Sex-Dependent Phenotypic and Histomorphometric Biomarkers in the APPswe/PS1dE9/Blg Mouse Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. The Laboratory Animals
2.2. Genotyping of Transgenic Animals
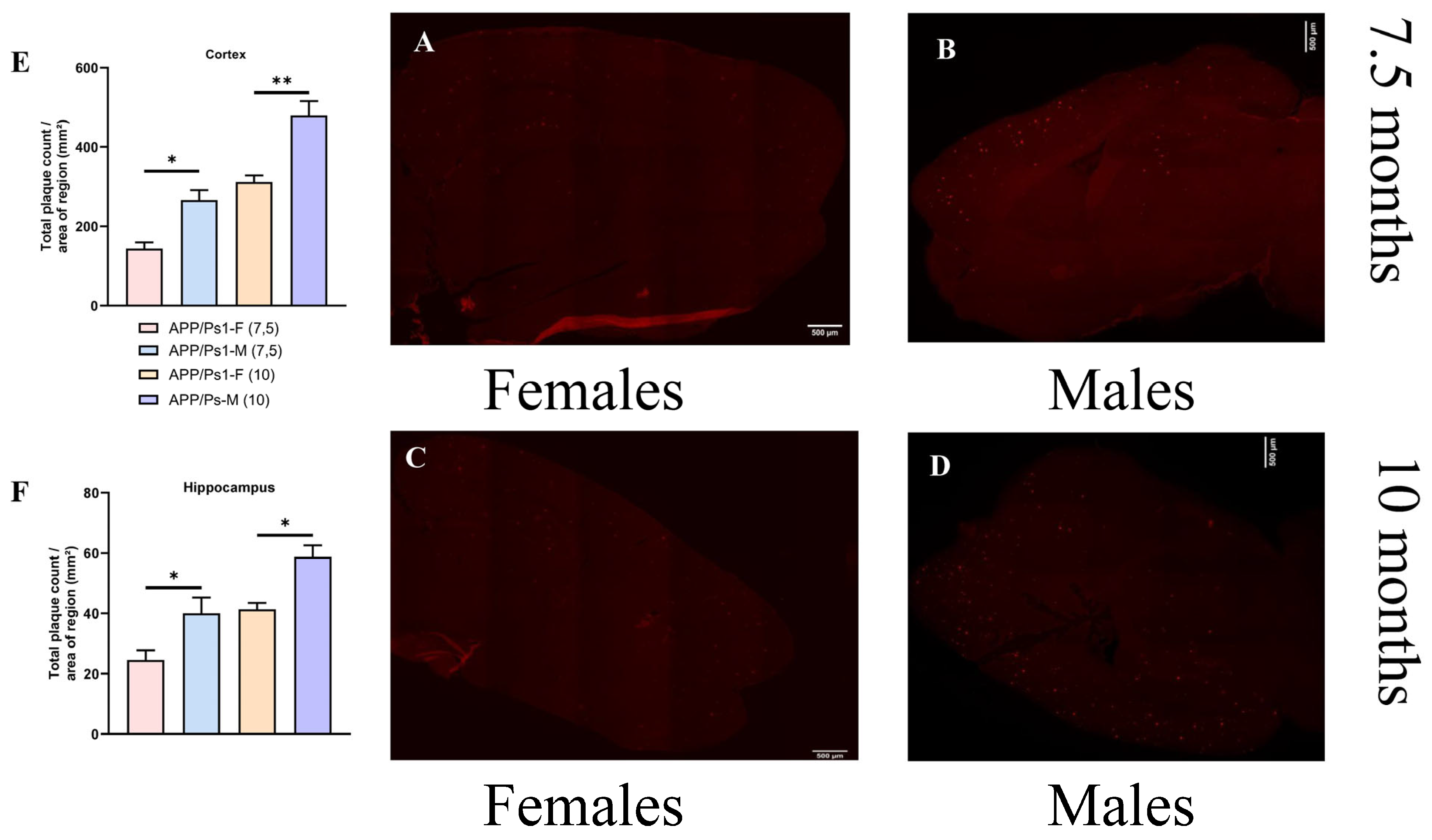
2.3. Histological Studies
2.4. Behavioral Testing
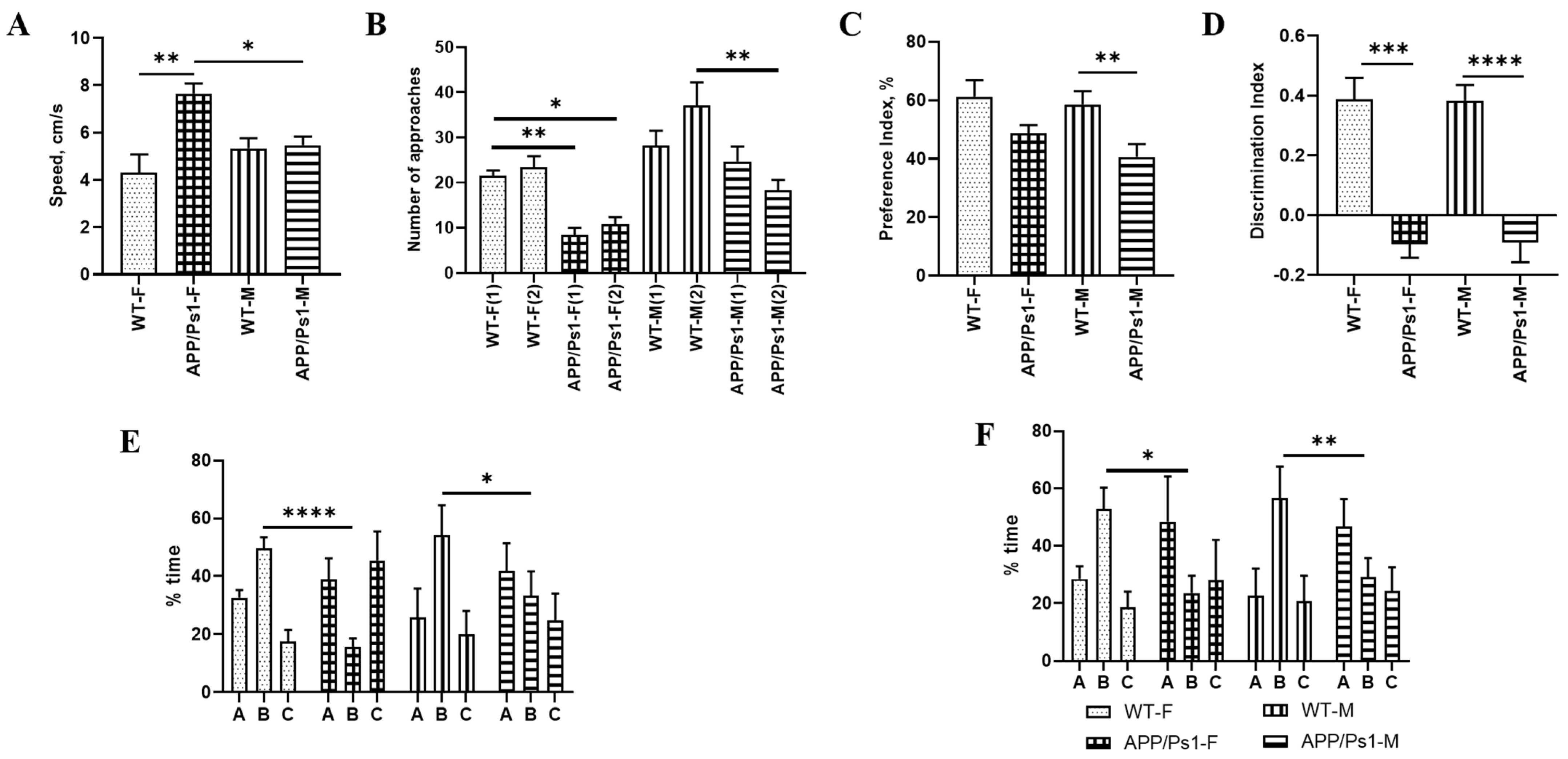
2.4.1. Open Field Test
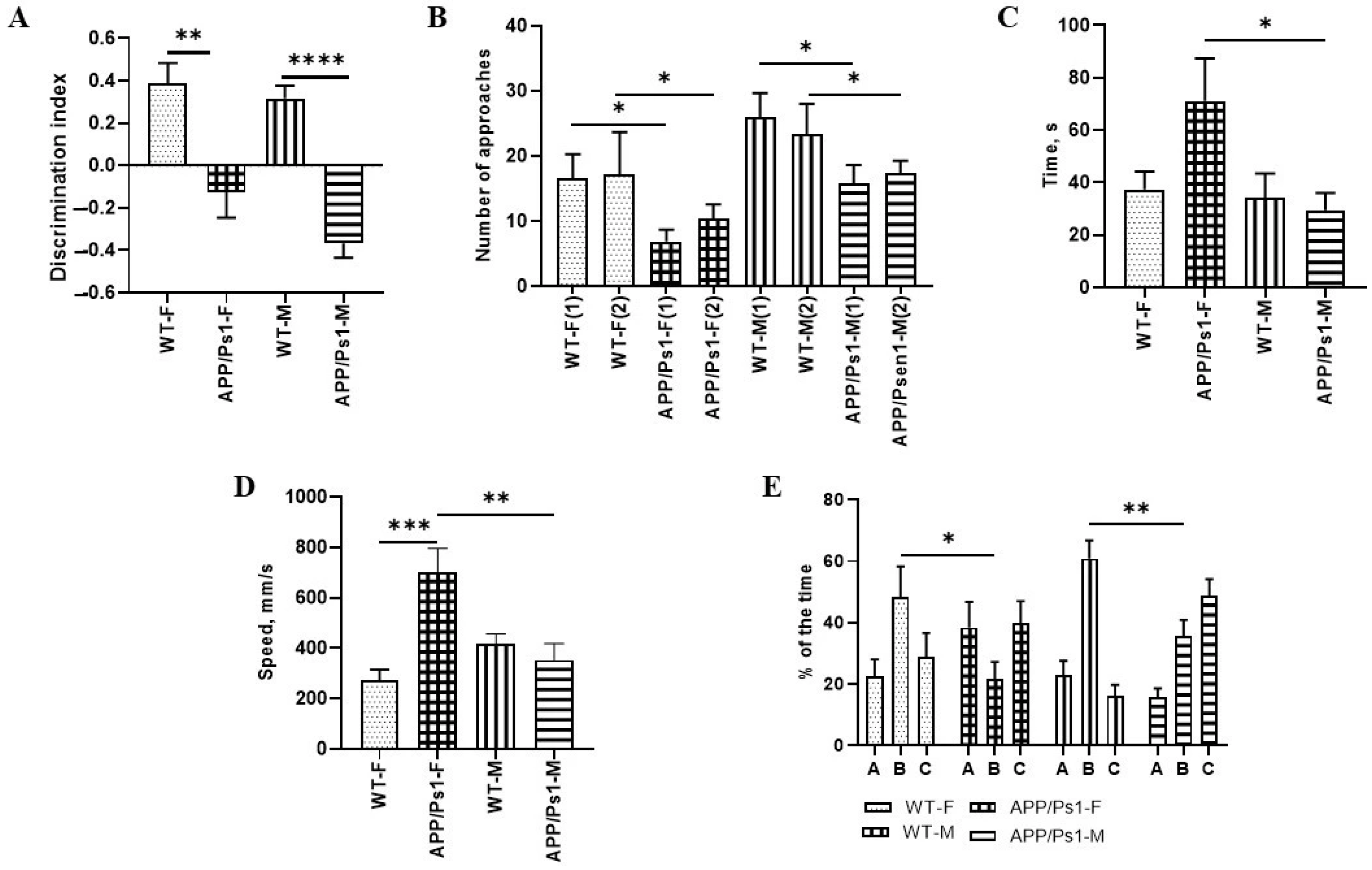
2.4.2. Novel Object Recognition Test
2.4.3. Y-Maze
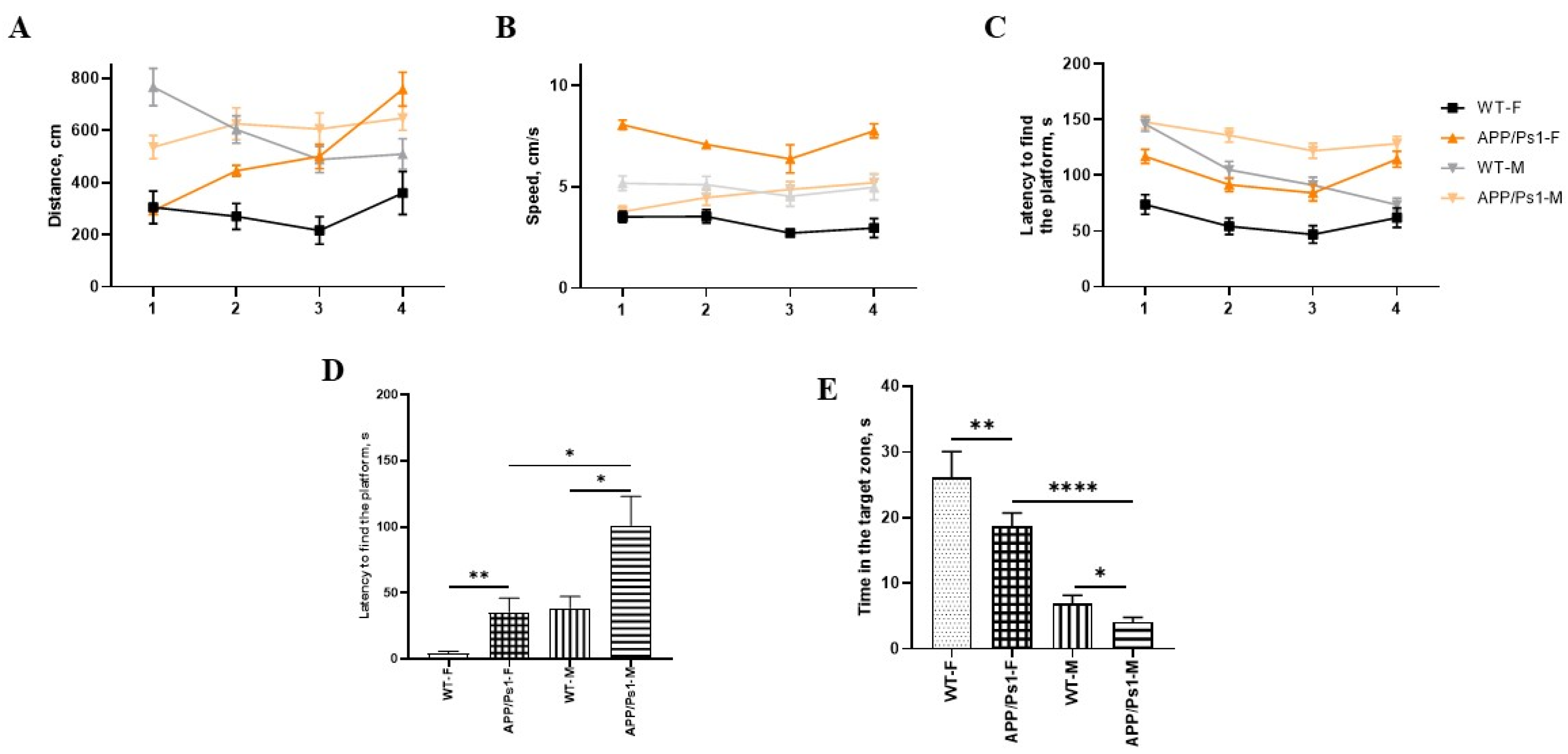
2.4.4. Barnes Maze Test
2.5. Statistical Analysis
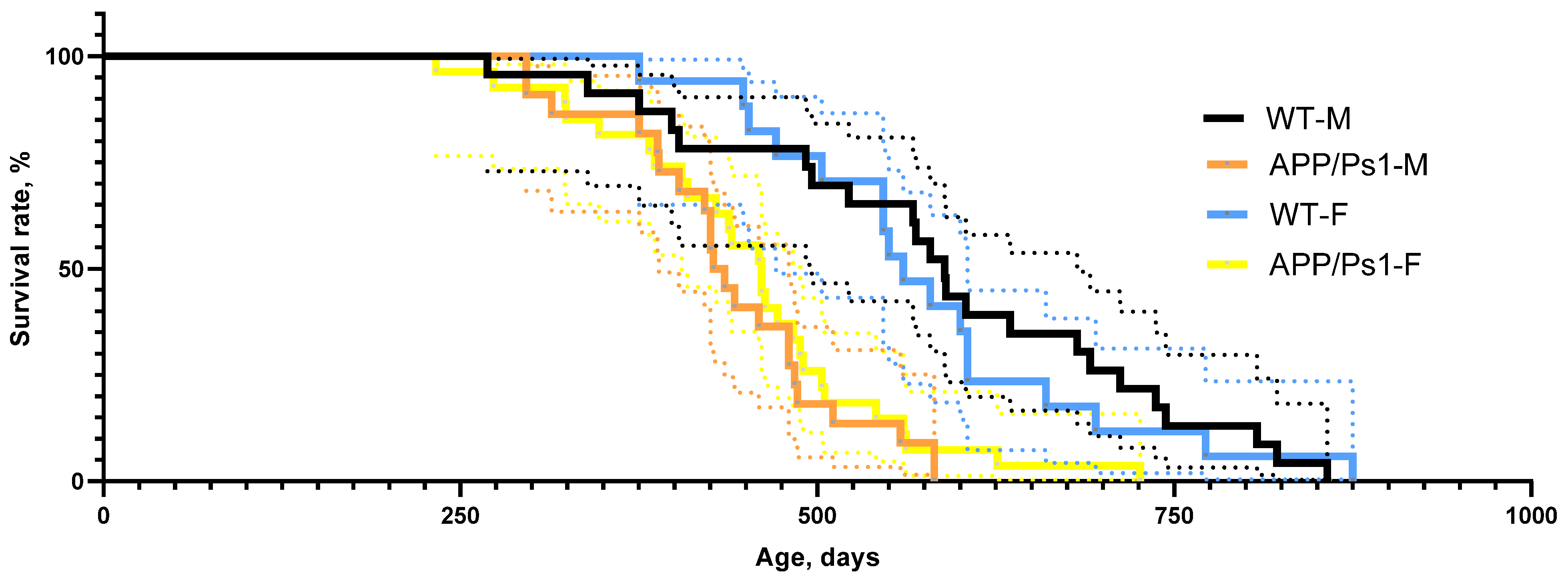
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease. |
| APP/Ps1 | APPswe/PS1dE9/Blg |
| Aβ | Amyloid beta |
| PET | Positron emission tomography |
| MRI | Magnetic Resonance Imaging |
| DI | Discrimination Index |
| RI | Recognition Index |
References
- Sakata, H.; Narasimhan, P.; Niizuma, K.; Maier, C.M.; Wakai, T.; Chan, P.H. Interleukin 6-Preconditioned Neural Stem Cells Reduce Ischaemic Injury in Stroke Mice. Brain 2012, 135, 3298–3310. [Google Scholar] [CrossRef]
- Zhang, S.; Danchuk, S.D.; Bonvillain, R.W.; Xu, B.; Scruggs, B.A.; Strong, A.L.; Semon, J.A.; Gimble, J.M.; Betancourt, A.M.; Sullivan, D.E.; et al. Interleukin 6 Mediates the Therapeutic Effects of Adipose-Derived Stromal/Stem Cells in Lipopolysaccharide-Induced Acute Lung Injury. Stem Cells 2014, 32, 1616–1628. [Google Scholar] [CrossRef]
- de Munter, J.P.J.M.; Shafarevich, I.; Liundup, A.; Pavlov, D.; Wolters, E.C.; Gorlova, A.; Veniaminova, E.; Umriukhin, A.; Kalueff, A.; Svistunov, A.; et al. Neuro-Cells Therapy Improves Motor Outcomes and Suppresses Inflammation during Experimental Syndrome of Amyotrophic Lateral Sclerosis in Mice. CNS Neurosci. Ther. 2020, 26, 504–517. [Google Scholar] [CrossRef]
- de Munter, J.P.J.M.; Mey, J.; Strekalova, T.; Kramer, B.W.; Wolters, E.C. Why Do Anti-Inflammatory Signals of Bone Marrow-Derived Stromal Cells Improve Neurodegenerative Conditions Where Anti-Inflammatory Drugs Fail? J. Neural Transm. 2020, 127, 715–727. [Google Scholar] [CrossRef]
