The Modulatory Effect of tDCS Onset Timing in Alleviating Vigilance Decrement
Abstract
1. Introduction
The Current Study
2. Materials and Methods
2.1. Participants
2.2. Vigilance Task: Visualized Modified Version of the Bakan Task
2.3. Subjective Mood Measurement
2.4. Stimulation Protocols and EEG Recording
2.4.1. Apparatus
2.4.2. tDCS Setup and EEG Recordings
2.5. Procedures and Pilot Study
2.5.1. Pilot Study and Its Results

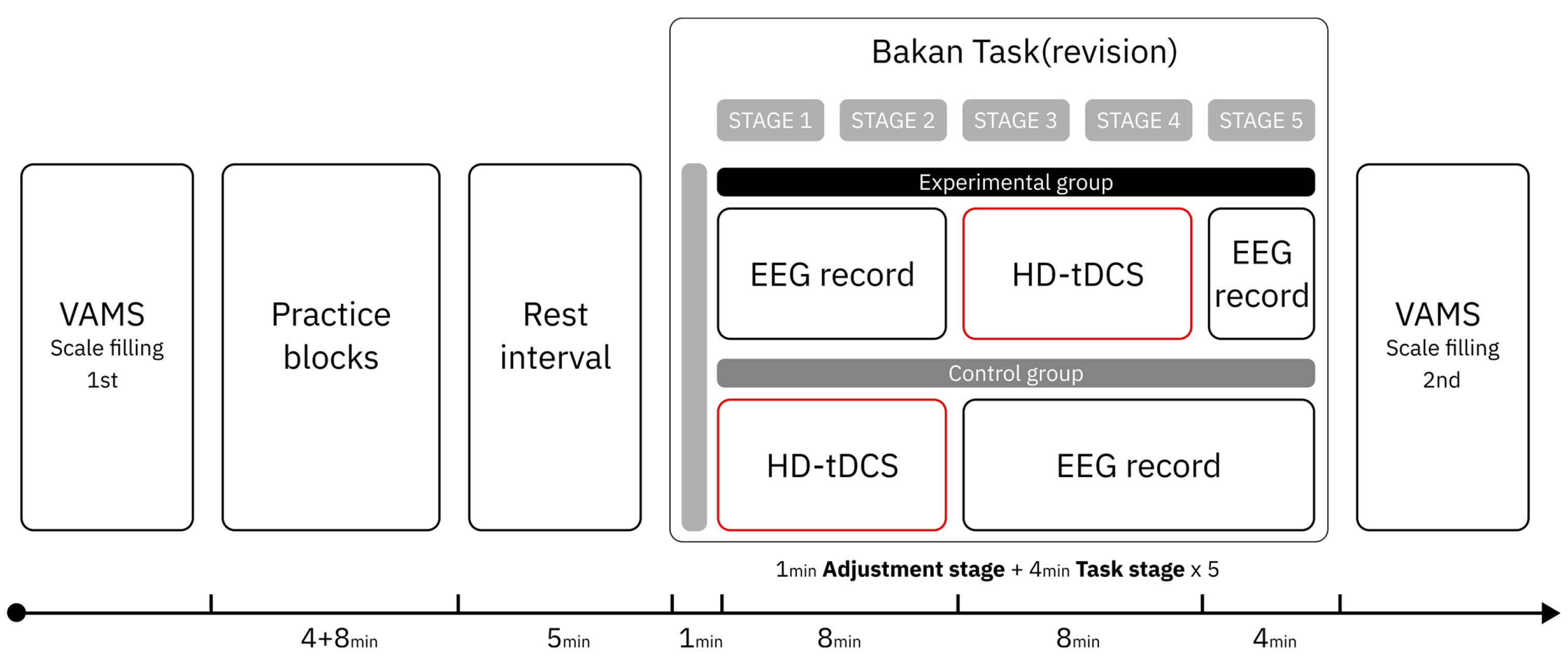
2.5.2. Experimental Procedures
2.6. Statistical Analyses
2.6.1. Behavioral Data
2.6.2. EEG Data
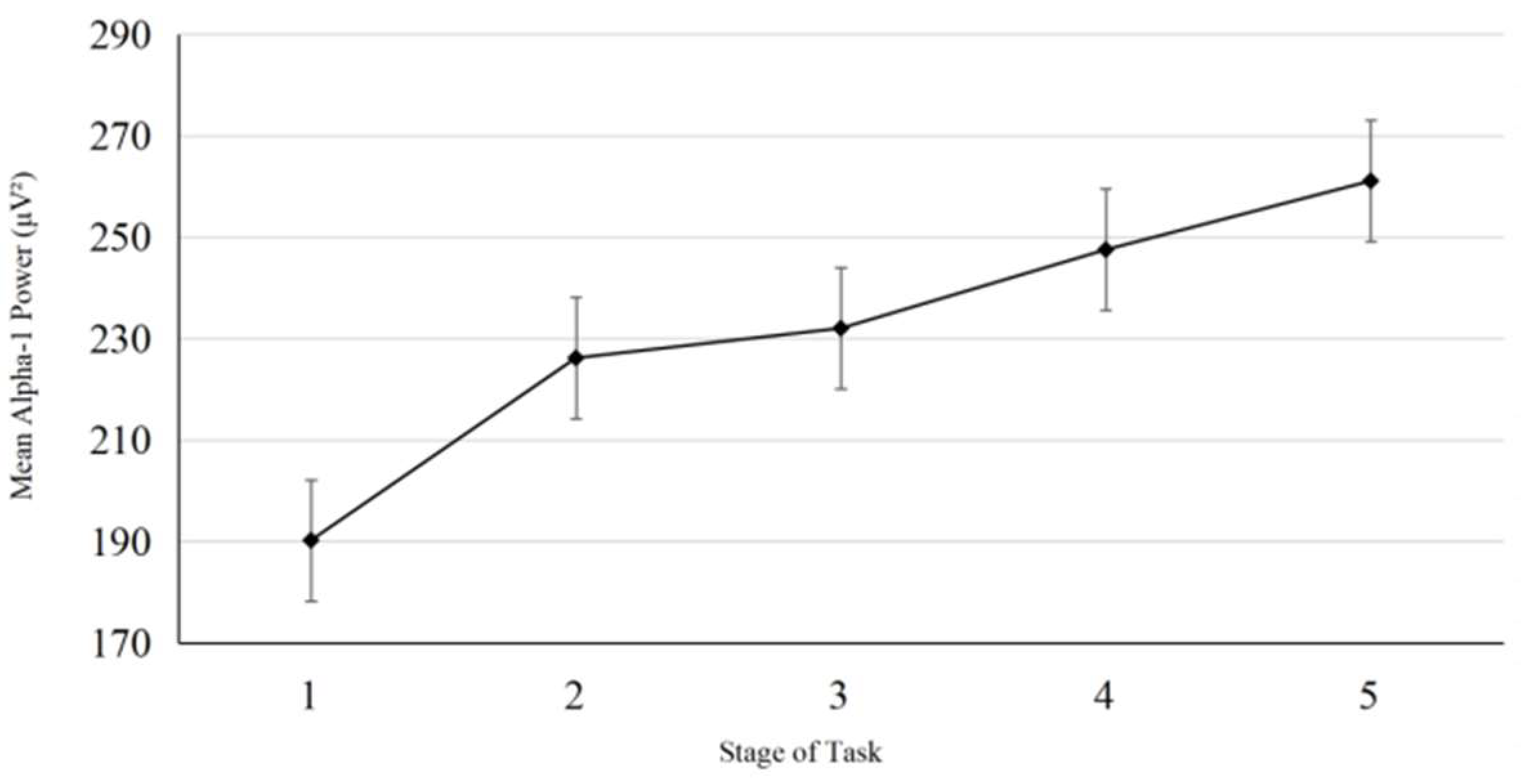
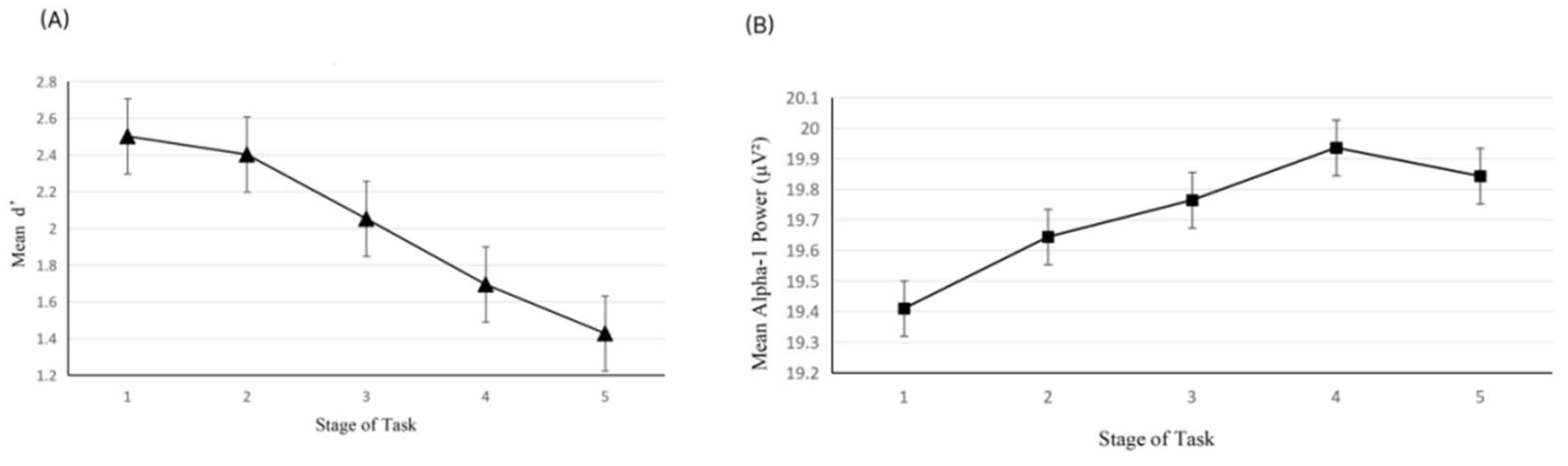
3. Results
3.1. Pre-Verification
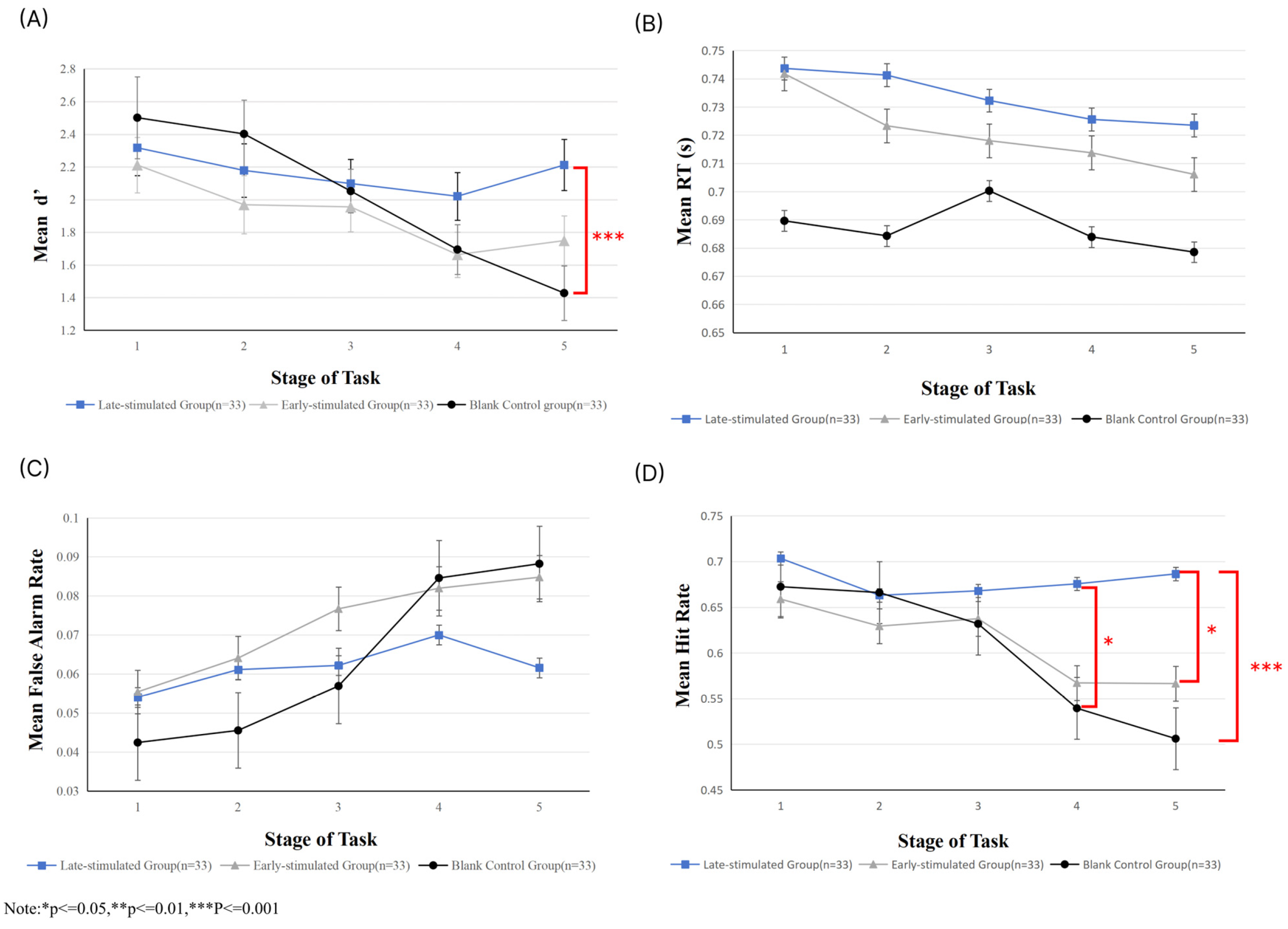
3.2. Behavioral Level Analysis
3.3. EEG Data Analysis
3.4. VAMS Score
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TDCS | Transcranial direct current stimulation |
| EEG | electroencephalographic |
References
- Morgan, K.; Johnson, A.J.; Miles, C. Chewing gum moderates the vigilance decrement. Br. J. Psychol. 2013, 105, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Luna, F.G.; Marino, J.; Roca, J.; Lupiáñez, J. Executive and arousal vigilance decrement in the context of the attentional networks: The ANTI-Vea task. J. Neurosci. Methods 2018, 306, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, V.; Tortajada, M.; Palmero, L.B.; Campoy, G.; Fuentes, L.J. Effects of transcranial alternating current stimulation over right-DLPFC on vigilance tasks depend on the arousal level. Sci. Rep. 2022, 12, 547. [Google Scholar] [CrossRef]
- Warm, J.S.; Parasuraman, R.; Matthews, G. Vigilance requires hard mental work and is stressful. Hum. Factors 2008, 50, 433–441. [Google Scholar] [CrossRef]
- Kharoufah, H.; Murray, J.; Baxter, G.; Wild, G. A review of human factors causations in commercial air transport accidents and incidents: From to 2000-2016. Prog. Aerosp. Sci. 2018, 99, 1–13. [Google Scholar] [CrossRef]
- Gök, F.; Koçbilek, Z.D. Examination of fatigue levels and factors affecting fatigue in operating room nurses. Perioper. Care Oper. Room Manag. 2022, 26, 100243. [Google Scholar] [CrossRef]
- Xie, T.; Ma, N. Tracking vigilance fluctuations in real-time: A sliding-window heart rate variability-based machine-learning approach. Sleep 2024, 48, zsae199. [Google Scholar] [CrossRef]
- Izzetoglu, K.; Yurtsever, G.; Bozkurt, A.; Bunce, S. Functional brain monitoring via NIR based optical spectroscopy. In Proceedings of the IEEE 29th Annual Northeast Bioengineering Conference, Nj Inst Technol, Newark, NJ, USA, 22–23 March 2003; pp. 335–336. [Google Scholar]
