Abstract
Glioblastoma (GBM) is the most common primary brain tumor in adults, with a median survival of 15–18 months. GBM cells, like all tumors, exhibit a metabolic shift known as the Warburg effect, favoring glycolysis even under normoxic conditions. GLUT1 is a primary glucose transporter in GBM cells and has been found to be overexpressed in these cells. The acidic microenvironment created by glycolysis facilitates immune evasion, therapy resistance, and tumor growth. Overexpression of GLUT1 is driven by hypoxia-inducible factor-1α (HIF-1α), c-Myc, and other pathways which have been correlated with tumor aggressiveness as well as poor prognosis Recent studies have highlighted the therapeutic potential of targeting GLUT1 in GBM. Preclinical research shows that GLUT1 inhibitors, such as WZB117 and BAY-876, effectively impair tumor metabolism, reduce cell viability, and improve survival in vitro and in animal models. GLUT1 expression also serves as a prognostic marker, with elevated levels linked to poor outcomes. This review highlights the importance of GLUT1 in GBM biology as a potential therapeutic target and biomarker.
1. Introduction
Glioblastoma (GBM) is among the most aggressive primary brain tumors in adults, with a 1-year overall survival rate of 53.7% in IDH wildtype and 76.3% in IDH mutant [1]. Despite extensive research into therapeutics capable of targeting GBM cells, these tumors rapidly develop resistance to various treatments, rendering them ineffective [2,3]. GBM cells, like many cancer cells, can proliferate without external stimuli and are highly metabolically active. They can switch from oxidative phosphorylation to anaerobic glycolysis to satisfy their metabolic needs—a shift towards pyruvate utilization historically known as the Warburg effect, a phenomenon observed in cancer cells [4]. This adaptation allows cancer cells to thrive independent of hypoxia or normoxic environments, which is a limiting factor in the metabolism of normal cells.
Over the last century, the discovery and study of proteins involved in this metabolic shift, such as glucose transporters (GLUTs), lactate dehydrogenases (LDHs), hexokinases (HKs), phosphofructokinases (PFKs), pyruvate kinase M2 (PKM2), and monocarboxylate transporters (MCTs) have been pivotal [5,6,7,8,9,10]. Long non-coding RNAs (lncRNAs), microRNAs (miRNAs), and c-Myc have also been identified as direct regulators of the Warburg effect [5,11,12]. This metabolic reprogramming contributes to an acidic tumor microenvironment, promotes resistance to therapies, facilitates gene mutations, supplies nutrients to the biomass, and affects interactions with the immune system [13]. Two key players in the regulation of this metabolic reprogramming are GLUTs and the hypoxia-inducible factors (HIFs).
In GBM, glucose transporters (mainly GLUT1 and GLUT3) regulate glucose uptake into cells. GLUT1 is specifically involved in the internalization of glucose in tumor cells [13]. HIFs are expressed under conditions of reduced oxygen concentration [14]. HIFs, in turn, enhances the expression of GLUT1, enabling cells to remain metabolically active in low-oxygen environments. This increased expression of GLUT1 in GBM cells has been correlated with tumor proliferation and poor patient survival outcomes [15,16]. In this literature review, we explore the role of GLUT1 in the progression of GBM, summarizing previous studies and their findings. We also briefly discuss the challenges and prospects of targeting GLUT1 as a therapeutic strategy.
1.1. The Role of GLUT1 in Glucose Transport, Cancer Metabolism, and Tumor Progression
GLUT1, encoded by the SLC2A1 gene in humans, is a member of the glucose transporter family, which allows the uptake of glucose into cells through facilitated diffusion [17]. This transporter enables efficient glucose utilization under hypoxic conditions. It is ubiquitously expressed and present in normal and cancerous cells, with notable abundance on erythrocytes and barrier cells such as those comprising the blood–brain barrier [17]. In its basal state, GLUT1 predominantly resides in the cytoplasm [18]. Upon specific cellular signals, it translocates to the plasma membrane, thereby permitting the influx of glucose into the cell [18]. This translocation is crucial for maintaining cellular energy homeostasis, particularly under conditions of reduced oxygen tension, where it ensures a continuous supply of glucose for glycolysis and subsequent energy production.
The expression of GLUT1 is an important hallmark of many cancers, such as breast cancer, ovarian cancer, prostate cancer, and GBM [19,20,21,22]. Different transcription factors, such as HIF-1α [23], c-Myc [24,25], K-Ras pathway [26], and PI3K/Akt [27,28] pathways, have been implicated in the upregulation of GLUT1 in hypoxic conditions [29]. Growth factors also play an important role in GLUT1 translocation via the PI3K/AKT pathway, which regulates cell proliferation and growth in many cancers [30].
Cancer cells have the ability to alter their metabolic functions under adverse conditions. This alteration allows for tumor cell proliferation and survival. Studies have shown that cancers that express more GLUT1 are more aggressive and are associated with poorer prognosis [21,31,32,33].
Warburg effect [34] allows tumor cells to efficiently utilize glucose for energy, producing lactate as a byproduct. Lactate is then excreted from the cells through lactic acid transporters [monocarboxylate transporters (MCT1-4)], accumulating lactate in the tumor microenvironment [6,35]. This accumulation of lactate results in an acidic environment, which has several effects. It suppresses local immune responses, thereby protecting the cancer cells from immune system attacks, promoting tumor growth and survival by creating conditions that are unfavorable for normal cells but advantageous for cancer cells [35]. The hypoxic environment and lactate build-up influence cell signaling and transcription processes, including the upregulation of HIF-1α and the activation of nuclear factor kappa-B and PI3K kinase pathways [36,37]. This, in turn, enhances GLUT1 expression and glucose uptake, promotes glycolysis, and further drives lactate production [38].
