Neurofeedback Training Modulates Brain Functional Networks and Improves Cognition in Amnestic Mild Cognitive Impairment Patients Aged 60–70 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Data Preprocessing
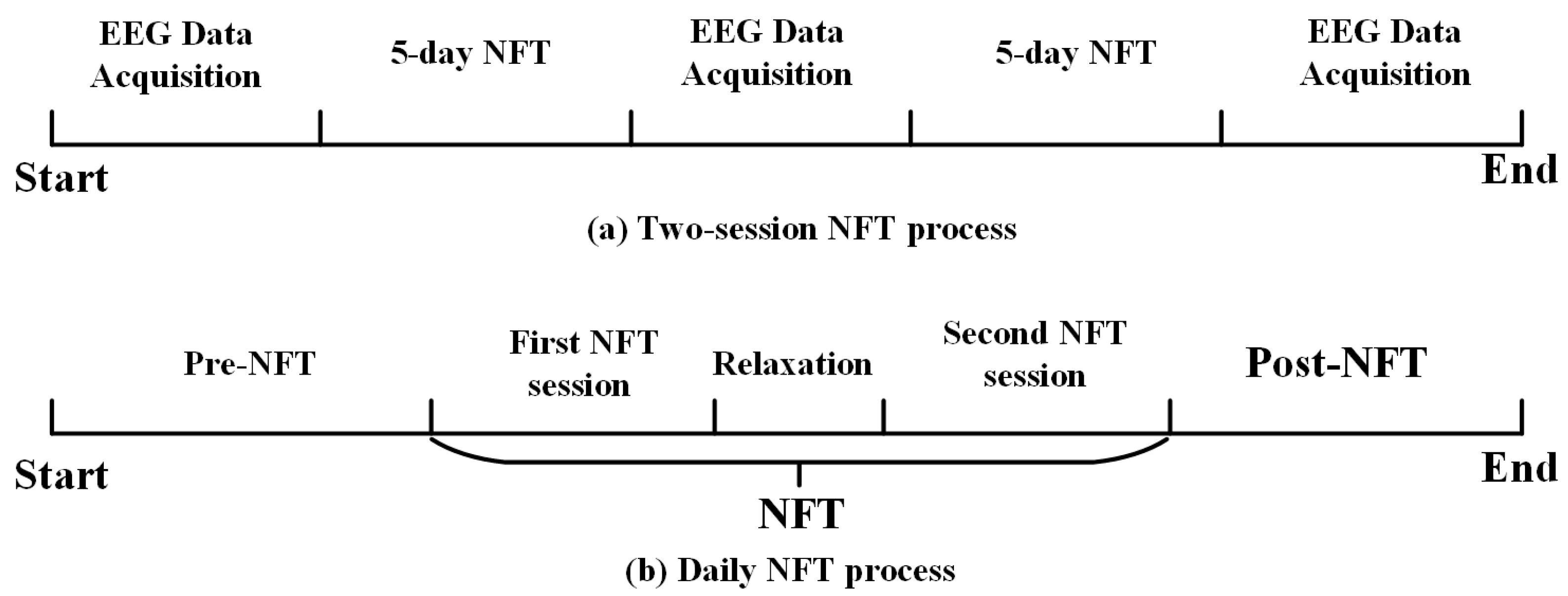
2.3. Neurofeedback Training Protocol
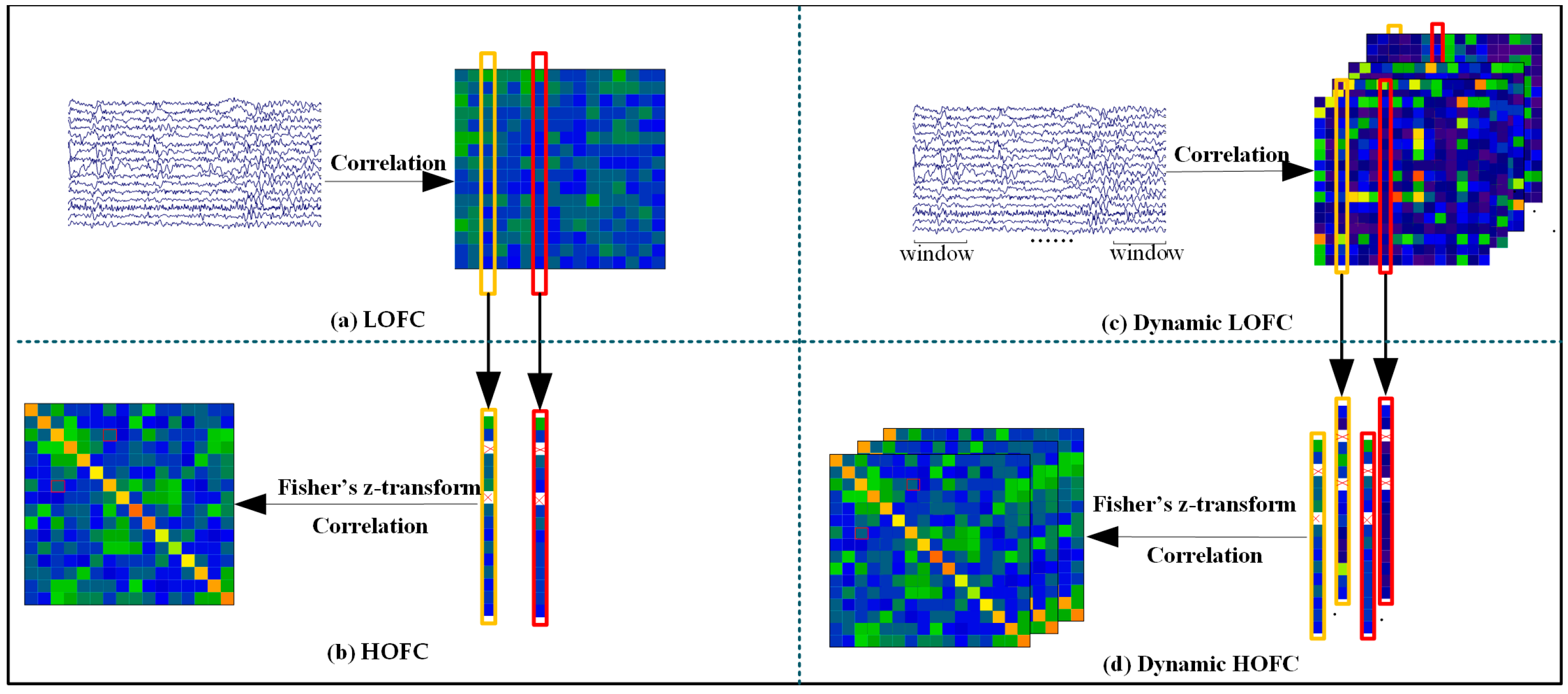
2.4. Construction of Brain Functional Networks
- Construction of Low- and High-Order Networks
- Construction of Dynamic High- and Low-Order Functional Networks
2.5. Statistical Analysis
3. Results
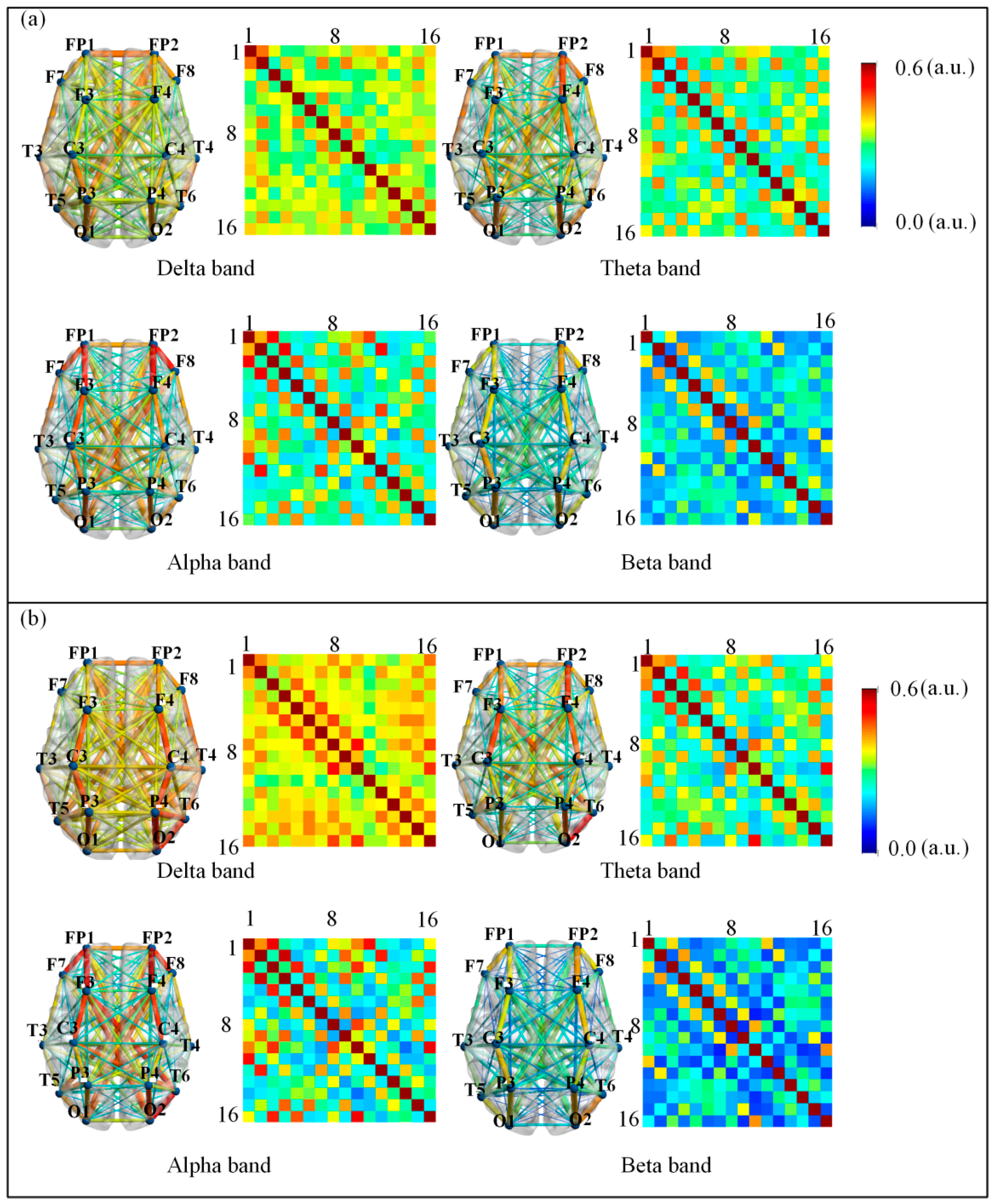
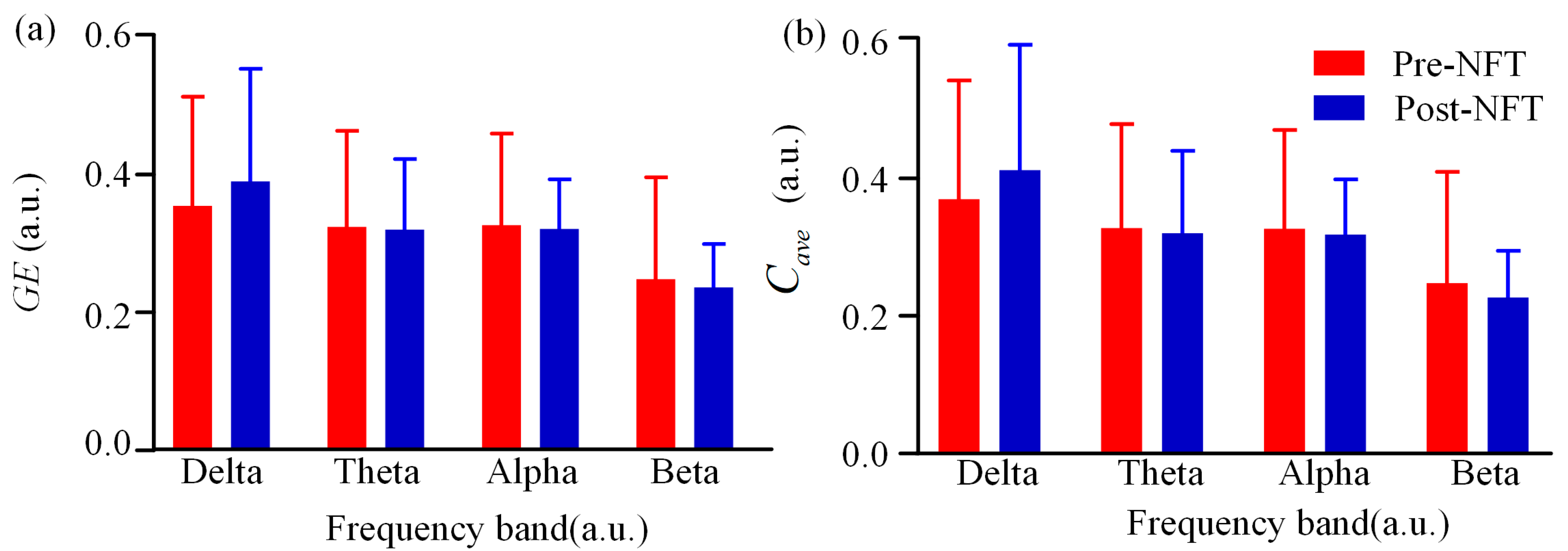
3.1. Low-Order Functional Brain Network Connectivity and Its Characteristics
3.2. High-Order Functional Network Connectivity and Its Characteristics
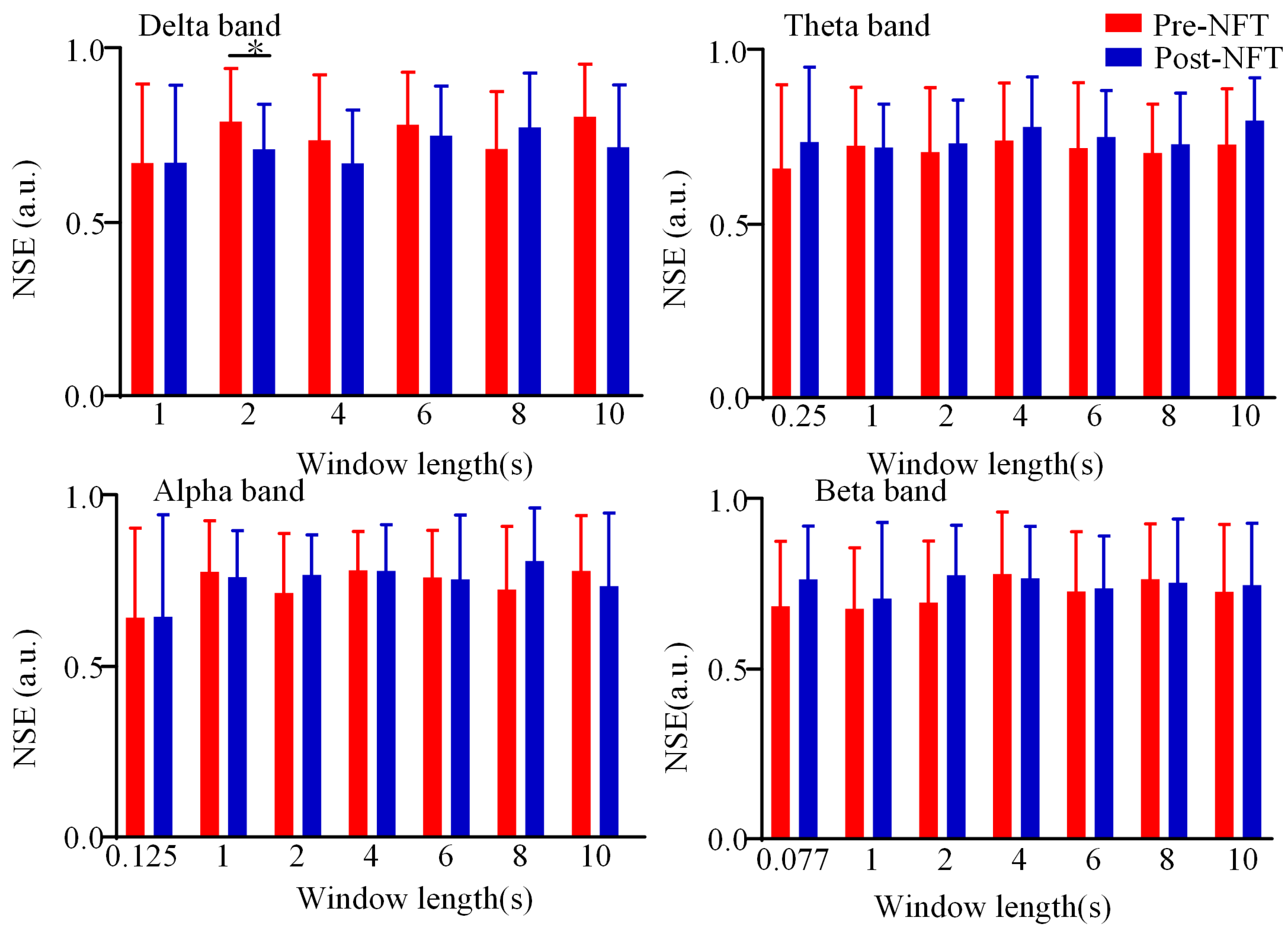
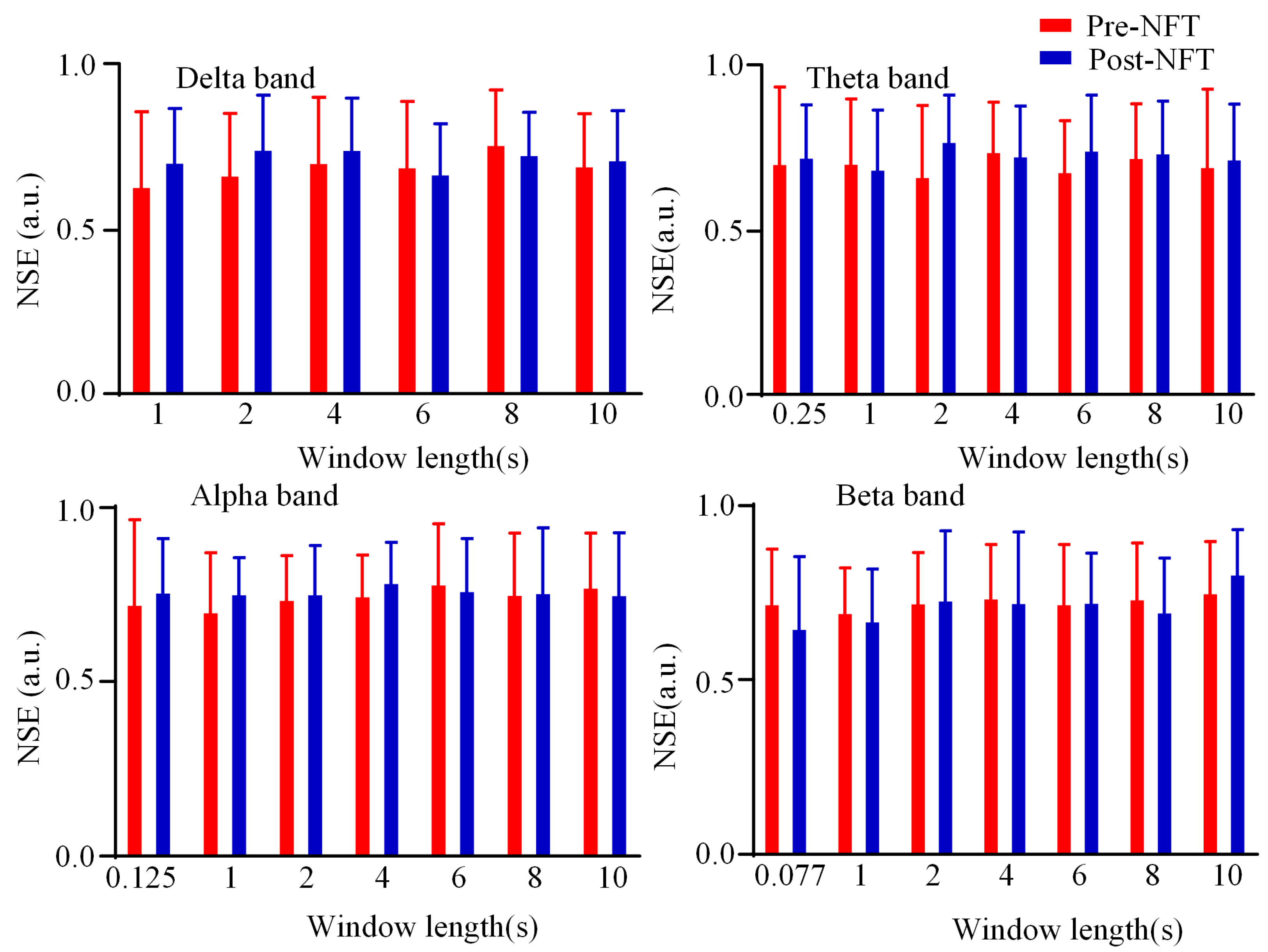
3.3. Dynamic Brain Functional Network State Entropy
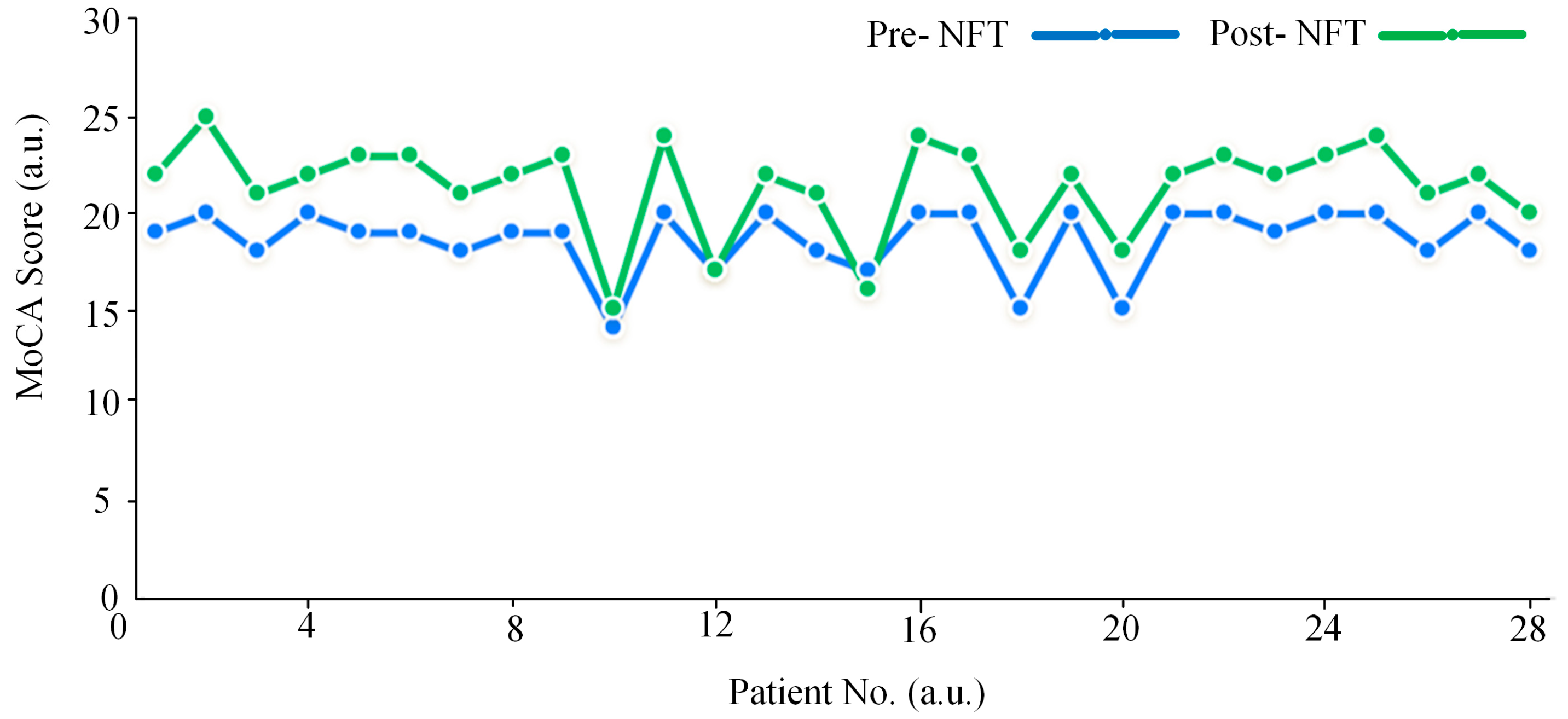
3.4. Analysis of MoCA Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Frontal (Pre-/Post-) | Occipital (Pre-/Post-) | Parietal (Pre-/Post-) | Temporal (Pre-/Post-) | |
|---|---|---|---|---|
| NE | 0.3384 ± 0.1685/ 0.3753 ± 0.1742 | 0.3274 ± 0.1582/ 0.3728 ± 0.1706 | 0.3375 ± 0.1639/ 0.3860 ± 0.1843 | 0.3391 ± 0.1660/ 0.3741 ± 0.1681 |
| NCC | 0.3712 ± 0.1788/ 0.4102 ± 0.1843 | 0.3593 ± 0.1679/ 0.4075 ± 0.1808 | 0.3701 ± 0.1741/ 0.4217 ± 0.1953 | 0.3720 ± 0.1761/ 0.4089 ± 0.1778 |
| Frontal (Pre-/Post-) | Occipital (Pre-/Post-) | Parietal (Pre-/Post-) | Temporal (Pre-/Post-) | |
|---|---|---|---|---|
| NE | 0.3048 ± 0.1505/ 0.3017 ± 0.1213 | 0.2958 ± 0.1352/ 0.2851 ± 0.0973 | 0.2956 ± 0.1444/ 0.2853 ± 0.1203 | 0.2949 ± 0.1453/ 0.2884 ± 0.1130 |