- Ma, J.X.; Shi, L.C.; Lu, B.L.; Ieee. Vigilance Estimation by Using Electrooculographic Features. In Proceedings of the 32nd Annual International Conference of the IEEE Engineering-in-Medicine-and-Biology-Society (EMBC 10), Buenos Aires, Argentina, 30 August–4 September 2010; pp. 6591–6594. [Google Scholar]
- Samima, S.; Sarma, M.; Samanta, D. Detecting Vigilance in People Performing Continual Monitoring Task. In Proceedings of the 9th International Conference on Intelligent Human Computer Interaction (IHCI), Evry, France, 11–13 December 2017; pp. 202–214. [Google Scholar]
- Wang, K.N.; Qiu, S.; Wei, W.; Zhang, C.C.; He, H.G.; Xu, M.P.; Ming, D.; Ieee. Vigilance Estimating in SSVEP-Based BCI Using Multimodal Signals. In Proceedings of the 43rd Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society (IEEE EMBC), Electr Network, Guadalajara, Mexico, 1–5 November 2021; pp. 5974–5978. [Google Scholar]
- Kamzanova, A.T.; Kustubayeva, A.M.; Matthews, G. Use of EEG Workload Indices for Diagnostic Monitoring of Vigilance Decrement. Hum. Factors 2014, 56, 1136–1149. [Google Scholar] [CrossRef]
- Luna, F.G.; Román-Caballero, R.; Barttfeld, P.; Lupiáñez, J.; Martín-Arévalo, E. A High -Definition tDCS and EEG study on attention and vigilance: Brain stimulation mitigates the executive but not the arousal vigilance decrement. Neuropsychologia 2020, 142, 107447. [Google Scholar] [CrossRef] [PubMed]
- Hemmerich, K.; Lupiñez, J.; Luna, F.G.; Martín-Arévalo, E. The mitigation of the executive vigilance decrement via HD-tDCS over the right posterior parietal cortex and its association with neural oscillations. Cerebral Cortex 2023, 33, 6761–6771. [Google Scholar] [CrossRef]
- Huang, J.; Yu, C.; Wang, Y.T.; Zhao, Y.; Liu, S.Q.; Mo, C.; Liu, J.; Zhang, L.; Shi, Y.C.; Acm. FOCUS: Enhancing Children’s Engagement in Reading by Using Contextual BCI Training Sessions. In Proceedings of the 32nd Annual ACM Conference on Human Factors in Computing Systems (CHI), Toronto, Canada, 26 April–1 May 2014; pp. 1905–1908. [Google Scholar]
- McIntire, L.K.; McKinley, R.A.; Goodyear, C.; Nelson, J. A Comparison of the Effects of Transcranial Direct Current Stimulation and Caffeine on Vigilance and Cognitive Performance During Extended Wakefulness. Brain Stimul. 2014, 7, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, C.; Blasco, E.; Luna, F.G.; Lupiáñez, J. Effects of caffeine intake and exercise intensity on executive and arousal vigilance. Sci. Rep. 2020, 10, 8393. [Google Scholar] [CrossRef]