1.2. Hypoxia and the Expression of GLUT1 in Cancer Cells
Under normal oxygen tension, HIF-1α is hydroxylated by prolyl hydroxylase enzymes, which target it for ubiquitination and subsequent proteasomal degradation [39,40]. However, under hypoxic conditions, the activity of prolyl hydroxylases (PHD1-3) and factor inhibiting HIF (FIH-1) is inhibited, leading to the stabilization of HIF-1α. HIF-1α translocates to the nucleus, where it dimerizes with HIF-1β, forming the active HIF-1 transcriptional complex [40]. This HIF-1 complex subsequently binds to specific DNA sequences known as hypoxia-responsive elements (HREs) which contain the core sequence 5′-[A/G]CGTG-3′ in the promoter regions of target genes [38,40]. HIF-1α directly induces the expression of a majority of glycolytic genes by binding to the HREs of the gene promoters (Figure 1). The GLUT1 gene, SLC2A1, also contains HREs in its promoter region and when the HIF-1 complex binds to these HREs, it recruits co-activator proteins which possess histone acetyltransferase activity [41]. The binding of HIF-1 and its co-activators to the HREs facilitates the transcription of the GLUT1 gene. The mature mRNA is exported from the nucleus to the cytoplasm, where it is translated into the GLUT1 protein. HIF-1α also regulates the transcription of other molecules, such as vascular endothelial growth factor (VEGF) and carbonic anhydrase IX, which have also been studied in relation to tumor grade and overall survival [42].
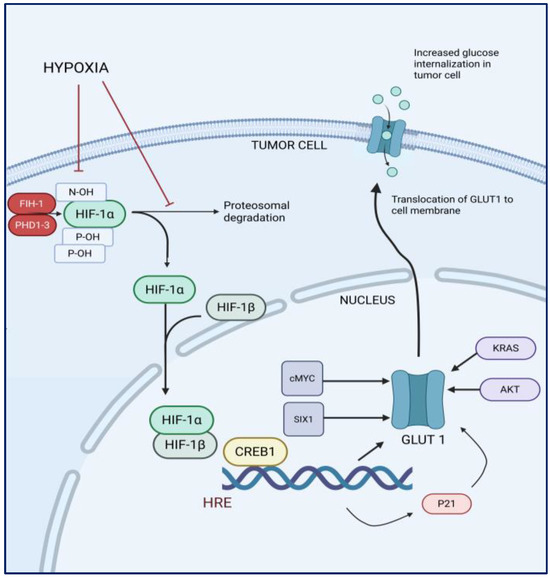
Figure 1.
Schematic of hypoxia-induced regulation of glucose uptake in tumor cells.
Other factors related to hypoxia, including the epidermal growth factor receptor (EGF-R)/KRAS pathway and AKT, also contribute to the upregulation of GLUT1 expression. Transcription factors c-Myc and SIX1 play a crucial role in the regulation of GLUT1 by binding to its upstream promoter regions, thereby enhancing its transcriptional activity. Conversely, the tumor suppressor protein p53 acts as a regulatory counterbalance by inhibiting the activation of the GLUT1 gene and obstructing the function of the GLUT1 protein [43,44].
1.3. The Role of GLUT1 in Glioblastoma
The expression of GLUT1 in GBM cells presents a compelling area of study, given the notable heterogeneity of these tumor cells. In GBM, GLUT1 expression is regulated at both genetic and post-translational levels [45,46]. Various genetic alterations, such as mutations and amplifications, can influence GLUT1 expression, leading to differential glucose uptake among tumor cells [33]. Additionally, post-translational modifications, including phosphorylation and ubiquitination, further modulate the activity and localization of GLUT1 within the cell.
Various mechanisms regulating GLUT1 expression and its pathways have been identified in the context of GBM. In 2022, Jin et al. found that p21 induced by HIF-1α under hypoxic conditions, leading to increased expression of GLUT1 and hence may have an impact on glycolysis-related genes [46]. Another study using the U87 glioma cell line identified GLUT1 as a key target gene regulated by CREB1 (cAMP-responsive element-binding protein 1) that has been implicated in many cancers [45]. It was further observed that both CREB1 and GLUT1 expression levels were significantly elevated in GBM compared to normal brain and WHO grade I, II and III glioma samples, with CREB1 positively influencing the transcriptional activity of GLUT1, thereby enhancing its expression [45].
Zhang et al. and colleagues investigated the mechanism of GLUT1 translocation and identified DHHC9, a palmitoyl transferase, as a key regulator in U87 and T98G glioma cell line [47]. They discovered that DHHC9 catalyzes the palmitoylation of GLUT1 at the cysteine residue Cys207. This post-translational modification is crucial for the translocation of GLUT1 from the intracellular compartments to the plasma membrane, where it facilitates glucose uptake. Inhibition of DHHC9 led to significantly reduced levels of GLUT1 on the cell surface. Consequently, the decreased GLUT1 expression impaired cell survival both in vitro and in vivo in athymic mice intracranially injected with U87, highlighting the importance of DHHC9 in regulating GLUT1 function and cellular metabolism [47].
Several studies have investigated the role of microRNAs (miRNAs) in regulating the expression of GLUT1 [48,49,50]. These miRNAs influence GLUT1 expression by targeting key pathways involved in its transcription and translation. For example, a study by Yin et al. (2020) demonstrated that overexpression of miR-181b in GBM cells significantly inhibited glucose metabolism and cell proliferation [51]. This effect was mediated through the downregulation of Specificity Protein 1 (SP1), a member of the Sp/KLF (Krüppel-like factor/Specificity Protein) family of transcription factors, which are known to regulate gene expression in both physiological and pathological contexts [51]. Abnormal N-glycosylation also plays a significant role in glioma progression, with N-acetylglucosaminyltransferase I (MGAT1) being crucial in converting high-mannose cores into complex or hybrid N-linked oligosaccharides [52]. Elevated MGAT1 expression has been observed in GBM compared to normal brain tissues [52]. Knockdown of MGAT1 impaired glioma cell proliferation and migration. Moreover, MGAT1 facilitated the complex N-glycosylation of GLUT1, increasing GLUT1 protein levels [52]. Furthermore, Cheng et al. demonstrated in an in vitro study using the T98G human GBM cell line that the transcription factor SREBP2 (sterol regulatory element-binding protein-2), known for promoting de novo cholesterol synthesis, also regulated GLUT1 expression. Elevated SREBP2 expression in T98G cells increased GLUT1 levels, significantly enhancing cell viability and migration [53]. These findings suggest a feedback mechanism that sustains high glucose and cholesterol levels, which are crucial for GBM cell survival.
Together, these complex regulatory mechanisms highlight the tightly controlled expression and function of GLUT1, reflecting its role in cellular metabolism and the adaptive response of GBM cells to their microenvironment.