| NCC | 0.3357 ± 0.1592/ 0.3325 ± 0.1293 | 0.3260 ± 0.1429/ 0.3148 ± 0.1037 | 0.3256 ± 0.1527/ 0.3147 ± 0.1282 | 0.3249 ± 0.1537/ 0.3180 ± 0.1203 |
| Frontal (Pre-/Post-) | Occipital (Pre-/Post-) | Parietal (Pre-/Post-) | Temporal (Pre-/Post-) | |
|---|---|---|---|---|
| NE | 0.3079 ± 0.1461/ 0.3074 ± 0.0888 | 0.2996 ± 0.1347/ 0.2999 ± 0.0881 | 0.2920 ± 0.1398/ 0.2768 ± 0.0727 | 0.2902 ± 0.1339/ 0.2786 ± 0.0737 |
| NCC | 0.3394 ± 0.1546/ 0.3394 ± 0.0952 | 0.3305 ± 0.1425/ 0.3313 ± 0.0945 | 0.3220 ± 0.1479/ 0.3061 ± 0.0779 | 0.3202 ± 0.1414/ 0.3080 ± 0.0789 |
| Frontal (Pre-/Post-) | Occipital (Pre-/Post-) | Parietal (Pre-/Post-) | Temporal (Pre-/Post-) | |
|---|---|---|---|---|
| NE | 0.2236 ± 0.1580/ 0.2089 ± 0.0625 | 0.2188 ± 0.1535/ 0.2001 ± 0.0551 | 0.2305 ± 0.1555/ 0.2061 ± 0.0750 | 0.2167 ± 0.1505/ 0.1965 ± 0.0637 |
| NCC | 0.2491 ± 0.1672/ 0.2339 ± 0.0671 | 0.2440 ± 0.1624/ 0.2244 ± 0.0591 | 0.2567 ± 0.1646/ 0.2309 ± 0.0807 | 0.2415 ± 0.1593/ 0.2202 ± 0.0685 |
Appendix B
| Frontal (Pre-/Post-) | Occipital (Pre-/Post-) | Parietal (Pre-/Post-) | Temporal (Pre-/Post-) | |
|---|---|---|---|---|
| NE | 0.4200 ± 0.1435/ 0.4474 ± 0.1668 | 0.4247 ± 0.1518/ 0.4547 ± 0.1745 | 0.4394 ± 0.1565/ 0.4509 ± 0.1877 | 0.4338 ± 0.1623/ 0.4409 ± 0.1656 |
| NCC | 0.4559 ± 0.1520/ 0.4851 ± 0.1765 | 0.4603 ± 0.1609/ 0.4922 ± 0.1851 | 0.4766 ± 0.1658/ 0.4889 ± 0.1991 | 0.4706 ± 0.1721/ 0.4779 ± 0.1752 |
| Frontal (Pre-/Post-) | Occipital (Pre-/Post-) | Parietal (Pre-/Post-) | Temporal (Pre-/Post-) | |
|---|---|---|---|---|
| NE | 0.3851 ± 0.1312/ 0.3958 ± 0.1265 | 0.3631 ± 0.1161/ 0.3808 ± 0.0896 | 0.3703 ± 0.1278/ 0.3705 ± 0.1293 | 0.3760 ± 0.1242/ 0.3749 ± 0.1062 |