- Nelson, J.T.; McKinley, R.A.; Golob, E.J.; Warm, J.S.; Parasuraman, R. Enhancing vigilance in operators with prefrontal cortex transcranial direct current stimulation (tDCS). Neuroimage 2014, 85, 909–917. [Google Scholar] [CrossRef]
- Jacoby, N.; Lavidor, M. Null tDCS Effects in a Sustained Attention Task: The Modulating Role of Learning. Front. Psychol. 2018, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- McIntire, L.K.; McKinley, R.A.; Nelson, J.M.; Goodyear, C. Transcranial direct current stimulation versus caffeine as a fatigue countermeasure. Brain Stimul. 2017, 10, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, G.S.; Stinear, C.M.; Searchfield, G.D. Transcranial Direct Current Stimulation Intensity and Duration Effects on Tinnitus Suppression. Neurorehabilit. Neural Repair 2013, 27, 164–172. [Google Scholar] [CrossRef]
- Furubayashi, T.; Terao, Y.; Arai, N.; Okabe, S.; Mochizuki, H.; Hanajima, R.; Hamada, M.; Yugeta, A.; Inomata-Terada, S.; Ugawa, Y. Short and long duration transcranial direct current stimulation (tDCS) over the human hand motor area. Exp. Brain Res. 2008, 185, 279–286. [Google Scholar] [CrossRef]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef]
- Thair, H.; Holloway, A.L.; Newport, R.; Smith, A.D. Transcranial Direct Current Stimulation (tDCS): A Beginner’s Guide for Design and Implementation. Front. Neurosci. 2017, 11, 641. [Google Scholar] [CrossRef]
- Navarro-López, V.; del Valle-Gratacós, M.; Fernández-Matías, R.; Carratalá-Tejada, M.; Cuesta-Gómez, A.; Molina-Rueda, F. The Long-Term Maintenance of Upper Limb Motor Improvements Following Transcranial Direct Current Stimulation Combined with Rehabilitation in People with Stroke: A Systematic Review of Randomized Sham-Controlled Trials. Sensors 2021, 21, 5216. [Google Scholar] [CrossRef]
- O’Neil-Pirozzi, T.M.; Doruk, D.; Thomson, J.M.; Fregni, F. Immediate memory and electrophysiologic effects of prefrontal cortex transcranial direct current stimulation on neurotypical individuals and individuals with chronic traumatic brain injury: A pilot study. Int. J. Neurosci. 2017, 127, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.O.; Macedo, M.; Monerat, V.K.V.; Ferreira, K.R.D.; dos Santos, M.E.C.; Esquirio, A.F.; Alves, A.L.G.; Gama, G.L.; Barbosa, M.A.; Barbosa, A.C. Does the Transcranial Direct Current Stimulation Selectively Modulate Prefrontal Cortex Hemodynamics? An Immediate Effect-Controlled Trial on People with and without Depression. Appl. Sci. 2024, 14, 7901. [Google Scholar] [CrossRef]