1.4. GLUT1 as a Therapeutic Target in Glioblastoma
Examining potential strategies to target GLUT1 in GBM reveals promising avenues to inhibit tumor growth. Current research has focused on developing and evaluating inhibitors that can specifically target GLUT1, given its critical role in the glycolytic metabolism of GBM and many other cancer cells. These inhibitors aim to disrupt glucose uptake in tumor cells, thereby impeding their energy supply and growth (Figure 2). The crystal structures of human GLUT1 and GLUT3 have elucidated the molecular intricacies of substrate binding and the transport mechanism. These structural insights suggest that inhibitors can be strategically designed to either obstruct substrate binding or disrupt the conformational changes necessary for the transporter’s alternating access mechanism.
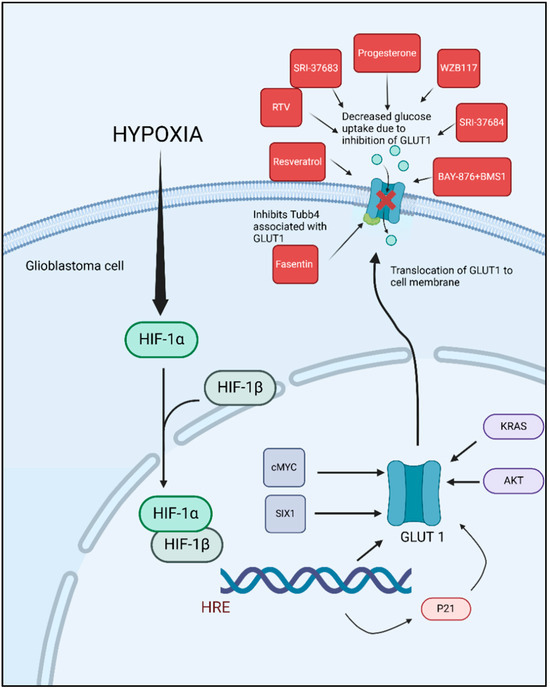
Figure 2.
Hypoxia-induced GLUT1 upregulation in glioblastoma and pharmacological inhibition of glucose uptake. GLUT1 inhibitors studied in preclinical GBM models.
The potential of targeting GLUT1 as a therapeutic approach in GBM is promising. Several studies have explored the inhibition of GLUT1, as researchers aimed to disrupt the glucose metabolism of tumor cells, thereby hindering their growth and survival (Table 1). One of these studies demonstrated that targeting GLUT1 and its associated protein TUBB4 with Fasentin significantly reduced cell viability, sphere formation ability, and cell aggressiveness in vitro in patient-derived GSC lines, GSC33 and GSC28 [16]. Fasentin’s inhibition of GLUT1 disrupts glucose uptake, which is critical for energy production and cell survival. Additionally, interfering with TUBB4 affects the structural integrity and function of the cytoskeleton, further impairing the cells’ ability to proliferate and form spheres, a characteristic of aggressive cancer cells [16].

Table 1.
Summary of in vitro and in vivo studies investigating drugs targeting GLUT1 in glioblastoma.
Many studies have explored the effects of chemotherapeutic drugs that act as GLUT1 inhibitors in GBM cell lines. For instance, Azzalin et al. evaluated the effects of Indinavir (IDV) and Ritonavir (RTV) in combination with TMZ and BCNU, in U87GM, Hu-197, GL261 and GBM-P1 (patient derived) cell lines, on the inhibition of the GLUT1/SLC2A transporter. They found that RTV, but not IDV, decreased glycolytic activity and cell growth in vitro. Furthermore, mice treated with RTV and BCNU showed improved survival compared to those treated with BCNU alone [54]. Another study conducted both in vitro and in vivo studies with the GL261 mouse glioma cell line, to evaluate the efficacy of BAY-876, a GLUT1 inhibitor. Their research demonstrated that BAY-876 significantly prolonged the survival of mice in an orthotropic GBM model. Furthermore, combining BAY-876 with the PD-1/PD-L1 blocker BMS-1 resulted in an even greater extension of survival, indicating a potential synergistic effect between GLUT1 inhibition and immune checkpoint blockade in the treatment of GBM [56]. In another study, the GLUT 1 inhibitors, SRI-37683 and SRI-37684 effectively decreased the glycolytic capacity, glycolytic reserve capacity, and overall cell survival of PDX GBM cell lines. The findings suggest that targeting GLUT with these compounds can significantly impair the metabolic function and viability of GBM cells [58]. Resveratrol, a natural phenol or polyphenol compound, has shown success in GBM therapy. It exerts its anti-tumor effects through multiple mechanisms, including the regulation of cell cycle progression and proliferation, modulation of autophagy, influence on the oxidative stress system, and activation of apoptosis pathways [70]. Multiple in vitro and in vivo studies have shown decreased tumor cell viability, decreased tumor growth and prolonged survival in the in vivo treatment cohorts [59,60,61,62,65] (Table 1) While studies have not yet demonstrated GLUT1 inhibition by resveratrol in GBM cells specifically, multiple studies in other cell lines like ovarian cancer cell lines [71,72] leukemia cell lines [73] and hypopharyngeal carcinoma [74] have shown that resveratrol can inhibit GLUT1. This suggests that resveratrol may have the potential to affect glycolysis in GBM and could be explored as a therapeutic option.
Currently, resveratrol is the only known GLUT1 inhibitor in the clinical trials phase for cancers such as multiple myeloma, gastrointestinal tumors, follicular lymphoma and breast cancer (Table 2). However, resveratrol is also a known inhibitor of various other pathways, such as STAT3, PI3K, AKT, mTOR, and NF-kB pathways that inhibit cancer progression, which is why it has been used in cancer research [63,66,67,69,71,72]. Over time, researchers discovered that resveretrol also inhibited GLUT1.

Table 2.
Clinical trials investigating the use of the GLUT1 inhibitor resveratrol in different cancers.