| NCC | 0.4192 ± 0.1388/ 0.4306 ± 0.1344 | 0.3957 ± 0.1236/ 0.4147 ± 0.0951 | 0.4031 ± 0.1349/ 0.4035 ± 0.1376 | 0.4092 ± 0.1304/ 0.4082 ± 0.1128 |
| Frontal (Pre-/Post-) | Occipital (Pre-/Post-) | Parietal (Pre-/Post-) | Temporal (Pre-/Post-) | |
|---|---|---|---|---|
| NE | 0.3662 ± 0.1419/ 0.3934 ± 0.0665 | 0.3651 ± 0.1462/ 0.3912 ± 0.0494 | 0.3523 ± 0.1405/ 0.3558 ± 0.0695 | 0.3666 ± 0.1399/ 0.3759 ± 0.0609 |
| NCC | 0.3992 ± 0.1501/ 0.4281 ± 0.0708 | 0.3978 ± 0.1548/ 0.4258 ± 0.0530 | 0.3842 ± 0.1486/ 0.3876 ± 0.0743 | 0.3995 ± 0.1478/ 0.4092 ± 0.0651 |
| Frontal (Pre-/Post-) | Occipital (Pre-/Post-) | Parietal (Pre-/Post-) | Temporal (Pre-/Post-) | |
|---|---|---|---|---|
| NE | 0.3455 ± 0.1442/ 0.3289 ± 0.0843 | 0.3417 ± 0.1507/ 0.3231 ± 0.0746 | 0.3222 ± 0.1433/ 0.3049 ± 0.0800 | 0.3380 ± 0.1448/ 0.3213 ± 0.0729 |
| NCC | 0.3775 ± 0.1524/ 0.3594 ± 0.0901 | 0.3733 ± 0.1595/ 0.3532 ± 0.0798 | 0.3522 ± 0.1514/ 0.3337 ± 0.0857 | 0.3693 ± 0.1530/ 0.3514 ± 0.0781 |
References
- Li, Y.; Shao, Y.; Wang, J.; Liu, Y.; Yang, Y.; Wang, Z.; Xi, Q. Machine learning based on functional and structural connectivity in mild cognitive impairment. Magn. Reason. Imaging 2024, 109, 10–17. [Google Scholar] [CrossRef]
- 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Cont-Richter, C.; Stute, N.; Galli, A.; Schulte, C.; Wojtecki, L.J.B.S. Transcranial Pulse Stimulation in Alzheimer’s: Long-Term Feasibility and a Multifocal Treatment Approach. Brain Sci. 2025, 15, 830. [Google Scholar] [CrossRef]
