---|
| Timed Up and Go (TUG) | Timed completion of rising from a chair, walking three meters, turning around, walking back to the chair, and sitting down. | Good test–retest reliability in PD (ICCs ≥ 0.80) [25,26] and excellent interrater reliability (r = 0.99) in PD [27] | Moderate to good convergent validity evidence in PD (correlated with the BBS (r = −0.78), fast gait speed (r = −0.69), and comfortable gait speed (r = −0.67) [28] | MDC = 4.85 s [29], also reported as MDC = 11 s [25] |
| Functional Reach Test (FRT) | Measurement of the maximum distance one can reach forward while standing in a fixed position. Provides a surrogate measure of dynamic balance and fall risk. | Excellent test–retest reliability in PD (ICC = 0.84) [30] and adequate intra-rater reliability (ICC = 0.74) [31] | Good predictive validity for future falls in PD [32,33] | MDC = 9 cm [31], also reported as MDC = 4.32 in patients with fall history [34], MDC = 8.07 in patients without fall history [34] |
| Berg Balance Scale (BBS) | A 14-item objective measure that assesses static balance and fall risk. | Excellent test–retest reliability (ICC = 0.94) [25] and excellent interrater reliability (ICC = 0.95) [35] | Excellent concurrent validity with the TUG (r = −0.78) and FRT (r = 0.50) [28] | MDC = 5 points [25] |
| Five Times Sit-to-Stand (5-STS) | Provides a method to quantify functional lower extremity strength and identify movement strategies to complete a transfer | Excellent test–retest reliability (ICC = 0.76) and excellent interrater reliability (ICC = 0.99) [36] | Excellent correlation with the MiniBEST test (r = 0.71) [36] | N/A |
| Activities Specific Balance Confidence Scale (ABC) | A self-report measure of balance confidence in performing various activities without losing balance or experiencing a sense of unsteadiness | Excellent test–retest reliability (ICC = 0.79) [29], (ICC = 0.96) [37] | Adequate concurrent validity with BBS (r = 0.505) [37] | MDC = 13 points [25] also reported as MDC = 11.12 points [29] |
| VSTBalance (cadence, gait speed) | Quantitative analysis of spatial and temporal gait measurements using machine vision | Good parallel forms reliability (r > 0.853) [38,39] | Excellent concurrent validity with Gait Rite (ICC > 0.971) [40] | N/A |
| Variable | T0 | T1 | T2 | p Value | Effect Size |
|---|---|---|---|---|---|
| Cadence (steps/min) | 104.2 (97.7–110.8) | 111.0 (103.4–118.6) | 110.5 (102.9–118.0) | 0.054 | 0.150 |
| Gait Speed (m/s) | 0.88 (0.794–0.956) | 1.0 (0.915–1.12) | 1.0 (0.932–1.07) | <0.001 | 0.372 |
| 5xSTS (sec) | 15.6 (13.9–17.2) | 13.4 (11.8–14.9) | 11.5 (10.0–13.1) | <0.001 | 0.736 |
| TUG (sec) | 11.0 (9.4–12.6) | 9.1 (7.9–10.3) | 8.1 (7.3–8.9) | <0.001 | 0.580 |
| BBS (total) | 40.5 (35.9–45.1) | 44.0 (39.8–48.2) | 46.8 (43.6–50.1) | <0.001 | 0.414 |
| FRT (cm) | 8.2 (6.8–9.3) | 8.4 (6.9–9.8) | 9.3 (7.9–10.6) | 0.215 | 0.082 |
| ABC (total) | 78.92 (74.7–83.3) | 79.71 (74.5–84.9) | 86.05 (81.5–90.6) | <0.001 | 0.442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, E.V.; Sawyer, K.E.; Smoliga, J.M. Feasibility of a Community-Based Boxing Program with Tailored Balance Training in Parkinson’s Disease: A Preliminary Study. Brain Sci. 2025, 15, 858. https://doi.org/10.3390/brainsci15080858
Papa EV, Sawyer KE, Smoliga JM. Feasibility of a Community-Based Boxing Program with Tailored Balance Training in Parkinson’s Disease: A Preliminary Study. Brain Sciences. 2025; 15(8):858. https://doi.org/10.3390/brainsci15080858
Chicago/Turabian StylePapa, Evan V., Kathryn E. Sawyer, and James M. Smoliga. 2025. "Feasibility of a Community-Based Boxing Program with Tailored Balance Training in Parkinson’s Disease: A Preliminary Study" Brain Sciences 15, no. 8: 858. https://doi.org/10.3390/brainsci15080858
APA StylePapa, E. V., Sawyer, K. E., & Smoliga, J. M. (2025). Feasibility of a Community-Based Boxing Program with Tailored Balance Training in Parkinson’s Disease: A Preliminary Study. Brain Sciences, 15(8), 858. https://doi.org/10.3390/brainsci15080858






