Integrated Workflow for Drug Repurposing in Glioblastoma: Computational Prediction and Preclinical Validation of Therapeutic Candidates
Abstract
1. Introduction
2. Materials and Methods
2.1. Glioma Biopsies Datasets
2.2. Drug Prediction Workflow
2.3. Potential Combination Analyses of Temozolomide with Other Drugs
2.4. Drugs
2.5. Cell Culture
2.6. In Vitro Validation of Therapeutic Potential
2.7. Wound Closure Assay
2.8. Statistical Analyses
3. Results
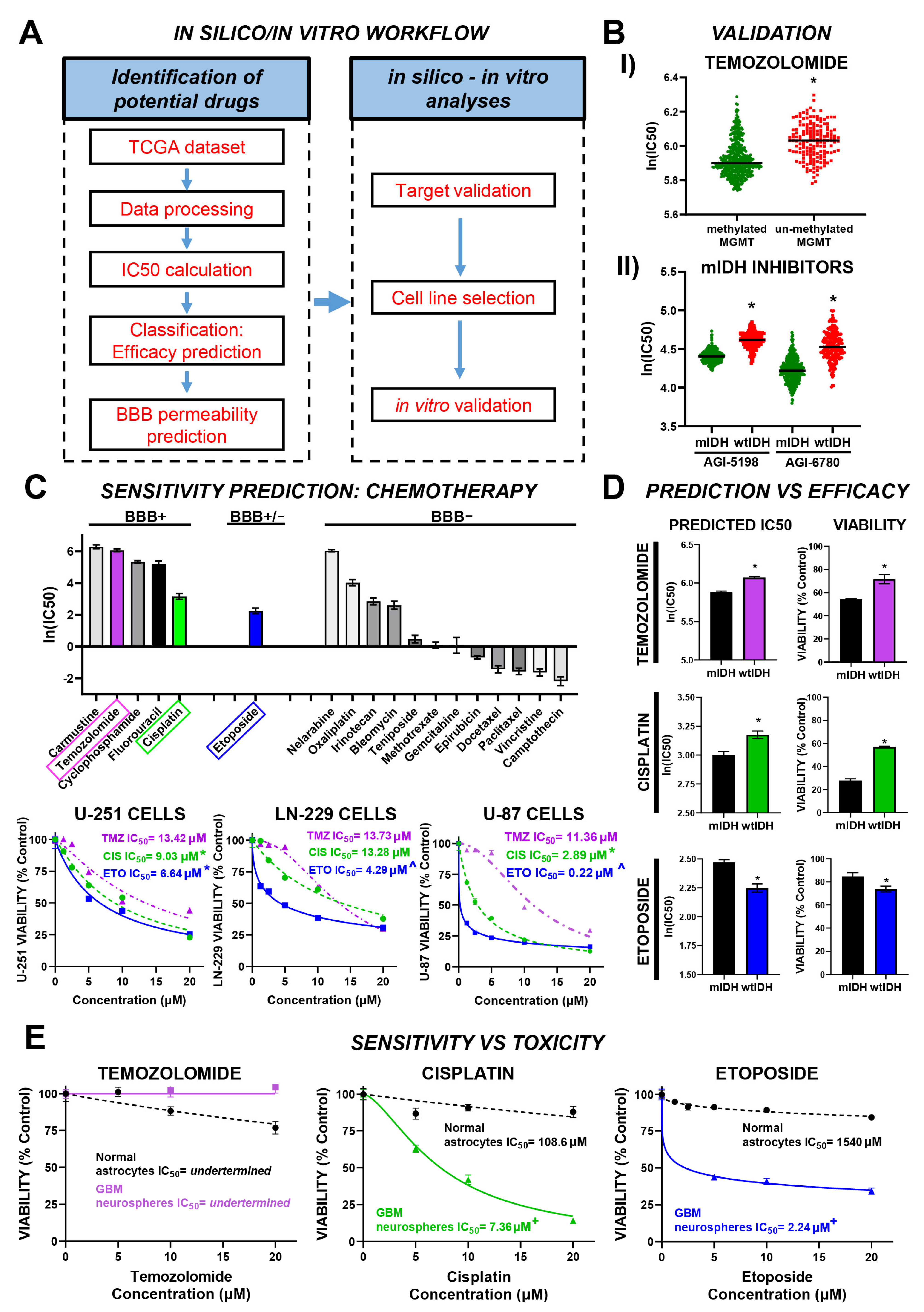
3.1. Efficacy Prediction Model for Chemotherapeutic Drugs with Potential Effect in GBM
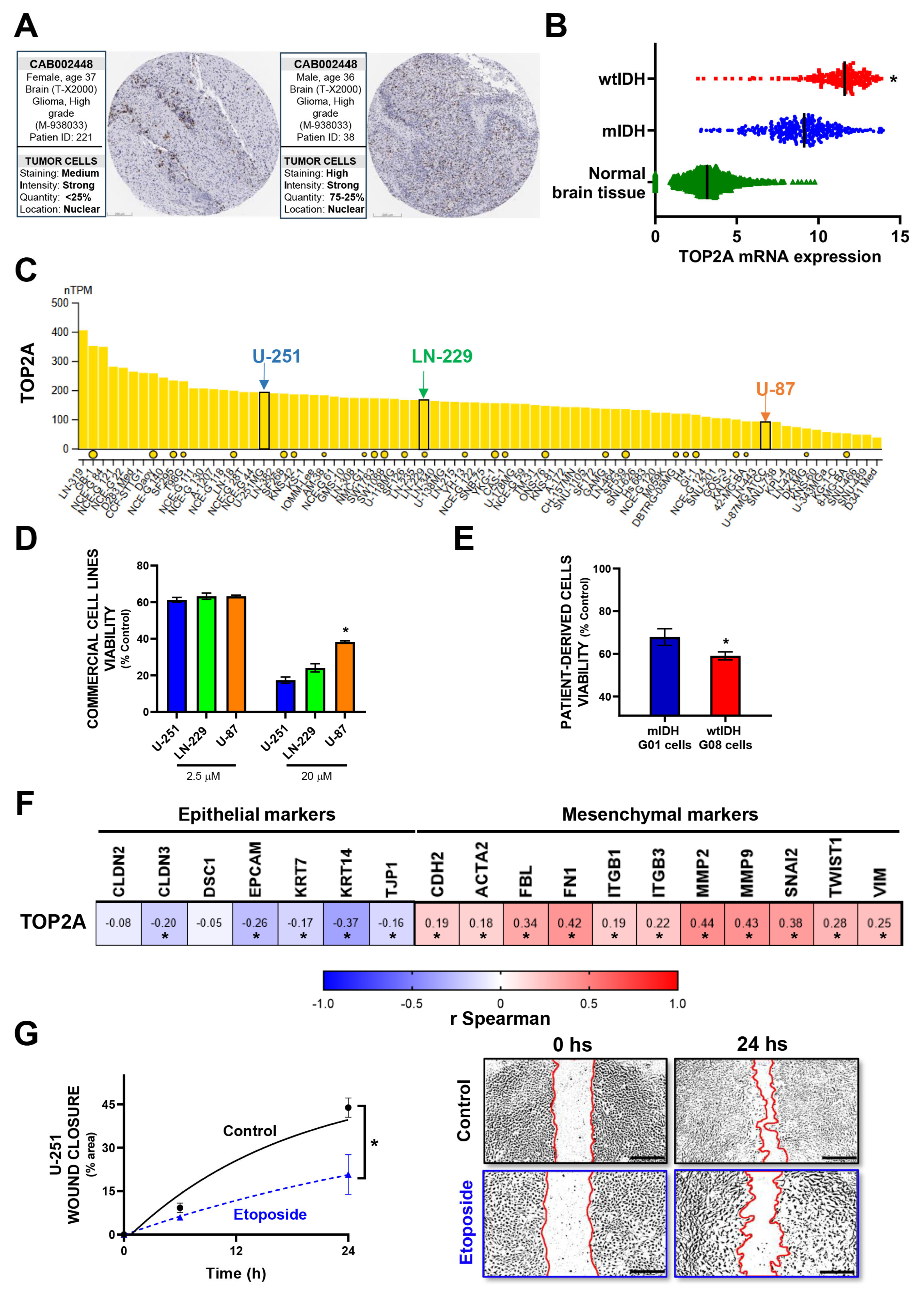
3.2. In Vitro Validation of Model-Predicted Etoposide Sensitivity in GBM Cells
3.3. Prediction Model for Alternative Drugs with Potential Therapeutic Effect in GBM
3.4. In Vitro Validation of Model-Predicted Daporinad Sensitivity in GBM Cells
3.5. Identification of Drug Combinations with Potential Therapeutic Efficacy in GBM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro-Oncol. 2023, 25 (Suppl. S4), iv1–iv99. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Reifenberger, G.; Wirsching, H.G.; Knobbe-Thomsen, C.B.; Weller, M. Advances in the molecular genetics of gliomas—Implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef]
- Masui, K.; Mischel, P.S.; Reifenberger, G. Molecular classification of gliomas. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 134, pp. 97–120. [Google Scholar]
- Wesseling, P.; Capper, D. WHO 2016 Classification of gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Weller, M.; Le Rhun, E.; Preusser, M.; Tonn, J.C.; Roth, P. How we treat glioblastoma. ESMO Open 2019, 4 (Suppl. S2), e000520. [Google Scholar] [CrossRef]
- Wen, J.; Chen, W.; Zhu, Y.; Zhang, P. Clinical features associated with the efficacy of chemotherapy in patients with glioblastoma (GBM): A surveillance, epidemiology, and end results (SEER) analysis. BMC Cancer 2021, 21, 81. [Google Scholar] [CrossRef]