- Xie, L.X.; Hu, P.N.; Guo, Z.L.; Chen, M.; Wang, X.; Du, X.Z.; Li, Y.; Chen, B.; Zhang, J.H.; Zhao, W.T.; et al. Immediate and long-term efficacy of transcranial direct current stimulation (tCDS) in obsessive-compulsive disorder, posttraumatic stress disorder and anxiety disorders: A systematic review and meta-analysis. Transl. Psychiatry 2024, 14, 343. [Google Scholar] [CrossRef]
- Leshikar, E.D.; Leach, R.C.; McCurdy, M.P.; Trumbo, M.C.; Sklenar, A.M.; Frankenstein, A.N.; Matzen, L.E. Transcranial direct current stimulation of dorsolateral prefrontal cortex during encoding improves recall but not recognition memory. Neuropsychologia 2017, 106, 390–397. [Google Scholar] [CrossRef]
- Bolognini, N.; Spandri, V.; Ferraro, F.; Salmaggi, A.; Molinari, A.C.L.; Fregni, F.; Maravita, A. Immediate and Sustained Effects of 5-Day Transcranial Direct Current Stimulation of the Motor Cortex in Phantom Limb Pain. J. Pain 2015, 16, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Liu, R.; Alonzo, A.; Green, M.; Loo, C.K. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: Effect of timing of stimulation. Exp. Brain Res. 2014, 232, 3345–3351. [Google Scholar] [CrossRef]
- Sriraman, A.; Oishi, T.; Madhavan, S. Timing-dependent priming effects of tDCS on ankle motor skill learning. Brain Res. 2014, 1581, 23–29. [Google Scholar] [CrossRef]
- Gan, T.; Huang, Y.Y.; Hao, X.; Hu, L.N.; Zheng, Y.; Yang, Z. Anodal tDCS Over the Left Frontal Eye Field Improves Sustained Visual Search Performance. Perception 2022, 51, 263–275. [Google Scholar] [CrossRef]
- Molero-Chamizo, A.; Bailén, J.R.A.; Béjar, T.G.; López, M.G.; Rodríguez, I.J.; Lérida, C.G.; Panal, S.P.; Angel, G.G.; Corchero, L.L.; Vega, M.J.R.; et al. Poststimulation time interval-dependent effects of motor cortex anodal tDCS on reaction-time task performance. Cogn. Affect. Behav. Neurosci. 2018, 18, 167–175. [Google Scholar] [CrossRef]
- Conradt, R.; Brandenburg, U.; Penzel, T.; Hasan, J.; Värri, A.; Peter, J.H. Vigilance transitions in reaction time test:: A method of describing the state of alertness more objectively. Clin. Neurophysiol. 1999, 110, 1499–1509. [Google Scholar] [CrossRef]
- Minkwitz, J.; Trenner, M.U.; Sander, C.; Olbrich, S.; Sheldrick, A.J.; Hegerl, U.; Himmerich, H. Time perception at different EEG-vigilance levels. Behav. Brain Funct. 2012, 8, 50. [Google Scholar] [CrossRef]
- Ross, H.A.; Russell, P.N.; Helton, W.S. Effects of breaks and goal switches on the vigilance decrement. Exp. Brain Res. 2014, 232, 1729–1737. [Google Scholar] [CrossRef]