- Facci, L.; Sandrini, L.; Bottini, G. Attentional Functioning in Healthy Older Adults and aMCI Patients: Results from the Attention Network Test with a Focus on Sex Differences. Brain Sci. 2025, 15, 770. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Alzheimer’s disease: From immunotherapy to immunoprevention. Cell 2023, 186, 4260–4270. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, X.; Zhou, H.; Lv, T.; Han, R.; Yang, Z.; Zheng, W.; Bai, F. Cortical plasticity, therapeutic effects, and neural circuit activity of angular gyrus rTMS in amnestic mild cognitive impairment. Clin. Neurophysiol. 2025, 174, 198–211. [Google Scholar] [CrossRef]
- Liu, S.; Ao, C.; Li, Z.; Chio, L.H.; Gu, Y.; Zhan, H.; Li, H.; Li, H.; Li, Z.; Wang, Q. Development and validation of a poly-somnography-based nomogram for predicting amnestic mild cognitive impairment in middle-aged and elderly people. Sleep Med. 2025, 132, 106564. [Google Scholar] [CrossRef]
- Batzikosta, A.; Moraitou, D.; Steiropoulos, P.; Papantoniou, G.; Kougioumtzis, G.A.; Katsouri, I.-G.; Sofologi, M.; Tsolaki, M. Examining specific theory-of-mind aspects in amnestic and non-amnestic mild cognitive impairment: Their relation-ships with sleep duration and cognitive planning. Brain Sci. 2025, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W. Pharmacotherapy for Dementia: A Practical Approach to the Use of Cholinesterase Inhibitors and Memantine. Drugs Aging 2016, 33, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Marlats, F.; Bao, G.; Chevallier, S.; Boubaya, M.; Azabou, E. SMR/Theta Neurofeedback Training Improves Cogni-tive Performance and EEG Activity in Elderly with Mild Cognitive Impairment: A Pilot Study. Front. Aging Neurosci. 2020, 12, 147. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, L.; Chen, H.; Liu, W.; Liang, Z. Improving attention through individualized fNIRS neurofeedback training: A pilot study. Brain Sci. 2022, 12, 862. [Google Scholar] [CrossRef]