- Cohen, M.H.; Johnson, J.R.; Pazdur, R. Food and Drug Administration Drug approval summary: Temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin. Cancer Res. 2005, 11 Pt 1, 6767–6771. [Google Scholar] [CrossRef]
- Olson, M.V. The human genome project. Proc. Natl. Acad. Sci. USA 1993, 90, 4338–4344. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets–update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Masica, D.L.; Karchin, R. Correlation of somatic mutation and expression identifies genes important in human glioblastoma progression and survival. Cancer Res. 2011, 71, 4550–4561. [Google Scholar] [CrossRef]
- Research, C.G.A.; Brat, D.J.; Verhaak, R.G.; Aldape, K.D.; Yung, W.K.; Salama, S.R.; Cooper, L.A.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar]
- Costello, J.C.; Heiser, L.M.; Georgii, E.; Gonen, M.; Menden, M.P.; Wang, N.J.; Bansal, M.; Ammad-ud-din, M.; Hintsanen, P.; Khan, S.A.; et al. A community effort to assess and improve drug sensitivity prediction algorithms. Nat. Biotechnol. 2014, 32, 1202–1212. [Google Scholar] [CrossRef]
- Aben, N.; Vis, D.J.; Michaut, M.; Wessels, L.F. TANDEM: A two-stage approach to maximize interpretability of drug response models based on multiple molecular data types. Bioinformatics 2016, 32, i413–i420. [Google Scholar] [CrossRef]
- Umbach, D.M.; Krahn, J.M.; Shats, I.; Li, X.; Li, L. Predicting tumor response to drugs based on gene-expression biomarkers of sensitivity learned from cancer cell lines. BMC Genom. 2021, 22, 272. [Google Scholar]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R., III; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Lv, M.; Pei, R.; Li, P.; Pei, Z.; Wang, Y.; Su, W.; Xie, X.-Q. AlzPlatform: An Alzheimer’s Disease Domain-Specific Chemogenomics Knowledgebase for Polypharmacology and Target Identification Research. J. Chem. Inf. Model. 2014, 54, 1050–1060. [Google Scholar] [CrossRef]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024, 52, W422–W431. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef]