- Wesensten, N.J.; Belenky, G.; Kautz, M.A.; Thorne, D.R.; Reichardt, R.M.; Balkin, T.J. Maintaining alertness and performance during sleep deprivation: Modafinil versus caffeine. Psychopharmacology 2002, 159, 238–247. [Google Scholar] [CrossRef]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Borghini, G.; Astolfi, L.; Vecchiato, G.; Mattia, D.; Babiloni, F. Measuring neurophysiological signals in aircraft pilots and car drivers for the assessment of mental workload, fatigue and drowsiness. Neurosci. Biobehav. Rev. 2014, 44, 58–75. [Google Scholar] [CrossRef]
- Boksem, M.A.S.; Meijman, T.F.; Lorist, M.M. Effects of mental fatigue on attention: An ERP study. Cogn. Brain Res. 2005, 25, 107–116. [Google Scholar] [CrossRef]
- Dissanayaka, C.; Ben-Simon, E.; Gruberger, M.; Maron-Katz, A.; Sharon, H.; Hendler, T.; Cvetkovic, D. Comparison between human awake, meditation and drowsiness EEG activities based on directed transfer function and MVDR coherence methods. Med. Biol. Eng. Comput. 2015, 53, 599–607. [Google Scholar] [CrossRef]
- Correa, A.G.; Orosco, L.; Laciar, E. Automatic detection of drowsiness in EEG records based on multimodal analysis. Med. Eng. Phys. 2014, 36, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.R.; Popovic, D.P.; Olmstead, R.E.; Stikic, M.; Levendowski, D.J.; Berka, C. Drowsiness/alertness algorithm development and validation using synchronized EEG and cognitive performance to individualize a generalized model. Biol. Psychol. 2011, 87, 241–250. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Liu, D.; Wang, Q.S.; Zhao, B.Q.; Bai, O.; Sun, J.W. Detection of alertness-related EEG signals based on decision fused BP neural network. Biomed. Signal Process. Control. 2022, 74, 103479. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhao, Y.; Zhang, J.; Zou, J.Z. Automatic detection of alertness/drowsiness from physiological signals using wavelet-based nonlinear features and machine learning. Expert Syst. Appl. 2015, 42, 7344–7355. [Google Scholar] [CrossRef]
- Pershin, I.; Candrian, G.; Münger, M.; Baschera, G.M.; Rostami, M.; Eich, D.; Müller, A. Vigilance described by the time-on-task effect in EEG activity during a cued Go/NoGo task. Int. J. Psychophysiol. 2023, 183, 92–102. [Google Scholar] [CrossRef]
- Shenhav, A.; Botvinick, M.M.; Cohen, J.D. The Expected Value of Control: An Integrative Theory of Anterior Cingulate Cortex Function. Neuron 2013, 79, 217–240. [Google Scholar] [CrossRef]