- Wizemann, T.M.; Pardue, M.L.; Institute of Medicine Committee on Understanding the Biology of Sex and Gender Differences (Eds.) Exploring the Biological Contributions to Human Health: Does Sex Matter? National Academies Press (US): Washington, DC, USA, 2001. [Google Scholar]
- de Munter, J.; Babaevskaya, D.; Wolters, E.C.; Pavlov, D.; Lysikova, E.; Kalueff, A.V.; Gorlova, A.; Oplatchikova, M.; Pomytkin, I.A.; Proshin, A. Molecular and Behavioural Abnormalities in the FUS-Tg Mice Mimic Frontotemporal Lobar Degeneration: Effects of Old and New Anti-Inflammatory Therapies. J. Cell. Mol. Med. 2020, 24, 10251–10257. [Google Scholar] [CrossRef] [PubMed]
- Sommerlad, A.; Kivimäki, M.; Larson, E.B.; Röhr, S.; Shirai, K.; Singh-Manoux, A.; Livingston, G. Social participation and risk of developing dementia. Nat. Aging 2023, 3, 532–545. [Google Scholar] [CrossRef]
- Ball, H.A.; McWhirter, L.; Ballard, C.; Bhome, R.; Blackburn, D.J.; Edwards, M.J.; Fleming, S.M.; Fox, N.C.; Howard, R.; Huntley, J.; et al. Functional cognitive disorder: Dementia’s blind spot. Brain 2020, 143, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Rahn, E.J.; Iannitti, T.; Donahue, R.R.; Taylor, B.K. Sex differences in a mouse model of multiple sclerosis: Neuropathic pain behavior in females but not males and protection from neurological deficits during proestrus. Biol. Sex Differ. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, G.V.; Frantsiyants, E.M.; Shikhlyarova, A.I.; Kaplieva, I.V.; Trepitaki, L.K.; Galina, A.V. On the variability of blood parameters and adaptation status of intact BALB/C mice of different sexes. South Russ. J. Oncol. 2023, 4, 13–22. [Google Scholar] [CrossRef]
- Jęśko, H.; Wencel, P.; Strosznajder, R.P.; Strosznajder, J.B. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem. Res. 2017, 42, 876–890. [Google Scholar] [CrossRef]
- Chiariello, A.; Valente, S.; Pasquinelli, G.; Baracca, A.; Sgarbi, G.; Solaini, G.; Medici, V.; Fantini, V.; Poloni, T.E.; Tognocchi, M.; et al. The expression pattern of GDF15 in human brain changes during aging and in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1058665. [Google Scholar] [CrossRef]