- Nunez, F.J.; Mendez, F.M.; Kadiyala, P.; Alghamri, M.S.; Savelieff, M.G.; Garcia-Fabiani, M.B.; Haase, S.; Koschmann, C.; Calinescu, A.A.; Kamran, N.; et al. IDH1-R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci. Transl. Med. 2019, 11, eaaq1427. [Google Scholar] [CrossRef]
- Videla Richardson, G.A.; Garcia, C.P.; Roisman, A.; Slavutsky, I.; Espinosa, D.D.F.; Romorini, L.; Miriuka, S.G.; Arakaki, N.; Martinetto, H.; Scassa, M.E.; et al. Specific Preferences in Lineage Choice and Phenotypic Plasticity of Glioma Stem Cells Under BMP4 and Noggin Influence. Brain Pathol. 2016, 26, 43–61. [Google Scholar] [CrossRef]
- Deweese, J.E.; Osheroff, N. The DNA cleavage reaction of topoisomerase II: Wolf in sheep’s clothing. Nucleic Acids Res. 2008, 37, 738–748. [Google Scholar] [CrossRef]
- Minematsu, T.; Sonoda, T.; Hashimoto, T.; Iwai, M.; Oppeneer, T.; Felder, L.; Shirai, N.; Miyashita, A.; Usui, T. Pharmacokinetics, distribution and excretion of YM155 monobromide, a novel small-molecule survivin suppressant, in male and pregnant or lactating female rats. Biopharm. Drug Dispos. 2012, 33, 160–169. [Google Scholar] [CrossRef]
- Householder, K.T.; DiPerna, D.M.; Yamaguchi, J.T.; Sanai, N.; Mehta, S.; Sirianni, R.W. Abstract B21: Use of polymeric nanoparticles for the delivery of YM155 to glioma cells in vitro and in vivo. Cancer Res. 2015, 75 (Suppl. S23), B21. [Google Scholar] [CrossRef]
- Hoadley, K.A.; Yau, C.; Wolf, D.M.; Cherniack, A.D.; Tamborero, D.; Ng, S.; Leiserson, M.D.M.; Niu, B.; McLellan, M.D.; Uzunangelov, V.; et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 2014, 158, 929–944. [Google Scholar] [CrossRef]