- Posner, M.I. Orienting of attention: Then and now. Q. J. Exp. Psychol. 2016, 69, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Bakan, P. Extraversion-introversion and improvement in an auditory vigilance task. Br. J. Psychol. 1959, 50, 325–332. [Google Scholar] [CrossRef]
- Temple, J.G.; Warm, J.S.; Dember, W.N.; Jones, K.T.S.; LaGrange, C.M.; Matthews, G. The effects of signal salience and caffeine on performance, workload, and stress in an abbreviated vigilance task. Hum. Factors 2000, 42, 183–194. [Google Scholar] [CrossRef] [PubMed]
- BOND, A.; LADER, M. The use of analogue scales in rating subjective feelings. Br. J. Med. Psychol. 1974, 47, 211–218. [Google Scholar] [CrossRef]
- Alam, M.; Truong, D.Q.; Khadka, N.; Bikson, M. Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Phys. Med. Biol. 2016, 61, 4506–4521. [Google Scholar] [CrossRef]
- Green, D.M.; Swets, J.A. Signal Detection Theory and Psychophysics; Wiley: Hoboken, NJ, USA, 1966. [Google Scholar]
- Corti, E.J.; Nguyen, A.T.; Marinovic, W.; Gasson, N.; Loftus, A.M. Anodal-TDCS over Left-DLPFC Modulates Motor Cortex Excitability in Chronic Lower Back Pain. Brain Sci. 2022, 12, 1654. [Google Scholar] [CrossRef]
- Falleti, M.G.; Maruff, P.; Collie, A.; Darby, D.G. Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. J. Clin. Exp. Neuropsychol. 2006, 28, 1095–1112. [Google Scholar] [CrossRef]
- Chuang, C.H.; Cao, Z.H.; King, J.T.; Wu, B.S.; Wang, Y.K.; Lin, C.T. Brain Electrodynamic and Hemodynamic Signatures Against Fatigue During Driving. Front. Neurosci. 2018, 12, 181. [Google Scholar] [CrossRef]
- Leproult, R.; Colecchia, E.F.; Berardi, A.M.; Stickgold, R.; Kosslyn, S.M.; Van Cauter, E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 284, R280–R290. [Google Scholar] [CrossRef]
- Babiloni, C.; Miniussi, C.; Babiloni, F.; Carducci, F.; Cincotti, F.; Del Percio, C.; Sirello, G.; Fracassi, C.; Nobre, A.C.; Rossini, P.M. Sub-second “temporal attention” modulates alpha rhythms. A high-resolution EEG study. Cogn. Brain Res. 2004, 19, 259–268. [Google Scholar] [CrossRef]
- Craig, A.; Tran, Y.; Wijesuriya, N.; Nguyen, H. Regional brain wave activity changes associated with fatigue. Psychophysiology 2012, 49, 574–582. [Google Scholar] [CrossRef]
- Bao, Z.J.; Burhan, A.; Frewen, P. Transcranial direct current stimulation over medial prefrontal cortex reduced alpha power and functional connectivity during somatic but not semantic self-referential processing. Neuroscience 2024, 553, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Arnau, S.; Löffler, C.; Rummel, J.; Hagemann, D.; Wascher, E.; Schubert, A.L. Inter-trial alpha power indicates mind wandering. Psychophysiology 2020, 57, e13581. [Google Scholar] [CrossRef] [PubMed]