- Lavy, Y.; Dwolatzky, T.; Kaplan, Z.; Guez, J.; Todder, D. Neurofeedback Improves Memory and Peak Alpha Frequency in Individuals with Mild Cognitive Impairment. Appl. Psychophysiol. Biofeedback 2019, 44, 41–49. [Google Scholar] [CrossRef]
- Su, R.; Li, X.; Liu, Y.; Cui, W.; Xie, P.; Han, Y. Evaluation of the brain function state during mild cognitive impair-ment based on weighted multiple multiscale entropy. Front. Aging Neurosci. 2021, 13, 625081. [Google Scholar] [CrossRef] [PubMed]
- Faucounau, V.; Wu, Y.H.; Boulay, M.; Rotrou, J.D.; Rigaud, A. Cognitive intervention programmes on patients affected by Mild Cognitive Impairment: A promising intervention tool for MCI? J. Nutr. Health Aging 2010, 14, 31–35. [Google Scholar] [CrossRef]
- Israsena, P.; Jirayucharoensak, S.; Hemrungrojn, S.; Pan-Ngum, S. Brain Exercising Games with Consumer-Grade Single-Channel Electroencephalogram Neurofeedback: Pre-Post Intervention Study. JMIR Serious Games 2021, 9, e26872. [Google Scholar] [CrossRef]
- Alatorre-Cruz, G.C.; Thalía, F.; Castro-Chavira, S.A.; González-López, M.; Sánchez-Moguel, M.S.; Sil-va-Pereyra, J. One-Year Follow-Up of Healthy Older Adults with Electroencephalographic Risk for Neurocogni-tive Disorder After Neurofeedback Training. J. Alzheimer’s Dis. 2022, 85, 1767–1781. [Google Scholar] [CrossRef]
- Klados, M.A.; Charis, S.; Frantzidis, C.A.; Evangelos, P.; Bamidis, P. Beta-Band Functional Connectivity is Reor-ganized in Mild Cognitive Impairment after Combined Computerized Physical and Cognitive Training. Front. Neurosci. 2016, 10, 55. [Google Scholar] [CrossRef]