- Ser, M.H.; Webb, M.J.; Sener, U.; Campian, J.L. Immune Checkpoint Inhibitors and Glioblastoma: A Review on Current State and Future Directions. J. Immunother. Precis. Oncol. 2023, 7, 97–110. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Li, X.; Zhao, H. Current state of immune checkpoints therapy for glioblastoma. Heliyon 2024, 10, e24729. [Google Scholar] [CrossRef] [PubMed]
- Szklener, K.; Bilski, M.; Nieoczym, K.; Mandziuk, D.; Mandziuk, S. Enhancing glioblastoma treatment through the integration of tumor-treating fields. Front. Oncol. 2023, 13, 1274587. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.R.; Khong, P.; Parkinson, J.F.; Howell, V.M.; Wheeler, H.R. Molecular heterogeneity in glioblastoma: Potential clinical implications. Front. Oncol. 2015, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef]
- Leonard, A.; Wolff, J.E. Etoposide improves survival in high-grade glioma: A meta-analysis. Anticancer Res. 2013, 33, 3307–3315. [Google Scholar]
- van der Meulen, M.; Chahal, M.; Mason, W.P. The Value of Etoposide for Recurrent Glioma. Can. J. Neurol. Sci. 2023, 51, 509–512. [Google Scholar] [CrossRef]
- Hollis, P.H.; Zappulla, R.A.; Spigelman, M.K.; Feuer, E.J.; Holland, J.F.; Malis, L.I. Effects of etoposide-induced blood-brain barrier disruption on brain water, intracranial pressure, and cerebral vasomotor tone. Exp. Neurol. 1988, 99, 428–439. [Google Scholar] [CrossRef]
- Darling, J.L.; Thomas, D.G. Response of short-term cultures derived from human malignant glioma to aziridinylbenzoquinone, etoposide and doxorubicin: An in vitro phase II trial. Anticancer. Drugs 2001, 12, 753–760. [Google Scholar] [CrossRef]
- Pavillard, V.; Kherfellah, D.; Richard, S.; Robert, J.; Montaudon, D. Effects of the combination of camptothecin and doxorubicin or etoposide on rat glioma cells and camptothecin-resistant variants. Br. J. Cancer 2001, 85, 1077–1083. [Google Scholar] [CrossRef]
- Combination Chemotherapy Plus Radiation Therapy in Treating Patients with Newly Diagnosed Glioblastoma Multiforme. Available online: https://clinicaltrials.gov/show/NCT00003996 (accessed on 1 March 2025).
