Neural Mechanisms of Role Reversal in Improvisational Music Psychodrama: An fNIRS Hyperscanning Study
Abstract
1. Introduction
2. Methods
2.1. Participants
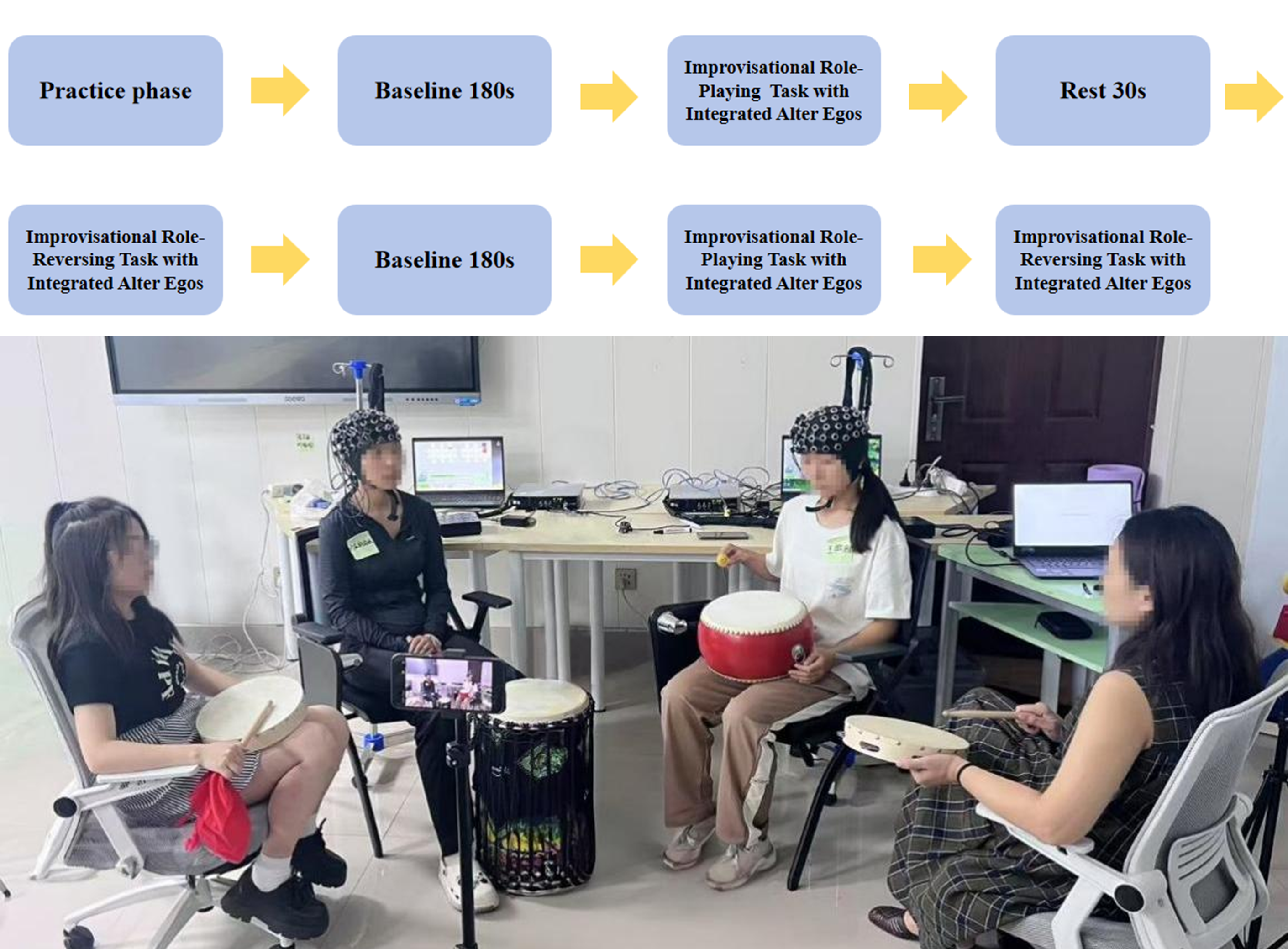
2.2. Experimental Tasks and Procedure
- (1)
- Pre-experiment Preparation: After determining role-playing themes and roles, participants completed open-ended prompts on paper templates to familiarize themselves with assigned themes and roles, followed by training in music psychodrama intervention.