- Francesco, B.; Matteo, M.; Mara, C.; Fulvia, A.; Roberta, P.; Roberta, A.; Augusto, C.G.; Claudia, R.; Giovanna, L.M.; Valeria, T. A Pilot Study on Brain Plasticity of Functional Connectivity Modulated by Cognitive Training in Mild Alzheimer’s Disease and Mild Cognitive Impairment. Brain Sci. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Samantha, S.; Abásolo, D. Distance-Based Lempel–Ziv Complexity for the Analysis of Electroencephalograms in Patients with Alzheimer’s Disease. Entropy 2017, 19, 129. [Google Scholar]
- Bahar-Fuchs, A.; Webb, S.; Bartsch, L.; Clare, L.; Rebok, G.; Cherbuin, N.; Anstey, K. Tailored and adaptive com-puterized cognitive training in older adults at risk for dementia: A randomized controlled trial. J. Alzheimer’s Dis. 2017, 60, 889–911. [Google Scholar] [CrossRef]
- Liu, Y.; Sourina, O.; Hou, X. Neurofeedback Games to Improve Cognitive Abilities. In Proceedings of the 2014 International Conference on Cyberworlds, Santander, Spain, 6–8 October 2014; pp. 161–168. [Google Scholar]
- Suhail, T.A.; Subasree, R.; Vinod, A.P.; Alladi, S. On the feasibility of an online brain-computer inter-face-based neurofeedback game for enhancing attention and working memory in stroke and mild cognitive impairment patients. Biomed. Phys. Eng. Express 2025, 11, 025049. [Google Scholar]
- Rui, S.; Haibo, Y.; Yina, Z.; Chenru, H.; Pengxiang, D.; Ping, X.; Yi, Y.; Xin, L. Changes in information segregation and integration of aMCI based on low- and high-order functional connectivity. Cereb. Cortex 2025, 35, bhaf001. [Google Scholar]