In addition, although targeting GLUT1 presents a promising therapeutic strategy, several challenges exist in developing effective drugs for it. The most important is that GLUT1 is ubiquitously expressed in many tissues, including normal cells and vital organs, which increases the risk of off-target effects and toxicity. Ensuring that drugs effectively reach and penetrate tumor cells while sparing healthy tissues poses a significant challenge, especially in the context of GBM due to the blood–brain barrier. In addition, designing drugs that specifically target GLUT1 without affecting other glucose transporters (e.g., GLUT2, GLUT3 or GLUT4) or essential cellular functions can be difficult. The expression of GLUT1 in GBM cells varies significantly, influenced by intra- and inter-tumor heterogeneity and the cells’ proximity to blood vessels. Future research should focus on overcoming these challenges by developing more selective GLUT1 inhibitors, exploring combination therapies that target multiple metabolic pathways, and investigating the mechanisms underlying resistance to GLUT1-targeted treatments. Additionally, advanced imaging techniques and molecular diagnostics can enhance the accuracy of GLUT1 expression assessment, providing more precise and real-time monitoring of therapeutic responses.
1.5. Clinical and Prognostic Implications of GLUT1 in GBM Therapy
GLUT1’s role in facilitating glucose uptake makes it a critical target for therapeutic interventions aimed at disrupting the metabolic pathways that GBM cells rely on for survival and growth. Targeting GLUT1 directly or modulating its regulatory pathways can potentially impair tumor metabolism and sensitize GBM cells to other treatments. Radiation is one of the mainstays in the treatment of GBM and irradiation can cause changes in tumor metabolism. GLUT1 has also been implicated in conferring radioresistance and playing a potential role in tumor recurrence [46].
In 2021, Prosniak et al. discovered that tumor cells characterized as GLUT1 positive and Nestin negative cells [GLUT1(+)/NES(−)], typically located far from blood vessels, exhibit natural resistance to conventional chemotherapy and radiation [79]. This resistance was attributed to their low proliferative capacity. A prior study in 2015 by Newman et al. showed that Dichloroacetate (DCA), a PDK inhibitor, modified GBM cell metabolism and caused cells to return to oxidative phosphorylation. Through reversal of this metabolic characteristic of GBM it was sensitized to radiation [80]. Furthermore, another study by Lan et al. showed that a drug, miR-448, negatively affecting glycolysis increased the radiosensitivity of GBM cells in both in vitro and in vivo models [81]. Another study revealed that a decrease in the heat shock protein 90 beta family member 1 leads to reduced GLUT1 localization on the plasma membrane and an ultimate decline in glycolytic activity, resulting in desensitization to radiotherapy [82].
Assessing GLUT1 expression levels can help evaluate the efficacy of such targeted therapies. For instance, a decrease in GLUT1 expression following treatment could indicate a successful disruption of tumor metabolism and a positive therapeutic response [83]. Conversely, as observed in colorectal and acute myeloid leukemia, persistent or increased GLUT1 expression may signal resistance to therapy, prompting the need for alternative or combination treatments [84,85].
Komaki et al. and colleagues observed an increased expression of GLUT1 in pseudo-palisaded and perivascular tumor cells and found its expression to be an independent predictor of worse prognosis in GBM patients [33]. Another study by Guda et al. obtained the mRNA expression data for GLUT1 from the TCGA (The Cancer Genome Atlas) data portal for their patient cohort and studied the correlation between GLUT1 expression and tumor aggressiveness and patient prognosis [16]. By using a data mining approach, they found that the mesenchymal subtype of GBM cells demonstrated the highest mRNA levels of GLUT1 in comparison to proneural, classical, and neural subtypes [16]. They further found that high-grade gliomas expressed more GLUT1 than low-grade gliomas, and patients who expressed higher GLUT1 levels had worse survivorship [16].
These studies highlight the prognostic implications of GLUT1, revealing its potential to predict disease outcomes and progression. Additionally, they highlight the importance of GLUT1 as a valuable biomarker in GBM diagnostics and therapy, emphasizing its role in identifying tumor characteristics, guiding treatment decisions, and monitoring therapeutic responses. The consistent association of elevated GLUT1 expression with aggressive tumor behavior and poor patient prognosis further reinforces its significance in GBM.
2. Conclusions and Future Directions
Glucose metabolism plays a crucial role in energy production, with glycolysis serving as the primary metabolic pathway for GBM cells. GLUT1 plays a critical role in tumor maintenance and progression, contributing to aggressiveness, radiation resistance, and recurrence, making it a promising therapeutic target. Preclinical trials in GBM cell lines have demonstrated encouraging results, showing that inhibiting GLUT1 can impair tumor growth and enhance treatment efficacy.
Most studies rely on commercially available GBM cell lines, which lack the heterogeneity seen in PDX. Future research should prioritize PDX models to improve clinical relevance. Given the heterogeneity in GBM tumor cells, focused ultrasound presents an innovative strategy to temporarily disrupt the BBB, enhancing targeted drug delivery of GLUT1 inhibitors. Beyond treatment, GLUT1’s prognostic value should be further validated and studied. Integrating it into a biomarker panel alongside other molecular indicators could refine glioma diagnosis and treatment stratification. A multi-pronged approach combining selective inhibition, advanced drug delivery, and biomarker integration is essential to the utility of GLUT1 as a therapeutic target in GBM.
However, to validate its therapeutic potential for GBM, it is essential to develop targeted drugs that selectively affect the tumor cells with limited off-target effects [86]. Targeting GLUT1 presents challenges due to systemic toxicity and BBB limitations, and there is a need for alternative strategies to target GLUT1 [87]. Given that GLUT1 is a primary glucose transporter at the blood–brain barrier, future studies should also consider the broader impact of GLUT1 inhibition on normal brain metabolism. Evaluating these systemic effects may provide additional insight into the complex role of GLUT1 in glioblastoma progression and therapeutic response.