- Stereotactic Radiology Versus Chemotherapy for Recurrent/Progressive Glioblastoma After Second-Line Chemotherapy. Available online: https://classic.clinicaltrials.gov/show/NCT05718466 (accessed on 1 March 2025).
- Standard Chemotherapy vs. Chemotherapy Guided by Cancer Stem Cell Test in Recurrent Glioblastoma. Available online: https://classic.clinicaltrials.gov/show/NCT03632135 (accessed on 1 March 2025).
- Mitusova, K.; Peltek, O.O.; Karpov, T.E.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Overcoming the blood-brain barrier for the therapy of malignant brain tumor: Current status and prospects of drug delivery approaches. J. Nanobiotechnology 2022, 20, 412. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, I.; Bouchard, E.D.; Beiggi, S.; Poeppl, A.G.; Johnston, J.B.; Gibson, S.B.; Banerji, V. On-target effect of FK866, a nicotinamide phosphoribosyl transferase inhibitor, by apoptosis-mediated death in chronic lymphocytic leukemia cells. Clin. Cancer Res. 2014, 20, 4861–4872. [Google Scholar] [CrossRef] [PubMed]
- Mutz, C.N.; Schwentner, R.; Aryee, D.N.T.; Bouchard, E.D.J.; Mejia, E.M.; Hatch, G.M.; Kauer, M.O.; Katschnig, A.M.; Ban, J.; Garten, A.; et al. EWS-FLI1 confers exquisite sensitivity to NAMPT inhibition in Ewing sarcoma cells. Oncotarget 2017, 8, 24679–24693. [Google Scholar] [CrossRef] [PubMed]
- A Phase I/II Study to Assess the Safety and Tolerability of APO866 for the Treatment of Refractory B-CLL. Available online: https://clinicaltrials.gov/show/NCT00435084 (accessed on 1 March 2025).
- A Study to Assess APO866 for the Treatment of Advanced Melanoma. Available online: https://clinicaltrials.gov/show/NCT00432107 (accessed on 1 March 2025).
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; de Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015, 11, 535–546. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Liu, X.; Mohseni, G.; Hao, X.; Ren, Y.; Xu, Y.; Gao, H.; Wang, Q.; Wang, Y. Mechanism research and treatment progress of NAD pathway related molecules in tumor immune microenvironment. Cancer Cell Int. 2022, 22, 242. [Google Scholar]
- Guo, Q.; Han, N.; Shi, L.; Yang, L.; Zhang, X.; Zhou, Y.; Yu, S.; Zhang, M. NAMPT: A potential prognostic and therapeutic biomarker in patients with glioblastoma. Oncol. Rep. 2019, 42, 963–972. [Google Scholar] [CrossRef]
- Lucena-Cacace, A.; Umeda, M.; Navas, L.E.; Carnero, A. NAMPT as a Dedifferentiation-Inducer Gene: NAD(+) as Core Axis for Glioma Cancer Stem-Like Cells Maintenance. Front. Oncol. 2019, 9, 292. [Google Scholar] [CrossRef]
- Panizza, E.; Regalado, B.D.; Wang, F.; Nakano, I.; Vacanti, N.M.; Cerione, R.A.; Antonyak, M.A. Proteomic analysis reveals microvesicles containing NAMPT as mediators of radioresistance in glioma. Life Sci. Alliance 2023, 6, e202201680. [Google Scholar] [CrossRef]
- Gujar, A.D.; Le, S.; Mao, D.D.; Dadey, D.Y.; Turski, A.; Sasaki, Y.; Aum, D.; Luo, J.; Dahiya, S.; Yuan, L.; et al. An NAD+-dependent transcriptional program governs self-renewal and radiation resistance in glioblastoma. Proc. Natl. Acad. Sci. USA 2016, 113, E8247–E8256. [Google Scholar] [CrossRef]
- Feng, J.; Yan, P.F.; Zhao, H.Y.; Zhang, F.C.; Zhao, W.H.; Feng, M. Inhibitor of Nicotinamide Phosphoribosyltransferase Sensitizes Glioblastoma Cells to Temozolomide via Activating ROS/JNK Signaling Pathway. Biomed. Res. Int. 2016, 2016, 1450843. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, L.; Wang, T.; Yang, J.; Zheng, S.; Tong, J.; Jiang, S.; Zhang, X.; Zhang, K. Recent advances of targeting nicotinamide phosphoribosyltransferase (NAMPT) for cancer drug discovery. Eur. J. Med. Chem. 2023, 258, 115607. [Google Scholar] [CrossRef] [PubMed]
- A Study of APO866 for the Treatment of Cutaneous T-Cell Lymphoma. Available online: https://clinicaltrials.gov/show/NCT00431912 (accessed on 1 March 2025).