- Sze, C.I.; Troncoso, J.C.; Kawas, C.; Mouton, P.; Price, D.L.; Martin, L.J. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1997, 56, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Elderman, M.; Hugenholtz, F.; Belzer, C.; Boekschoten, M.; van Beek, A.; de Haan, B.; Savelkoul, H.; de Vos, P.; Faas, M. Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol. Sex Differ. 2018, 9, 26. [Google Scholar] [CrossRef]
- Bozon, B.; Davis, S.; Laroche, S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron 2003, 40, 695–701. [Google Scholar] [CrossRef]
- Qin, X.; Wang, Y.; Paudel, H.K. Early Growth Response 1 (Egr-1) Is a Transcriptional Activator of β-Secretase 1 (BACE-1) in the Brain. J. Biol. Chem. 2016, 291, 22276–22287. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS 2019, 16, 3. [Google Scholar] [CrossRef]
- Jackson, K.; Barisone, G.A.; Diaz, E.; Jin, L.W.; DeCarli, C.; Despa, F. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann. Neurol. 2013, 74, 517–526. [Google Scholar] [CrossRef]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2010, 35, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, N.M.; Chen, H.-C.; Lechner, M.G.; Su, M.A. Sex Differences in Immunity. Annu. Rev. Immunol 2022, 40, 75–94. [Google Scholar] [CrossRef]
- Mogil, J.S.; Chanda, M.L. The case for the inclusion of female subjects in basic science studies of pain. Pain 2005, 117, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S.; Wilson, S.G.; Chesler, E.J.; Rankin, A.L.; Nemmani, K.V.; Lariviere, W.R.; Groce, M.K.; Wallace, M.R.; Kaplan, L.; Staud, R.; et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc. Natl. Acad. Sci. USA 2003, 100, 4867–4872. [Google Scholar] [CrossRef]
- Cahill, L. A half-truth is a whole lie: On the necessity of investigating sex influences on the brain. Endocrinology 2012, 153, 2541–2543. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]