- Ke, Z.; Yinghua, W.; Gaoxiang, O.; Zhijie, B.; Lei, W.; Xiaoli, L. Complex network analysis of resting state EEG in amnestic mild cognitive impairment patients with type 2 diabetes. Front. Comput. Neurosci. 2015, 9, 133. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Surya, D.; Puthankattil, S.D. Puthankattil Complex network analysis of MCI-AD EEG signals under cognitive and resting state. Brain Res. 2020, 1735, 146743. [Google Scholar]
- Zeng, L.; Wang, C.; Sun, K.; Pu, Y.; Gao, Y.; Wang, H.; Liu, X.; Wen, Z. Upregulation of a small-world brain network improves inhibitory control: An fNIRS neurofeedback training study. Brain Sci. 2023, 13, 1516. [Google Scholar] [CrossRef]
- Tazaki, M. Effects of neurofeedback on MCI and AD. Front. Hum. Neurosci. 2024, 17, 1331436. [Google Scholar]
- Trambaiolli, L.R.; Cassani, R.; Mehler, D.M.A.; Falk, T.H. Neurofeedback and the Aging Brain: A Systematic Review of Training Protocols for Dementia and Mild Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 682683. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Li, X.-D.; Cui, W. Neurofeedback Training for Brain Functional Connectivity Improvement in Mild Cognitive. J. Med. Biol. Eng. 2020, 40, 484–495. [Google Scholar] [CrossRef]
- Kabbara, A.; Eid, H.; El Falou, W.; Khalil, M.; Wendling, F.; Hassan, M. Reduced integration and improved segregation of functional brain networks in Alzheimer’s disease. J. Neural Eng. 2018, 15, 026023. [Google Scholar] [CrossRef] [PubMed]
- Vilou, I.; Varka, A.; Parisis, D.; Afrantou, T.; Ioannidis, P. EEG-Neurofeedback as a Potential Therapeutic Approach for Cognitive Deficits in Patients with Dementia, Multiple Sclerosis, Stroke and Traumatic Brain Injury. Life 2023, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, A.; Di Lorenzo, F.; Manzoli, L.; Flacco, M.E.; Koch, G. Blood phosphorylated Tau217 distinguishes amyloid-positive from amyloid-negative subjects in the Alzheimer’s disease continuum. A systematic review and meta-analysis. J. Neurol. 2025, 272, 252. [Google Scholar] [CrossRef] [PubMed]