A potential approach is by using alternative targets like key regulators of GLUT1 translocation and expression, such as chaperones or membrane trafficking facilitators, for more selective inhibition. For instance, inhibitors of MGAT1 and DHHC9 can indirectly target the expression of GLUT1. In 2022, GAL, a long non-coding RNA (lncRNA) regulating GLUT1 expression, was identified as a negative regulator of GLUT1. Given the tissue-specific expression patterns of lncRNAs demonstrated in the study, targeting GAL in GBM may offer a selective strategy to suppress GLUT1 expression in tumor cells. Additionally, miR-181b, a microRNA shown to downregulate GLUT1, may present a promising tool to suppress GLUT1 function post-transcriptionally. Research into findings tumor-specific untranslated regions (UTRs) that are GLUT1/SLC2A1-specific can allow for targeted tumor GLUT1 suppression without affecting other normal cells. Developing mutation-activated GLUT1 inhibitors that selectively target GBM-specific mutations such as EGFRvIII, IDH1, and PTEN loss, reducing toxicity in normal tissues. Another strategy involves combination therapy, integrating GLUT1 inhibitors with mTOR inhibitors, autophagy blockers, anti-angiogenic agents, or immunotherapies to prevent metabolic adaptation. Immunotherapy in particular could be a promising avenue. A recent report by Jackson et al. highlighted the expression of GLUT1 in myeloid derived suppressor cells (MDSCs) especially in the early-stage MDSCs, an important immunosuppressive population within the GBM microenvironment [88]. GLUT1 can potentially be used to target this population of cells with checkpoint inhibitors to enhance the function of T-cells. Given the central role of metabolic pathways in GBM progression, targeting alternative Warburg effect regulators like HK2, LDHA, or MCT1, which are more tumor-specific, may offer greater therapeutic benefits. Future research should prioritize GBM-specific inhibitors, metabolic alternatives, combination therapies, and optimized drug delivery systems to enhance efficacy while minimizing systemic side effects.
Author Contributions
Conceptualization, J.R.-T. and C.B.; resources, F.R.; writing—original draft preparation, F.R.; writing—review and editing, F.R., S.S. and A.S.-F.; visualization.; supervision, J.R.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors have no relevant conflicts of interest.
References
- Price, M.; Ryan, K.; Shoaf, M.L.; Neff, C.; Iorgulescu, J.B.; Landi, D.B.; Cioffi, G.; A Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S.; et al. Childhood, adolescent, and adult primary brain and central nervous system tumor statistics for practicing healthcare providers in neuro-oncology, CBTRUS 2015–2019. Neurooncol. Pract. 2023, 11, 5–25. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xue, Q.; Liu, K.; Ge, W.; Liu, W.; Wang, J.; Zhang, M.; Li, Q.-Y.; Cai, D.; Shan, C.; et al. Dimethylaminomicheliolide (DMAMCL) suppresses the proliferation of glioblastoma cells via targeting pyruvate kinase 2 (PKM2) and rewiring aerobic glycolysis. Front. Oncol. 2019, 9, 993. [Google Scholar] [CrossRef]
- Miranda-Gonçalves, V.; Gonçalves, C.S.; Granja, S.; de Castro, J.V.; Reis, R.M.; Costa, B.M.; Baltazar, F. MCT1 is a new prognostic biomarker and its therapeutic inhibition boosts response to temozolomide in human glioblastoma. Cancers 2021, 13, 3468. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, A.; Samsó, P.; Fontova, P.; Simon-Molas, H.; Manzano, A.; Castaño, E.; Rosa, J.L.; Martinez-Outshoorn, U.; Ventura, F.; Navarro-Sabaté, À.; et al. TGF-β1 targets smad, p38 MAPK, and PI 3K/akt signaling pathways to induce PFKFB 3 gene expression and glycolysis in glioblastoma cells. FEBS J. 2017, 284, 3437–3454. [Google Scholar] [CrossRef]
- Wolf, A.; Agnihotri, S.; Micallef, J.; Mukherjee, J.; Sabha, N.; Cairns, R.; Hawkins, C.; Guha, A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 2011, 208, 313–326. [Google Scholar] [CrossRef]
- Li, J.; Zhu, S.; Tong, J.; Hao, H.; Yang, J.; Liu, Z.; Wang, Y. Suppression of lactate dehydrogenase A compromises tumor progression by downregulation of the warburg effect in glioblastoma. Neuroreport 2016, 27, 110–115. [Google Scholar] [CrossRef]
- Sanzey, M.; Abdul Rahim, S.A.; Oudin, A.; Dirkse, A.; Kaoma, T.; Vallar, L.; Herold-Mende, C.; Bjerkvig, R.; Golebiewska, A.; Niclou, S.P. Comprehensive analysis of glycolytic enzymes as therapeutic targets in the treatment of glioblastoma. PLoS ONE 2015, 10, e0123544. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Q.; Guo, F.; Guo, S.; Yang, B.; Liu, B.; Li, P.; Li, J.; Guan, S.; Liu, X. Long noncoding RNA PCED1B-AS1 promotes the warburg effect and tumorigenesis by upregulating HIF-1α in glioblastoma. Cell Transpl. 2020, 29, 0963689720906777. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Liao, Y.; Zhao, H.; Zhang, J.; Mu, K. ANXA2P2/miR-9/LDHA axis regulates warburg effect and affects glioblastoma proliferation and apoptosis. Cell Signal 2020, 74, 109718. [Google Scholar] [CrossRef]
- Bache, M.; Rot, S.; Keßler, J.; Güttler, A.; Wichmann, H.; Greither, T.; Wach, S.; Taubert, H.; Söling, A.; Bilkenroth, U.; et al. mRNA expression levels of hypoxia-induced and stem cell-associated genes in human glioblastoma. Oncol. Rep. 2015, 33, 3155–3161. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.J.; Ratcliffe, P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004, 5, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X. Greasy GLUT1 maintains glioblastoma malignancy. Mol. Cell Oncol. 2021, 8, 2009423. [Google Scholar] [CrossRef]