- Shmigerova, V.S.; Stepenko, Y.V.; Kurbatova, A.A.; Zhunusov, N.S.; Lyapkalov, N.S.; Sviridova, M.S.; Avtina, T.V.; Nesterov, A.V.; Popov, A.A.; Nesterova, N.I.; et al. Investigation of the pharmacological activity of the tetrapeptide HAEE, zinc, and human serum albumin in a transgenic mouse model with tau protein overexpression (P301S). Res. Results Pharmacol. 2025, 11, 49–57. [Google Scholar] [CrossRef]
- de Munter, J.P.J.M.; Tsoy, A.; Sitdikova, K.; Wolters, E.C.; Chaprov, K.; Yenkoyan, K.B.; Torosyan, H.; Askarova, S.; Anthony, D.C.; Strekalova, T. Therapeutic Effects of Neuro-Cells on Amyloid Pathology, BDNF Levels, and Insulin Signalling in APPswe/PSd1E9 Mice. Cells 2025, 14, 1293. [Google Scholar] [CrossRef] [PubMed]
- de Munter, J.; Chaprov, K.; Lang, E.; Sitdikova, K.; Wolters, E.C.; Svirin, E.; Kassenova, A.; Tsoy, A.; Kramer, B.W.; Askarova, S.; et al. Neuro-Cells Mitigate Amyloid Plaque Formation and Behavioral Deficits in the APPswe/PS1dE9 Model of Alzheimer Disease While Also Reducing IL-6 Production in Human Monocytes. Cells 2025, 14, 1168. [Google Scholar] [CrossRef]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef]
- Strekalova, T.; Svirin, E.; Veniaminova, E.; Kopeikina, E.; Veremeyko, T.; Yung, A.W.Y.; Proshin, A.; Walitza, S.; Anthony, D.C.; Lim, L.W. ASD-like behaviors, a dysregulated inflammatory response and decreased expression of PLP1 characterize mice deficient for sialyltransferase ST3GAL5. Brain Behav. Immun.-Health 2021, 16, 100306. [Google Scholar] [CrossRef]
- Veniaminova, E.; Oplatchikova, M.; Bettendorff, L.; Kotenkova, E.; Lysko, A.; Vasilevskaya, E.; Kalueff, A.V.; Fedulova, L.; Umriukhin, A.; Lesch, K.-P. Prefrontal cortex inflammation and liver pathologies accompany cognitive and motor deficits following Western diet consumption in non-obese female mice. Life Sci. 2020, 241, 117163. [Google Scholar] [CrossRef]