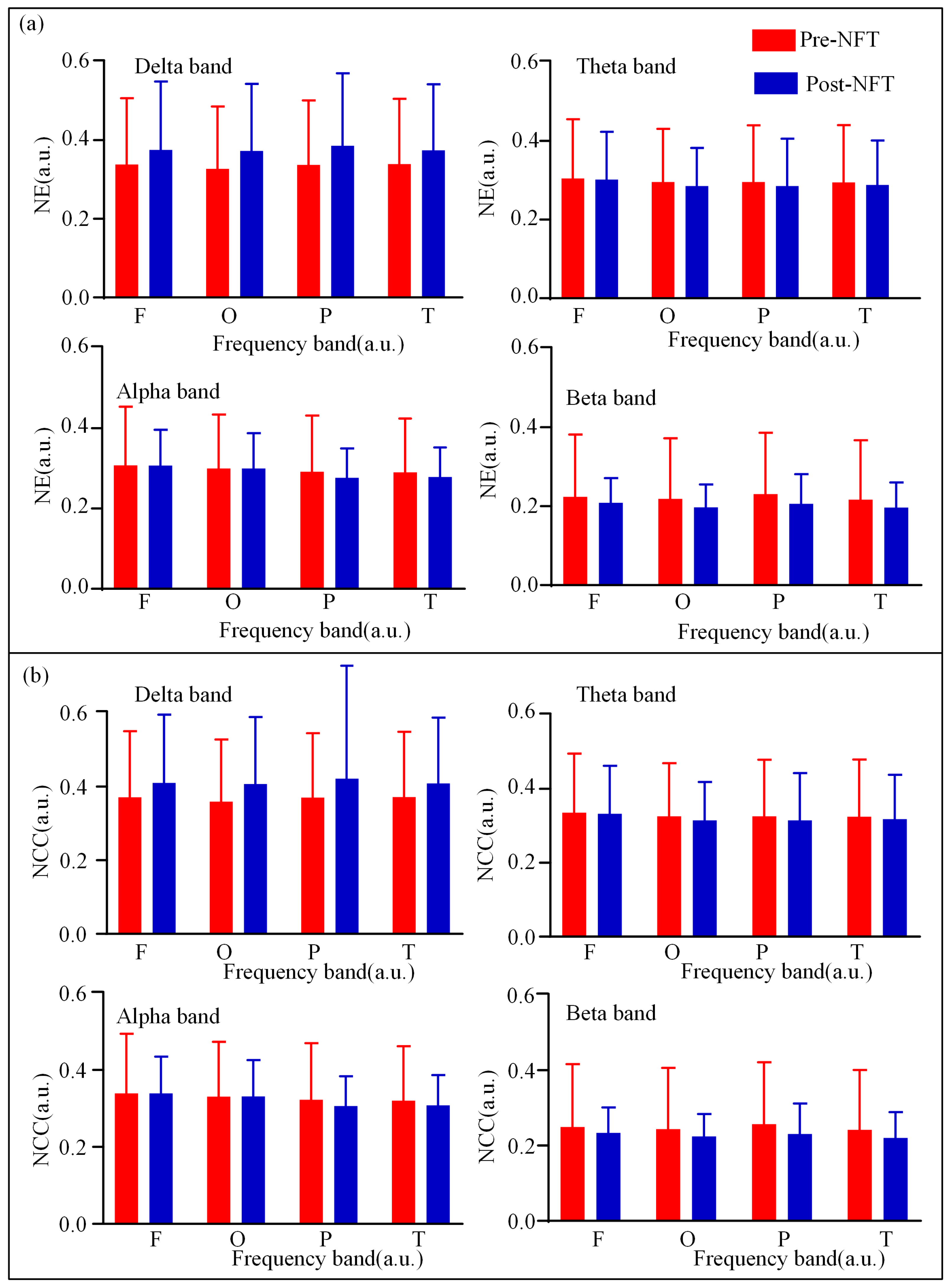
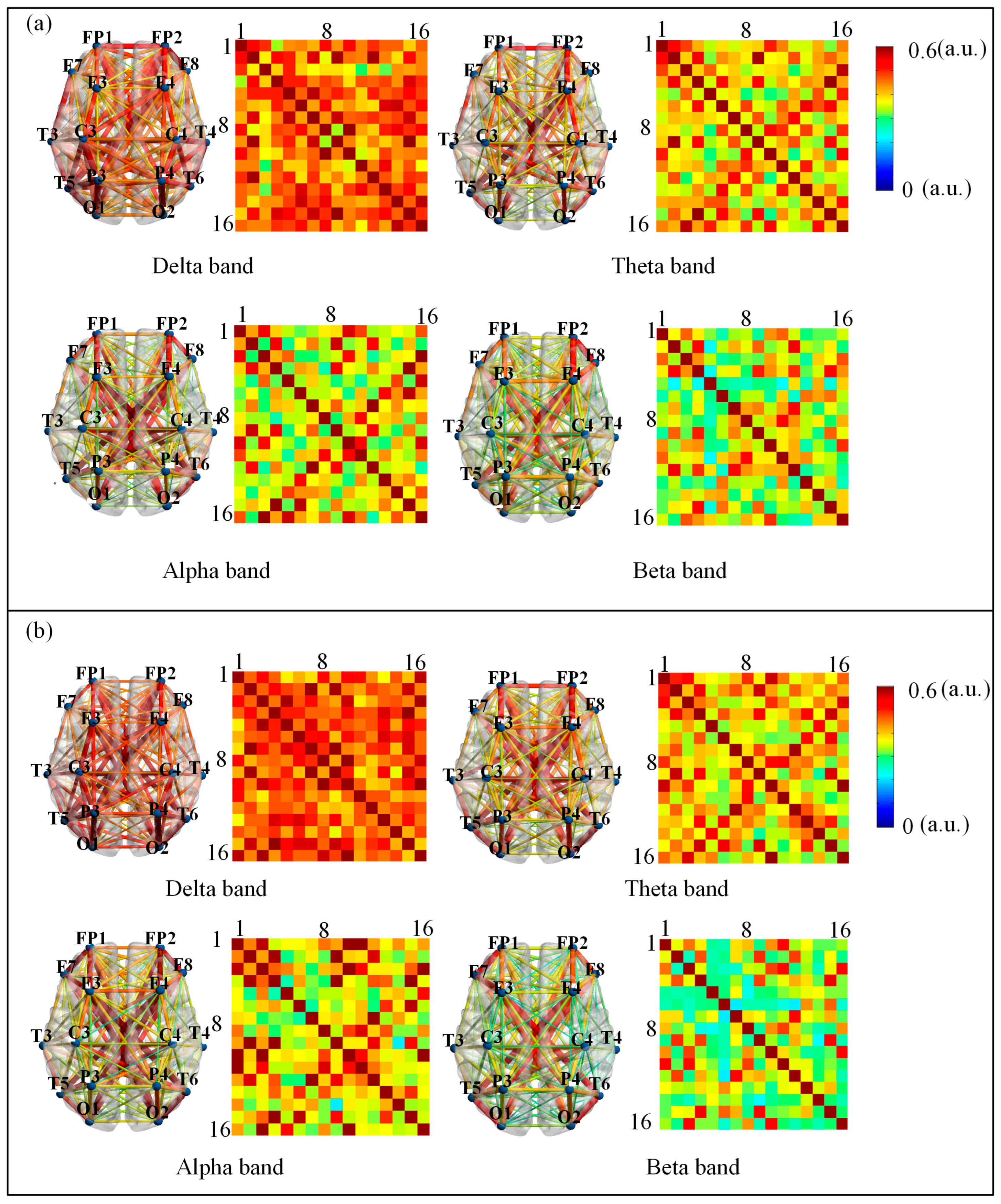
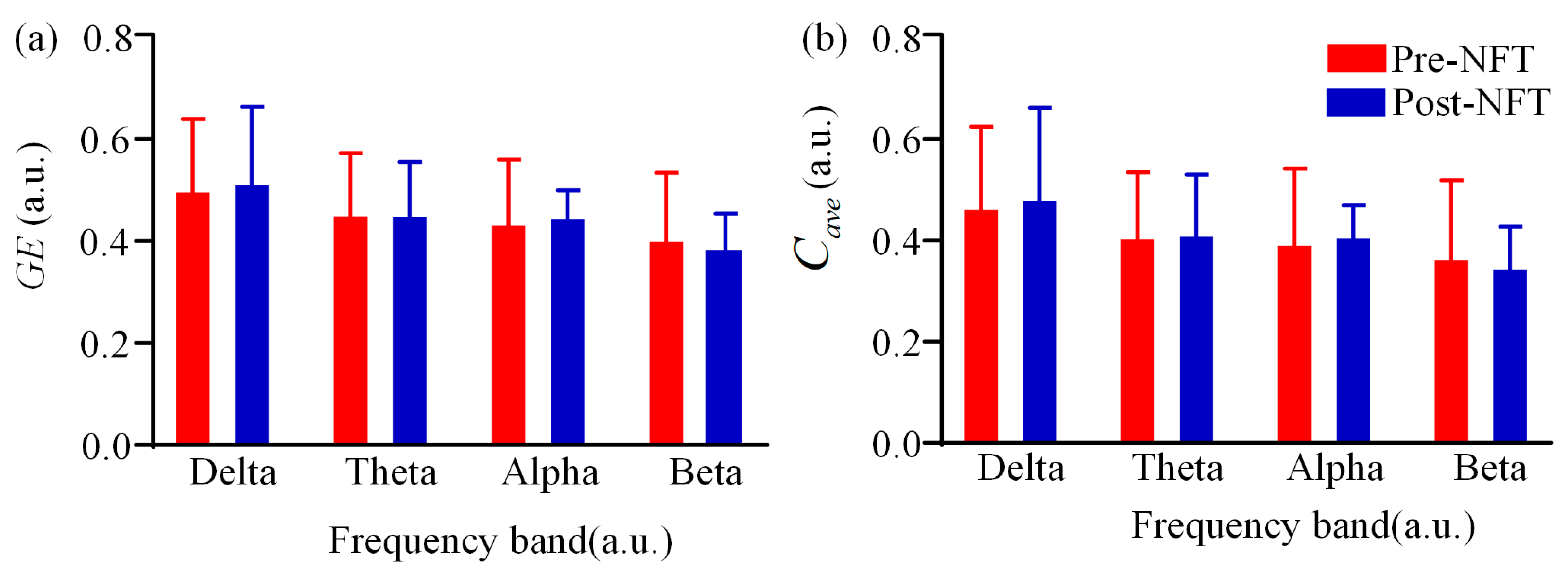
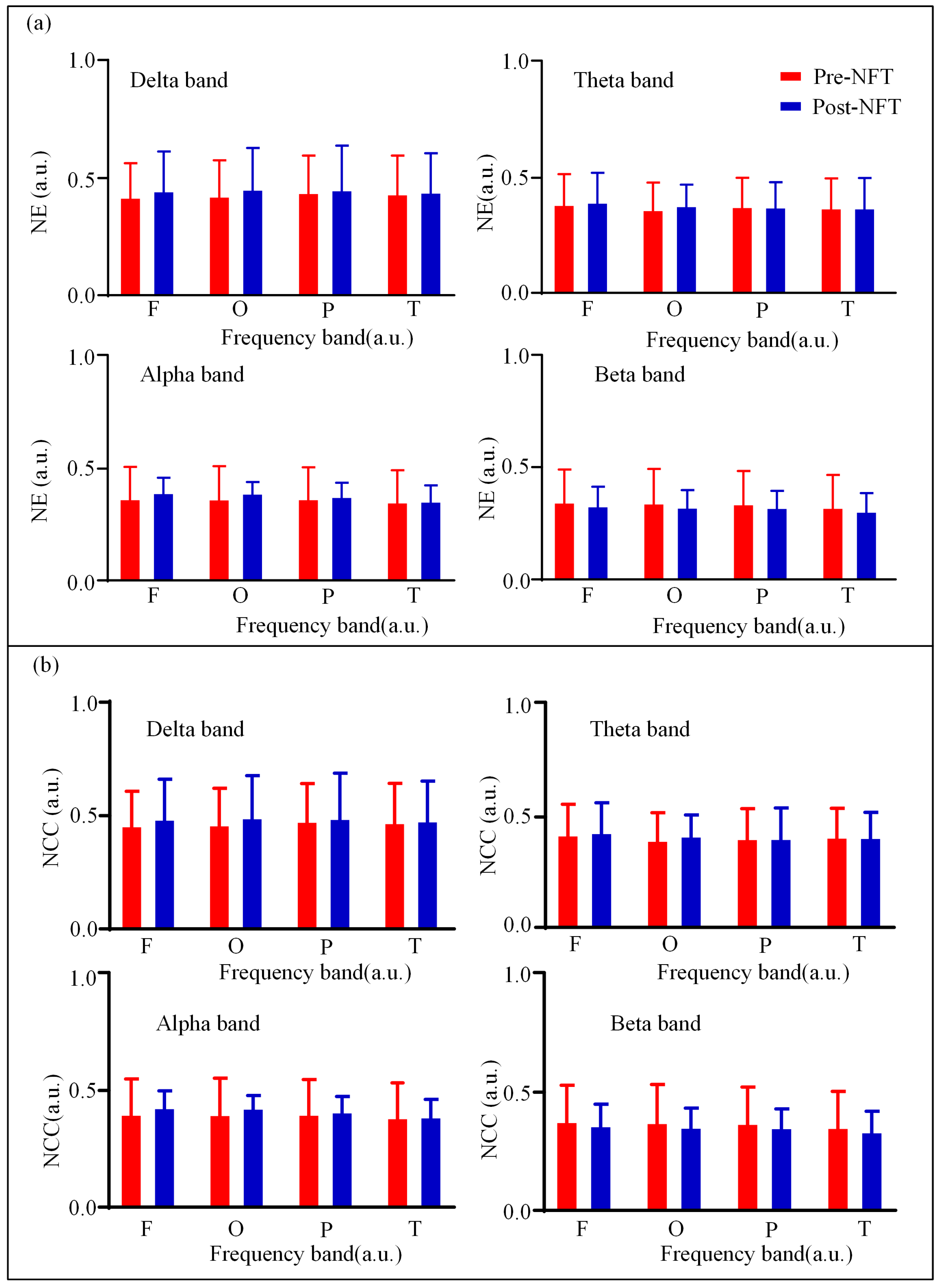
| Characteristics | aMCI (n = 28) |
|---|---|
| Age (mean ± SD) | 65.12 ± 4.31 |
| Gender (male/female) | 16/12 |
| Educational level (mean ± SD) | 12.63 ± 2.30 |
| Medical history | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, R.; Li, X.; Xie, P.; Yuan, Y. Neurofeedback Training Modulates Brain Functional Networks and Improves Cognition in Amnestic Mild Cognitive Impairment Patients Aged 60–70 Years. Brain Sci. 2025, 15, 1243. https://doi.org/10.3390/brainsci15111243
Su R, Li X, Xie P, Yuan Y. Neurofeedback Training Modulates Brain Functional Networks and Improves Cognition in Amnestic Mild Cognitive Impairment Patients Aged 60–70 Years. Brain Sciences. 2025; 15(11):1243. https://doi.org/10.3390/brainsci15111243
Chicago/Turabian StyleSu, Rui, Xin Li, Ping Xie, and Yi Yuan. 2025. "Neurofeedback Training Modulates Brain Functional Networks and Improves Cognition in Amnestic Mild Cognitive Impairment Patients Aged 60–70 Years" Brain Sciences 15, no. 11: 1243. https://doi.org/10.3390/brainsci15111243
APA StyleSu, R., Li, X., Xie, P., & Yuan, Y. (2025). Neurofeedback Training Modulates Brain Functional Networks and Improves Cognition in Amnestic Mild Cognitive Impairment Patients Aged 60–70 Years. Brain Sciences, 15(11), 1243. https://doi.org/10.3390/brainsci15111243