- Guda, M.R.; Labak, C.M.; Omar, S.I.; Asuthkar, S.; Airala, S.; Tuszynski, J.; Tsung, A.J.; Velpula, K.K. GLUT1 and TUBB4 in glioblastoma could be efficacious targets. Cancers 2019, 11, 1308. [Google Scholar] [CrossRef]
- Deng, D.; Xu, C.; Sun, P.; Wu, J.; Yan, C.; Hu, M.; Yan, N. Crystal structure of the human glucose transporter GLUT1. Nature 2014, 510, 121–125. [Google Scholar] [CrossRef]
- Egert, S.; Nguyen, N.; Schwaiger, M. Myocardial glucose transporter GLUT1: Translocation induced by insulin and ischemia. J. Mol. Cell Cardiol. 1999, 31, 1337–1344. Available online: https://www.sciencedirect.com/science/article/pii/S0022282899909653 (accessed on 28 September 2024). [CrossRef]
- Rudlowski, C.; Moser, M.; Becker, A.J.; Rath, W.; Buttner, R.; Schroder, W.; Schurmann, A. GLUT1 mRNA and protein expression in ovarian borderline tumors and cancer. Oncology 2004, 66, 404–410. [Google Scholar] [CrossRef]
- Meziou, S.; Ringuette Goulet, C.; Hovington, H.; Lefebvre, V.; Lavallée, E.; Bergeron, M.; Brisson, H.; Champagne, A.; Neveu, B.; Lacombe, D.; et al. GLUT1 expression in high-risk prostate cancer: Correlation with 18F-FDG-PET/CT and clinical outcome. Prostate Cancer Prostatic Dis. 2020, 23, 441–448. [Google Scholar] [CrossRef]
- Krzeslak, A.; Wojcik-Krowiranda, K.; Forma, E.; Jozwiak, P.; Romanowicz, H.; Bienkiewicz, A.; Brys, M. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol. Oncol. Res. 2012, 18, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yongzhi, H.; Chen, S.; Luo, X.; Lin, Y.; Zhou, Y.; Jin, H.; Hou, B.; Deng, Y.; Tu, L.; et al. The prognostic value of GLUT1 in cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 43356–43367. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Gao, P.; Liu, Y.; Semenza, G.L.; Dang, C.V. Hypoxia-inducible factor 1 and dysregulated c-myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol. Cell Biol. 2007, 27, 7381–7393. [Google Scholar] [CrossRef]
- Gordan, J.D.; Bertout, J.A.; Hu, C.; Diehl, J.A.; Simon, M.C. HIF-2α promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 2007, 11, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Koshiji, M.; Kageyama, Y.; Pete, E.A.; Horikawa, I.; Barrett, J.C.; Huang, L.E. HIF-1α induces cell cycle arrest by functionally counteracting myc. EMBO J. 2004, 23, 1949–1956. [Google Scholar] [CrossRef]
- Zeng, M.; Kikuchi, H.; Pino, M.S.; Chung, D.C. Hypoxia activates the K-ras proto-oncogene to stimulate angiogenesis and inhibit apoptosis in colon cancer cells. PLoS ONE 2010, 5, e10966. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.; Kim, Y.W.; Han, S.K.; Shim, Y.; Yoo, C. Hypoxia confers protection against apoptosis via PI3K/akt and ERK pathways in lung cancer cells. Cancer Lett. 2006, 242, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Nan, K. Activation of PI3 kinase/akt/HIF-1α pathway contributes to hypoxia-induced epithelial-mesenchymal transition and chemoresistance in hepatocellular carcinoma. Int. J. Oncol. 2012, 40, 461–468. [Google Scholar]
- Wofford, J.A.; Wieman, H.L.; Jacobs, S.R.; Zhao, Y.; Rathmell, J.C. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of akt to support T-cell survival. Blood J. Am. Soc. Hematol. 2008, 111, 2101–2111. [Google Scholar] [CrossRef]
- Melstrom, L.G.; Salabat, M.R.; Ding, X.-Z.; Milam, B.M.; Strouch, M.; Pelling, J.C.; Bentrem, D.J. Apigenin inhibits the GLUT-1 glucose transporter and the phosphoinositide 3-kinase/akt pathway in human pancreatic cancer cells. Pancreas 2008, 37, 426–431. [Google Scholar] [CrossRef]
- Grimm, M.; Munz, A.; Teriete, P.; Nadtotschi, T.; Reinert, S. GLUT-1/TKTL1 coexpression predicts poor outcome in oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Jung, W.; Koo, J.S. The expression of metabolism-related proteins in phyllodes tumors. Tumor Biol. 2013, 34, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Komaki, S.; Sugita, Y.; Furuta, T.; Yamada, K.; Moritsubo, M.; Abe, H.; Akiba, J.; Miyagi, N.; Nakamura, H.; Miyoshi, H.; et al. Expression of GLUT1 in pseudopalisaded and perivascular tumor cells is an independent prognostic factor for patients with glioblastomas. J. Neuropathol. Exp. Neurol. 2019, 78, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Khan, F.; Lin, Y.; Ali, H.; Pang, L.; Dunterman, M.; Hsu, W.-H.; Frenis, K.; Rowe, R.G.; Wainwright, D.A.; McCortney, K.; et al. Lactate dehydrogenase A regulates tumor-macrophage symbiosis to promote glioblastoma progression. Nat. Commun. 2024, 15, 1987. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Chaumeil, M.M.; Ward, C.S.; Haas-Kogan, D.A.; James, C.D.; Ronen, S.M. Reduced phosphocholine and hyperpolarized lactate provide magnetic resonance biomarkers of PI3K/akt/mTOR inhibition in glioblastoma. Neurooncology 2012, 14, 315–325. [Google Scholar] [CrossRef]
- Lim, K.S.; Lim, K.J.; Price, A.C.; Orr, B.A.; Eberhart, C.G.; Bar, E.E. Inhibition of monocarboxylate transporter-4 depletes stem-like glioblastoma cells and inhibits HIF transcriptional response in a lactate-independent manner. Oncogene 2014, 33, 4433–4441. [Google Scholar] [CrossRef]
- Semenza, G.L.; Jiang, B.-H.; Leung, S.W.; Passantino, R.; Concordet, J.-P.; Maire, P.; Giallongo, A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 1996, 271, 32529–32537. [Google Scholar] [CrossRef]
- Fong, G.; Takeda, K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 2008, 15, 635–641. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE 2007, 2007, cm8. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 and tumor progression: Pathophysiology and therapeutics. Trends Mol. Med. 2002, 8, S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Peters, H.; St Croix, B.; Haroon, Z.A.; Dewhirst, M.W.; Strausberg, R.L.; Kaanders, J.H.; Van Der Kogel, A.J.; Riggins, G.J. Transcriptional response to hypoxia in human tumors. J. Natl. Cancer Inst. 2001, 93, 1337–1343. [Google Scholar]