- Becker, J.B.; Prendergast, B.J.; Liang, J.W. Female rats are not more variable than male rats: A meta-analysis of neuroscience studies. Biol Sex Differ. 2016, 26, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Slunt, H.H.; Ratovitski, T.; Jenkins, N.A.; Copeland, N.G.; Borchelt, D.R. Co-expression of multiple transgenes in mouse CNS: A comparison of strategies. Biomol. Eng. 2001, 17, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Costa-Nunes, J.P.; Cline, B.H.; Araújo-Correia, M.; Valença, A.; Markova, N.; Dolgov, O.; Kubatiev, A.; Yeritsyan, N.; Steinbusch, H.W.; Strekalova, T. Animal Models of Depression and Drug Delivery with Food as an Effective Dosing Method: Evidences from Studies with Celecoxib and Dicholine Succinate. Biomed. Res. Int. 2015, 2015, 596126. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Costa-Nunes, J.P.; Veniaminova, E.; Kubatiev, A.; Lesch, K.-P.; Chekhonin, V.P.; Evans, M.C.; Steinbusch, H.W.M. Insulin receptor sensitizer, dicholine succinate, prevents both Toll-like receptor 4 (TLR4) upregulation and affective changes induced by a high-cholesterol diet in mice. J. Affect. Disord. 2016, 196, 109–116. [Google Scholar] [CrossRef]
- Patrakhanov, E.A. Effectiveness of the tetrapeptide HAEE: An innovative approach to Alzheimer’s treatment in experimentation. Res. Results Pharmacol. 2024, 10, 107–111. [Google Scholar] [CrossRef]
- Strekalova, T.; Pavlov, D.; Trofimov, A.; Anthony, D.C.; Svistunov, A.; Proshin, A.; Umriukhin, A.; Lyundup, A.; Lesch, K.P.; Cespuglio, R. Hippocampal Over-Expression of Cyclooxygenase-2 (COX-2) Is Associated with Susceptibility to Stress-Induced Anhedonia in Mice. Int. J. Mol. Sci. 2022, 23, 2061. [Google Scholar] [CrossRef]
- Aleksandrova, Y.; Semakov, A.; Tsypyshev, D.; Chaprov, K.; Klochkov, S.; Neganova, M. Neuroprotective Effects and Cognitive Enhancement of Allomargaritarine in 5xFAD Alzheimer’s Disease Mice Model. OBM Neurobiol 2024, 8, 1–33. [Google Scholar] [CrossRef]
- Sun, Y.; Kakinen, A.; Zhang, C.; Yang, Y.; Faridi, A.; Davis, T.P.; Cao, W.; Ke, P.C.; Ding, F. Amphiphilic surface chemistry of fullerenols is necessary for inhibiting the amyloid aggregation of alpha-synuclein NACore. Nanoscale 2019, 11, 11933–11945. [Google Scholar] [CrossRef] [PubMed]