- Linnhoff, S.; Wolter-Weging, J.; Zaehle, T. Objective electrophysiological fatigability markers and their modulation through tDCS. Clin. Neurophysiol. 2021, 132, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.M.; Klein, M.I. The Abbreviated Vigilance Task and Its Attentional Contributors. Hum. Factors 2019, 61, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Helton, W.S.; Matthews, G.; Warm, J.S. Stress state mediation between environmental variables and performance: The case of noise and vigilance. Acta Psychol. 2009, 130, 204–213. [Google Scholar] [CrossRef]
- Coffman, B.A.; Trumbo, M.C.; Clark, V.P. Enhancement of object detection with transcranial direct current stimulation is associated with increased attention. Bmc Neurosci. 2012, 13, 108. [Google Scholar] [CrossRef]
- Roy, L.B.; Sparing, R.; Fink, G.R.; Hesse, M.D. Modulation of attention functions by anodal tDCS on right PPC. Neuropsychologia 2015, 74, 96–107. [Google Scholar] [CrossRef]
- Lo, O.Y.; van Donkelaar, P.; Chou, L.S. Effects of transcranial direct current stimulation over right posterior parietal cortex on attention function in healthy young adults. Eur. J. Neurosci. 2019, 49, 1623–1631. [Google Scholar] [CrossRef]
- Vergallito, A.; Feroldi, S.; Pisoni, A.; Lauro, L.J.R. Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors. Brain Sci. 2022, 12, 522. [Google Scholar] [CrossRef]
- Bell, S.B.; Turner, B.; Sawaki, L.; DeWall, N. When brain stimulation backfires: The effects of prefrontal cortex stimulation on impulsivity. Soc. Cogn. Affect. Neurosci. 2022, 17, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Miles, C.; Haddrell, B.; Harrison, E.; Osborne, L.; Wilson, N.; Jenks, R. The effect of chewing gum on physiological and self-rated measures of alertness and daytime sleepiness. Physiol. Behav. 2012, 105, 815–820. [Google Scholar] [CrossRef]
- Dockree, P.M.; Kelly, S.P.; Roche, R.A.P.; Hogan, M.J.; Reilly, R.B.; Robertson, I.H. Behavioural and physiological impairments of sustained attention after traumatic brain injury. Cogn. Brain Res. 2004, 20, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Adolfsdottir, S.; Sorensen, L.; Lundervold, A.J. The attention network test: A characteristic pattern of deficits in children with ADHD. Behav. Brain Funct. 2008, 4, 9. [Google Scholar] [CrossRef]