- Schwartzenberg-Bar-Yoseph, F.; Armoni, M.; Karnieli, E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004, 64, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liang, Y.; Kang, L.; Liu, Y.; Gao, S.; Chen, S.; Li, Y.; You, W.; Dong, Q.; Hong, T.; et al. Transcriptional regulation of the warburg effect in cancer by SIX1. Cancer Cell 2018, 33, 368–385.e7. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Mi, Y.; Chen, F.; Du, D. CREB1 regulates glucose transport of glioma cell line U87 by targeting GLUT1. Mol Cell Biochem. 2017, 436, 79–86. [Google Scholar] [CrossRef]
- Jin, X.; Kuang, Y.; Li, L.; Li, H.; Zhao, T.; He, Y.; Di, C.; Kang, J.; Yuan, L.; Yu, B.; et al. A positive feedback circuit comprising p21 and HIF-1α aggravates hypoxia-induced radioresistance of glioblastoma by promoting Glut1/LDHA-mediated glycolysis. FASEB J. 2022, 36, e22229. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Yang, F.; Chen, C.; Liu, P.; Ren, Y.; Sun, P.; Wang, Z.; You, Y.; Zeng, Y.-X.; et al. DHHC9-mediated GLUT1 S-palmitoylation promotes glioblastoma glycolysis and tumorigenesis. Nat. Commun. 2021, 12, 5872–5874. [Google Scholar] [CrossRef]
- Guo, H.; Nan, Y.; Zhen, Y.; Zhang, Y.; Guo, L.; Yu, K.; Huang, Q.; Zhong, Y. miRNA-451 inhibits glioma cell proliferation and invasion by downregulating glucose transporter 1. Tumor Biol. 2016, 37, 13751–13761. [Google Scholar] [CrossRef]
- Nie, S.; Li, K.; Huang, Y.; Hu, Q.; Gao, X.; Jie, S. miR-495 mediates metabolic shift in glioma cells via targeting Glut1. J. Craniofacial Surg. 2015, 26, e155–e158. [Google Scholar] [CrossRef]
- Zhang, R.; Luo, H.; Wang, S.; Chen, W.; Chen, Z.; Wang, H.-W.; Chen, Y.; Yang, J.; Zhang, X.; Wu, W.; et al. MicroRNA-377 inhibited proliferation and invasion of human glioblastoma cells by directly targeting specificity protein 1. Neurooncology 2014, 16, 1510–1522. [Google Scholar] [CrossRef]
- Yin, J.; Shi, Z.; Wei, W.; Lu, C.; Wei, Y.; Yan, W.; Li, R.; Zhang, J.; You, Y.; Wang, X. MiR-181b suppress glioblastoma multiforme growth through inhibition of SP1-mediated glucose metabolism. Cancer Cell Int. 2020, 20, 69. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhu, H.; Chen, X.; Tian, M.; Wei, Y.; Gong, Y.; Jiang, J. N-acetylglucosaminyltransferase I promotes glioma cell proliferation and migration through increasing the stability of the glucose transporter GLUT1. FEBS Lett. 2020, 594, 358–366. [Google Scholar] [CrossRef]
- Cheng, C.; Tu, J.; Hu, Z.; Chen, Y.; Wang, Y.; Zhang, T.; Zhang, C.; Li, C.; Wang, Y.; Niu, C. SREBP2/Rab11s/GLUT1/6 network regulates proliferation and migration of glioblastoma. Pathol. Res. Pract. 2022, 240, 154176. Available online: https://www.sciencedirect.com/science/article/pii/S0344033822004204 (accessed on 27 September 2024). [CrossRef] [PubMed]
- Azzalin, A.; Nato, G.; Parmigiani, E.; Garello, F.; Buffo, A.; Magrassi, L. Inhibitors of GLUT/SLC2A enhance the action of BCNU and temozolomide against high-grade gliomas. Neoplasia 2017, 19, 364–373. [Google Scholar] [CrossRef]
- Atif, F.; Yousuf, S.; Espinosa-Garcia, C.; Sergeeva, E.; Stein, D.G. Progesterone treatment attenuates glycolytic metabolism and induces senescence in glioblastoma. Sci. Rep. 2019, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, D.; Ruan, Z.; Zhou, J.; Sun, W.; Rao, B.; Xu, H. Metabolism/immunity dual-regulation thermogels potentiating immunotherapy of glioblastoma through lactate-excretion inhibition and PD-1/PD-L1 blockade. Adv. Sci. 2024, 11, e2310163. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Okada, M.; Suzuki, S.; Seino, M.; Seino, S.; Takeda, H.; Kitanaka, C. Targeting the facilitative glucose transporter GLUT1 inhibits the self-renewal and tumor-initiating capacity of cancer stem cells. Oncotarget 2015, 6, 651–661. [Google Scholar] [CrossRef]
- Landis, C.J.; Zhang, S.; Benavides, G.A.; Scott, S.E.; Li, Y.; Redmann, M.; Tran, A.N.; Otamias, A.; Darley-Usmar, V.; Napierala, M.; et al. Identification of compounds that decrease glioblastoma growth and glucose uptake in vitro. ACS Chem. Biol. 2018, 13, 2048–2057. [Google Scholar] [CrossRef]
- Leone, S.; Fiore, M.; Lauro, M.G.; Pino, S.; Cornetta, T.; Cozzi, R. Resveratrol and X rays affect gap junction intercellular communications in human glioblastoma cells. Mol. Carcinog. 2008, 47, 587–598. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, Y.; Huang, P.; Chiou, G.; Tseng, L.; Chiou, S.; Chen, M.; Shih, Y.; Chang, C.; Hsu, C.; et al. Resveratrol suppresses tumorigenicity and enhances radiosensitivity in primary glioblastoma tumor initiating cells by inhibiting the STAT3 axis. J. Cell Physiol. 2012, 227, 976–993. [Google Scholar] [CrossRef]
- Wang, L.; Long, L.; Wang, W.; Liang, Z. Resveratrol, a potential radiation sensitizer for glioma stem cells both in vitro and in vivo. J. Pharmacol. Sci. 2015, 129, 216–225. [Google Scholar] [CrossRef]
- Khoei, S.; Shoja, M.; Mostaar, A.; Faeghi, F. Effects of resveratrol and methoxyamine on the radiosensitivity of iododeoxyuridine in U87MG glioblastoma cell line. Exp. Biol. Med. 2016, 241, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, Y.; Günaydın, C.; Yalçın, F.; Nazıroğlu, M.; Braidy, N. Resveratrol enhances apoptotic and oxidant effects of paclitaxel through TRPM2 channel activation in DBTRG glioblastoma cells. Oxidative Med. Cell. Longev. 2019, 2019, 4619865. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lin, H.; Zhang, X.; Li, J. Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway. Oncol. Rep. 2012, 27, 2050–2056. [Google Scholar] [PubMed]