- Shmigerova, V.S.; Stepienko, Y.V.; Pokrovskaya, T.G. Effect of the pharmaceutical composition HAEE-Zn-HSA on spatial learning and memory in transgenic mice P301S Tau. Exp. Clin. Pharmacol. 2023, 11, 161. [Google Scholar] [CrossRef]
- Malatynska, E.; Steinbusch, H.W.M.; Redkozubova, O.; Bolkunov, A.; Kubatiev, A.; Yeritsyan, N.B.; Vignisse, J.; Bachurin, S.; Strekalova, T. Anhedonic-like traits and lack of affective deficits in 18-month-old C57BL/6 mice: Implications for modeling elderly depression. Exp. Gerontol. 2012, 47, 552–564. [Google Scholar] [CrossRef]
- Schroeter, C.A.; Gorlova, A.; Sicker, M.; Umriukhin, A.; Burova, A.; Shulgin, B.; Morozov, S.; Costa-Nunes, J.P.; Strekalova, T. Unveiling the Mechanisms of a Remission in Major Depressive Disorder (MDD)-like Syndrome: The Role of Hippocampal Palmitoyltransferase Expression and Stress Susceptibility. Biomolecules 2025, 15, 67. [Google Scholar] [CrossRef]
- Strekalova, T.; Anthony, D.C.; Dolgov, O.; Anokhin, K.; Kubatiev, A.; Steinbusch, H.M.; Schroeter, C. The differential effects of chronic imipramine or citalopram administration on physiological and behavioral outcomes in naïve mice. Behav. Brain Res. 2013, 245, 101–106. [Google Scholar] [CrossRef]
- Pokrovsky, V.M.; Deikin, A.V.; Zhang, T.; Verlov, N.A.; Konevega, A.L.; Korokin, M.V. The influence of exogenous recombinant HSP 70 on the alteration of membrane stiffness in hippocampal neurons following the modeling of neonatal hypoxic-ischemic injury in mice. Res. Results Pharmacol. 2024, 10, 87–97. [Google Scholar] [CrossRef]
- Ferretti, M.T.; Iulita, M.F.; Cavedo, E.; Chiesa, P.A.; Schumacher Dimech, A.; Santuccione Chadha, A.; Baracchi, F.; Girouard, H.; Misoch, S.; Giacobini, E.; et al. Sex differences in Alzheimer disease—The gateway to precision medicine. Nat. Rev. Neurol. 2018, 14, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Cline, B.H.; Steinbusch, H.W.M.; Malin, D.; Revishchin, A.V.; Pavlova, G.V.; Cespuglio, R.; Strekalova, T. The neuronal insulin sensitizer dicholine succinate reduces stress-induced depressive traits and memory deficit: Possible role of insulin-like growth factor 2. BMC Neurosci. 