- Shats, I.; Williams, J.G.; Liu, J.; Makarov, M.V.; Wu, X.; Lih, F.B.; Deterding, L.J.; Lim, C.; Xu, X.; Randall, T.A.; et al. Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway. Cell Metab. 2020, 31, 564–579. [Google Scholar] [CrossRef] [PubMed]
- ElMokh, O.; Matsumoto, S.; Biniecka, P.; Bellotti, A.; Schaeuble, K.; Piacente, F.; Gallart-Ayala, H.; Ivanisevic, J.; Stamenkovic, I.; Nencioni, A.; et al. Gut microbiota severely hampers the efficacy of NAD-lowering therapy in leukemia. Cell Death Dis. 2022, 13, 320. [Google Scholar] [CrossRef]
- Perryman, R.; Chau, T.W.; De-Felice, J.; O’Neill, K.; Syed, N. Distinct Capabilities in NAD Metabolism Mediate Resistance to NAMPT Inhibition in Glioblastoma. Cancers 2024, 16, 2054. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, N.; Pérez Küper, M.; Garcia Fallit, M.; Agudelo, J.A.P.; Nicola Candia, A.; Suarez Velandia, M.; Romero, A.C.; Videla Richardson, G.; Candolfi, M. Integrated Workflow for Drug Repurposing in Glioblastoma: Computational Prediction and Preclinical Validation of Therapeutic Candidates. Brain Sci. 2025, 15, 637. https://doi.org/10.3390/brainsci15060637
Gonzalez N, Pérez Küper M, Garcia Fallit M, Agudelo JAP, Nicola Candia A, Suarez Velandia M, Romero AC, Videla Richardson G, Candolfi M. Integrated Workflow for Drug Repurposing in Glioblastoma: Computational Prediction and Preclinical Validation of Therapeutic Candidates. Brain Sciences. 2025; 15(6):637. https://doi.org/10.3390/brainsci15060637
Chicago/Turabian StyleGonzalez, Nazareno, Melanie Pérez Küper, Matías Garcia Fallit, Jorge A. Peña Agudelo, Alejandro Nicola Candia, Maicol Suarez Velandia, Ana Clara Romero, Guillermo Videla Richardson, and Marianela Candolfi. 2025. "Integrated Workflow for Drug Repurposing in Glioblastoma: Computational Prediction and Preclinical Validation of Therapeutic Candidates" Brain Sciences 15, no. 6: 637. https://doi.org/10.3390/brainsci15060637
APA StyleGonzalez, N., Pérez Küper, M., Garcia Fallit, M., Agudelo, J. A. P., Nicola Candia, A., Suarez Velandia, M., Romero, A. C., Videla Richardson, G., & Candolfi, M. (2025). Integrated Workflow for Drug Repurposing in Glioblastoma: Computational Prediction and Preclinical Validation of Therapeutic Candidates. Brain Sciences, 15(6), 637. https://doi.org/10.3390/brainsci15060637