- Li, H.; Liu, Y.; Jiao, Y.; Guo, A.; Xu, X.; Qu, X.; Wang, S.; Zhao, J.; Li, Y.; Cao, Y. Resveratrol sensitizes glioblastoma-initiating cells to temozolomide by inducing cell apoptosis and promoting differentiation. Oncol. Rep. 2016, 35, 343–351. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Bu, X.; Yang, B.; Wang, B.; Hu, S.; Yan, Z.; Gao, Y.; Han, S.; Qu, M. Resveratrol restores sensitivity of glioma cells to temozolamide through inhibiting the activation of wnt signaling pathway. J. Cell Physiol. 2019, 234, 6783–6800. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Wu, M.; Wu, J.; Liu, J. Synergistic effects of resveratrol and temozolomide against glioblastoma cells: Underlying mechanism and therapeutic implications. Cancer Manag. Res. 2020, 12, 8341–8354. [Google Scholar] [CrossRef]
- Yuan, Y.; Xue, X.; Guo, R.; Sun, X.; Hu, G. Resveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathway. CNS Neurosci. Ther. 2012, 18, 536–546. [Google Scholar] [CrossRef]
- Lin, C.-J.; Lee, C.-C.; Shih, Y.-L.; Lin, T.-Y.; Wang, S.-H.; Lin, Y.-F.; Shih, C.-M. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic. Biol. Med. 2012, 52, 377–391. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Mortezazadeh, T.; Aryafar, T.; Gharepapagh, E.; Majdaeen, M.; Farhood, B. Therapeutic potentials of resveratrol in combination with radiotherapy and chemotherapy during glioblastoma treatment: A mechanistic review. Cancer Cell Int. 2021, 21, 391. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, L.; Luo, Y.; Li, X.; Chen, G.; Wang, Y. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J. Cell Biochem. 2018, 119, 6162–6172. [Google Scholar] [CrossRef] [PubMed]
- Kueck, A.; Opipari, A.W., Jr.; Griffith, K.A.; Tan, L.; Choi, M.; Huang, J.; Wahl, H.; Liu, J.R. Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol. Oncol. 2007, 107, 450–457. [Google Scholar] [CrossRef]
- Salas, M.; Obando, P.; Ojeda, L.; Ojeda, P.; Pérez, A.; Vargas-Uribe, M.; Rivas, C.I.; Vera, J.C.; Reyes, A.M. Resolution of the direct interaction with and inhibition of the human GLUT1 hexose transporter by resveratrol from its effect on glucose accumulation. Am. J. Physiol. Cell Physiol. 2013, 305, 90. [Google Scholar] [CrossRef] [PubMed]
- Kleszcz, R.; Paluszczak, J.; Krajka-Kuźniak, V.; Baer-Dubowska, W. The inhibition of c-MYC transcription factor modulates the expression of glycolytic and glutaminolytic enzymes in FaDu hypopharyngeal carcinoma cells. Adv. Clin. Exp. Med. 2018, 27, 735–742. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, R.F.; Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar] [CrossRef]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef]
- Resveratrol in Treating Patients with Colorectal Cancer That Can be Removed by Surgery. Available online: https://clinicaltrials.gov/study/NCT00433576 (accessed on 28 September 2024).
- Prosniak, M.; Kenyon, L.C.; Hooper, D.C. Glioblastoma contains topologically distinct proliferative and metabolically defined subpopulations of nestin- and Glut1-expressing cells. J. Neuropathol. Exp. Neurol. 2021, 80, 674–684. [Google Scholar] [CrossRef]
- Shen, H.; Hau, E.; Joshi, S.; Dilda, P.J.; McDonald, K.L. Sensitization of glioblastoma cells to irradiation by modulating the glucose metabolism. Mol. Cancer Ther. 2015, 14, 1794–1804. [Google Scholar] [CrossRef]
- Lan, F.; Qin, Q.; Yu, H.; Yue, X. Effect of glycolysis inhibition by miR-448 on glioma radiosensitivity. J. Neurosurg. JNS 2020, 132, 1456–1464. Available online: https://thejns.org/view/journals/j-neurosurg/132/5/article-p1456.xml (accessed on 28 September 2024). [CrossRef]
- Li, Y.; Ge, Y.; Zhao, M.; Ding, F.; Wang, X.; Shi, Z.; Ge, X.; Wang, X.; Qian, X. HSP90B1-mediated plasma membrane localization of GLUT1 promotes radioresistance of glioblastomas. J. Biomed. Res. 2023, 37, 326. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, C.; Kamitori, K.; Hossain, A.; Hoshikawa, H.; Katagi, A.; Dong, Y.; Sui, L.; Tokuda, M.; Yamaguchi, F. D-allose inhibits cancer cell growth by reducing GLUT1 expression. Tohoku J. Exp. Med. 2016, 238, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ning, K.; Lu, T.; Hua, D. Elevated expression of TrpC5 and GLUT1 is associated with chemoresistance in colorectal cancer. Oncol. Rep. 2017, 37, 1059–1065. [Google Scholar] [CrossRef]
- Song, K.; Li, M.; Xu, X.J.; Xuan, L.; Huang, G.N.; Song, X.L.; Liu, Q.F. HIF-1α and GLUT1 gene expression is associated with chemoresistance of acute myeloid leukemia. Asian Pac. J. Cancer Prev. 2014, 15, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, R.; Van Solinge, W.W. The energy-less red blood cell is lost: Erythrocyte enzyme abnormalities of glycolysis. Blood 2005, 106, 4034–4042. [Google Scholar] [CrossRef]
- Evans, A.; Bates, V.; Troy, H.; Hewitt, S.; Holbeck, S.; Chung, Y.-L.; Phillips, R.; Stubbs, M.; Griffiths, J.; Airley, R. Glut-1 as a therapeutic target: Increased chemoresistance and HIF-1-independent link with cell turnover is revealed through COMPARE analysis and metabolomic studies. Cancer Chemother. Pharmacol. 2008, 61, 377–393. [Google Scholar] [CrossRef]
- Jackson, C.; Cherry, C.; Bom, S.; Dykema, A.G.; Wang, R.; Thompson, E.; Zhang, M.; Li, R.; Ji, Z.; Hou, W.; et al. Distinct myeloid-derived suppressor cell populations in human glioblastoma. Science 2025, 387, eabm5214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).