2012, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, I.L.; Gaisina, G.G.; Klen, E.E.; Tkachenko, L.A. Assessment of the 3-substituted thietane-1,1-dioxide derivative antidepressant effect using rat model of depression induced by reserpine. Res. Results Pharmacol. 2025, 11, 13–26. [Google Scholar] [CrossRef]
- Askarova, S.; Sitdikova, K.; Kassenova, A.; Chaprov, K.; Svirin, E.; Tsoy, A.; de Munter, J.; Gorlova, A.; Litavrin, A.; Deikin, A.; et al. Distinctive Effects of Fullerene C60 and Fullerenol C60(OH)24 Nanoparticles on Histological, Molecular and Behavioral Hallmarks of Alzheimer’s Disease in APPswe/PS1E9 Mice. Antioxidants 2025, 14, 834. [Google Scholar] [CrossRef]
- Yamago, S.; Tokuyama, H.; Nakamura, E.; Kikuchi, K.; Kananishi, S.; Sueki, K.; Nakahara, H.; Enomoto, S.; Ambe, F. In vivo biological behavior of a water-miscible fullerene: 14C labeling, absorption, distribution, excretion and acute toxicity. Chem. Biol. 1995, 2, 385–389. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Brown, R.E. Optimization of apparatus design and behavioral measures for the assessment of visuo-spatial learning and memory of mice on the Barnes maze. Learn. Mem. 2013, 20, 85–96. [Google Scholar] [CrossRef]
- Avdeeva, N.V. Novel mGluR4 agonist Rapitalam ameliorates motor dysfunction in mice with tau-associated neurodegeneration. Res. Results Pharmacol. 2024, 6, 9–17. [Google Scholar] [CrossRef]
- Shea, T.; Ortiz, D. 17 β-Estradiol alleviates synergistic oxidative stress resulting from folate deprivation and amyloid-β treatment. J. Alzheimer’s Dis. 2003, 5, 323–327. [Google Scholar] [CrossRef]
- Frick, K.M. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm. Behav. 2015, 74, 4–18. [Google Scholar] [CrossRef]