- Brosnan, M.B.; Arvaneh, M.; Harty, S.; Maguire, T.; O’Connell, R.; Robertson, I.H.; Dockree, P.M. Prefrontal Modulation of Visual Processing and Sustained Attention in Aging, a tDCS-EEG Coregistration Approach. J. Cogn. Neurosci. 2018, 30, 1630–1645. [Google Scholar] [CrossRef] [PubMed]


| Participants (n = 117) | |
|---|---|
| Age: mean years ± SD (range) | 20.56 ± 1.48 (18–25) |
| Gender | |
| Female | 58 |
| Male | 59 |
| Education | |
| Bachelor’s degree | 64 |
| Postgraduate degree | 53 |
| Handedness | |
| Right-handed | 117 |
| Left-handed | 0 |
| Mixed-handed | 0 |
| Visual Acuity | |
| Normal or Corrected-to-Normal Vision | 117 |
| Color Vision | Normal, n = 117 |
| Factors | Numbers | Scales | |
|---|---|---|---|
| vigilance | 1 | Alert | Drowsy |
| 2 | Attentive | Dreamy | |
| 3 | Energetic | Lethargic | |
| 4 | Clear-headed | Muzzy | |
| 5 | Well-coordinated | Clumsy | |
| 6 | Quick-witted | Mentally slow | |
| 7 | Strong | Feeble | |
| 8 | Interested | Bored | |
| 9 | Proficient | Incompetent | |
| Contentedness | 10 | Happy | Sad |
| 11 | Amicable | Antagonistic | |
| 12 | Tranquil | Troubled | |
| 13 | Contented | Discontented | |
| 14 | Gregarious | Withdrawn | |
| Calmness | 15 | Calm | Excited |
| 16 | Relaxed | Tense | |
| Group (alpha-1 Power) | M | SD | t | p | Cohen’s d |
|---|---|---|---|---|---|
| Blank Control Group Stage III | 20.3 | 2.19 | −0.135 | 0.893 | −0.33 |
| Early-stimulated Group Stage III | 20.22 | 2.88 | |||
| Blank Control Group Stage V | 20.40 | 3.11 | −3.24 | 0.002 ** | −0.797 |
| Late-stimulated Group Stage V | 18.39 | 1.75 | |||
| Stage III differential | 0.85 | 3.55 | 2.17 | 0.034 * | 0.533 |
| Stage V differential | 2.01 | 3.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Chen, Y.; Wu, S.; Xia, T. The Modulatory Effect of tDCS Onset Timing in Alleviating Vigilance Decrement. Brain Sci. 2025, 15, 1085. https://doi.org/10.3390/brainsci15101085
Pan Z, Chen Y, Wu S, Xia T. The Modulatory Effect of tDCS Onset Timing in Alleviating Vigilance Decrement. Brain Sciences. 2025; 15(10):1085. https://doi.org/10.3390/brainsci15101085
Chicago/Turabian StylePan, Zelin, Yang Chen, Shanghong Wu, and Tiansheng Xia. 2025. "The Modulatory Effect of tDCS Onset Timing in Alleviating Vigilance Decrement" Brain Sciences 15, no. 10: 1085. https://doi.org/10.3390/brainsci15101085
APA StylePan, Z., Chen, Y., Wu, S., & Xia, T. (2025). The Modulatory Effect of tDCS Onset Timing in Alleviating Vigilance Decrement. Brain Sciences, 15(10), 1085. https://doi.org/10.3390/brainsci15101085