- Distech, C.M.; Berletch, J.B. X-chromosome inactivation and escape. J. Genet. 2015, 94, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Rots, D.; Jakub, T.E.; Keung, C.; Jackson, A.; Banka, S.; Pfundt, R.; de Vries, B.B.; van Jaarsveld, R.H.; Hopman, S.M.; van Binsbergen, E.; et al. The clinical and molecular spectrum of the KDM6B-related neurodevelopmental disorder. Am. J. Hum. Genet. 2023, 110, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Wang, Z.J.; Cai, H.Y. Sex Differences in Neuropathology and Cognitive Behavior in APP/PS1/tau Triple-Transgenic Mouse Model of Alzheimer’s Disease. Neurosci. Bull. 2018, 34, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A. Studying both sexes: A guiding principle for biomedicine. FASEB J. 2016, 30, 519–524. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzubova, E.; Radchenko, A.; Pokrovskii, M.; Shcheblykina, O.; Chaprov, K.; Nesterov, A.; Avtina, T.; Pokrovskii, V.; Korokin, M. Sex-Dependent Phenotypic and Histomorphometric Biomarkers in the APPswe/PS1dE9/Blg Mouse Model of Alzheimer’s Disease. Brain Sci. 2025, 15, 1237. https://doi.org/10.3390/brainsci15111237
Kuzubova E, Radchenko A, Pokrovskii M, Shcheblykina O, Chaprov K, Nesterov A, Avtina T, Pokrovskii V, Korokin M. Sex-Dependent Phenotypic and Histomorphometric Biomarkers in the APPswe/PS1dE9/Blg Mouse Model of Alzheimer’s Disease. Brain Sciences. 2025; 15(11):1237. https://doi.org/10.3390/brainsci15111237
Chicago/Turabian StyleKuzubova, Elena, Alexandra Radchenko, Mikhail Pokrovskii, Olesya Shcheblykina, Kirill Chaprov, Arkadii Nesterov, Tatiana Avtina, Vladimir Pokrovskii, and Mikhail Korokin. 2025. "Sex-Dependent Phenotypic and Histomorphometric Biomarkers in the APPswe/PS1dE9/Blg Mouse Model of Alzheimer’s Disease" Brain Sciences 15, no. 11: 1237. https://doi.org/10.3390/brainsci15111237
APA StyleKuzubova, E., Radchenko, A., Pokrovskii, M., Shcheblykina, O., Chaprov, K., Nesterov, A., Avtina, T., Pokrovskii, V., & Korokin, M. (2025). Sex-Dependent Phenotypic and Histomorphometric Biomarkers in the APPswe/PS1dE9/Blg Mouse Model of Alzheimer’s Disease. Brain Sciences, 15(11), 1237. https://doi.org/10.3390/brainsci15111237