- (2)
- Baseline (3 min): Participants sat quietly with eyes closed, performing neutral breathing exercises.
- (3)
- Task Phase (6 min, comprising two 3-min blocks with a 30-s rest):
- (4)
- Resting Phase (3 min): Participants sat quietly with eyes open, refraining from active task-related thinking.
- (5)
- Pre- and post-experiment, participants rated negative emotions on a 5-point Likert scale (1 = completely inconsistent, 5 = completely consistent). The total duration of the experimental protocol (including baseline, task, and rest phases) was approximately 18.5 min, with 12 min of valid interactive recording.
2.3. fNIRS Data Acquisition
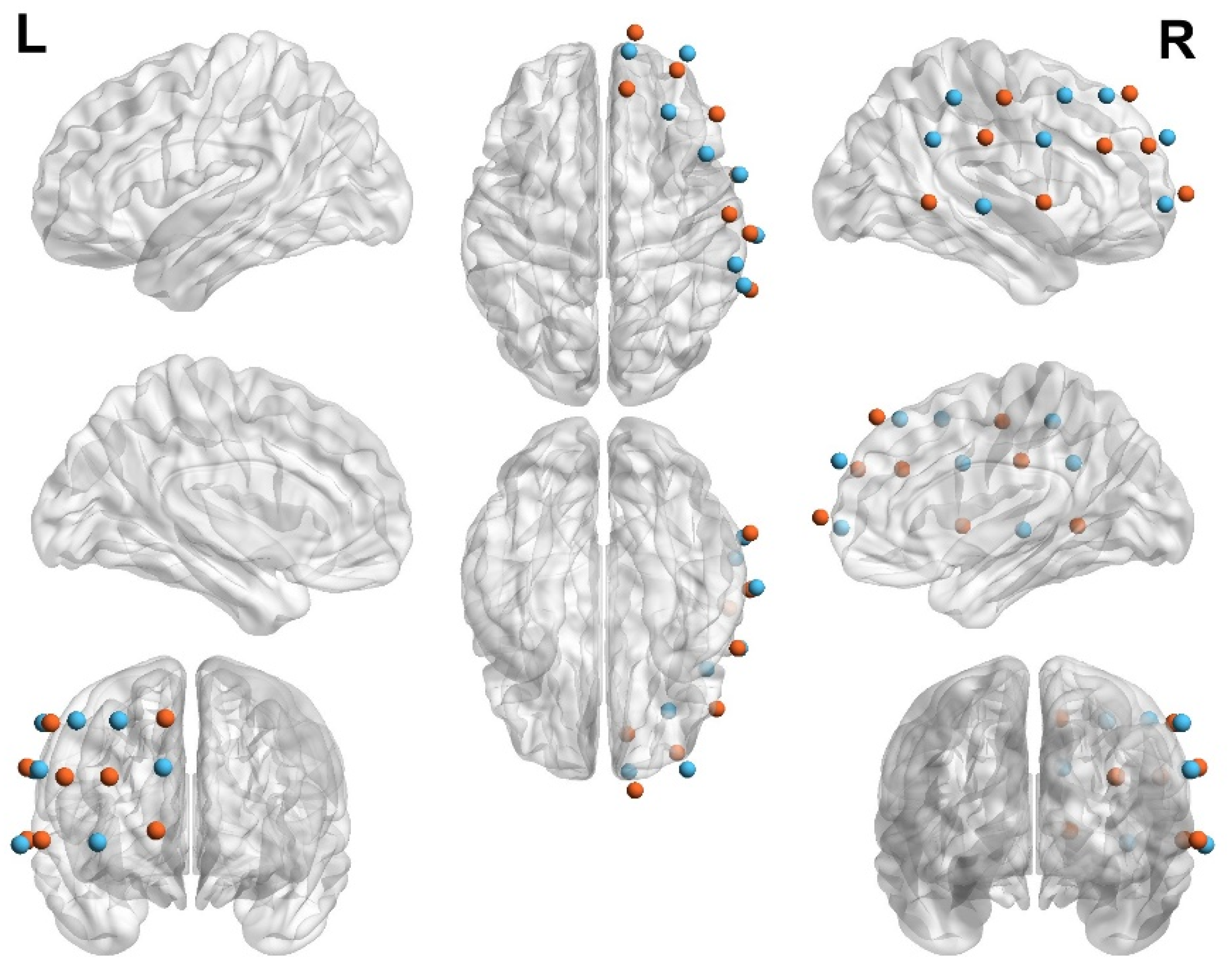
2.4. Data Analysis
2.4.1. Behavioral Data Analysis
2.4.2. fNIRS Data Analysis
3. Results
3.1. Behavioral Results
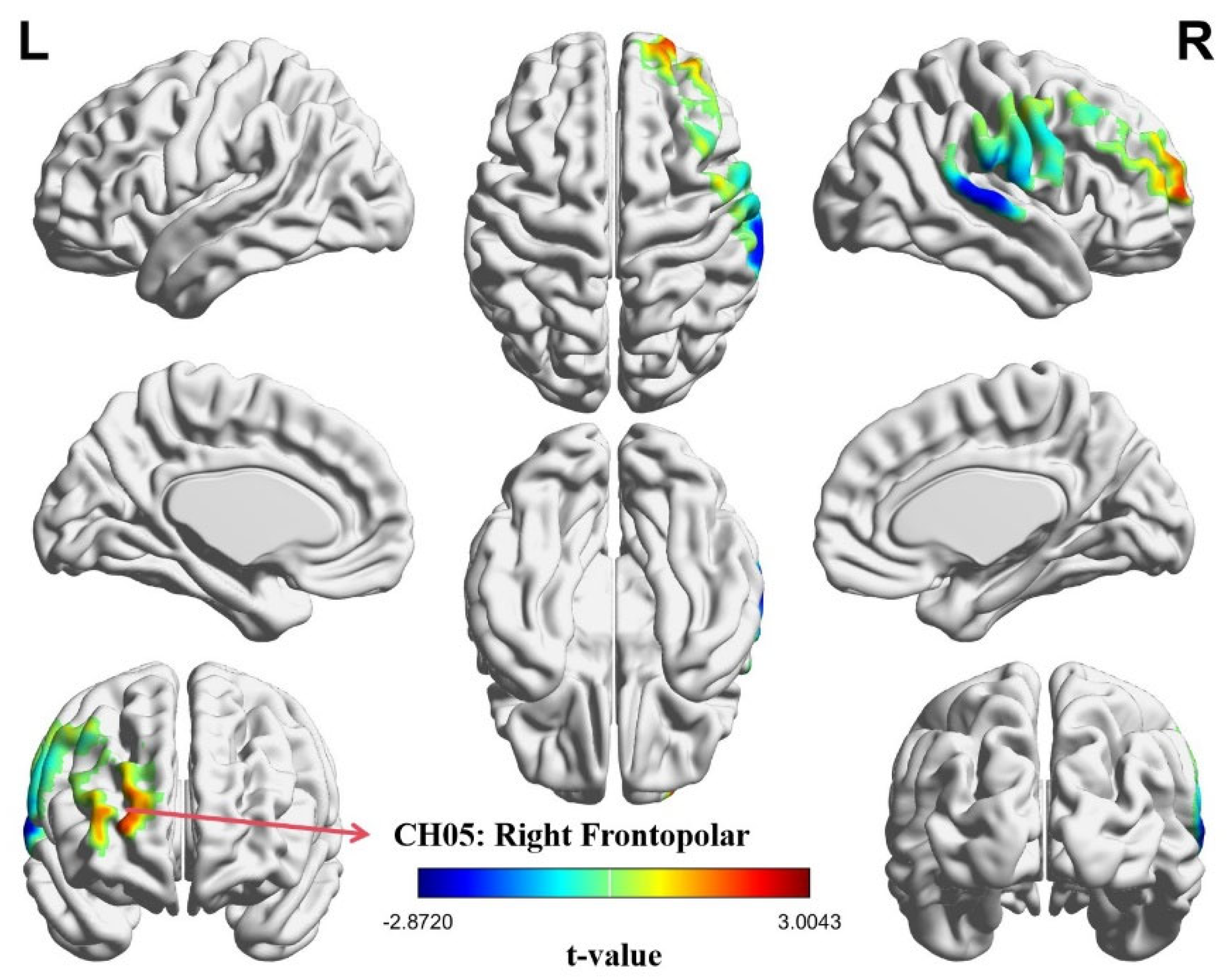
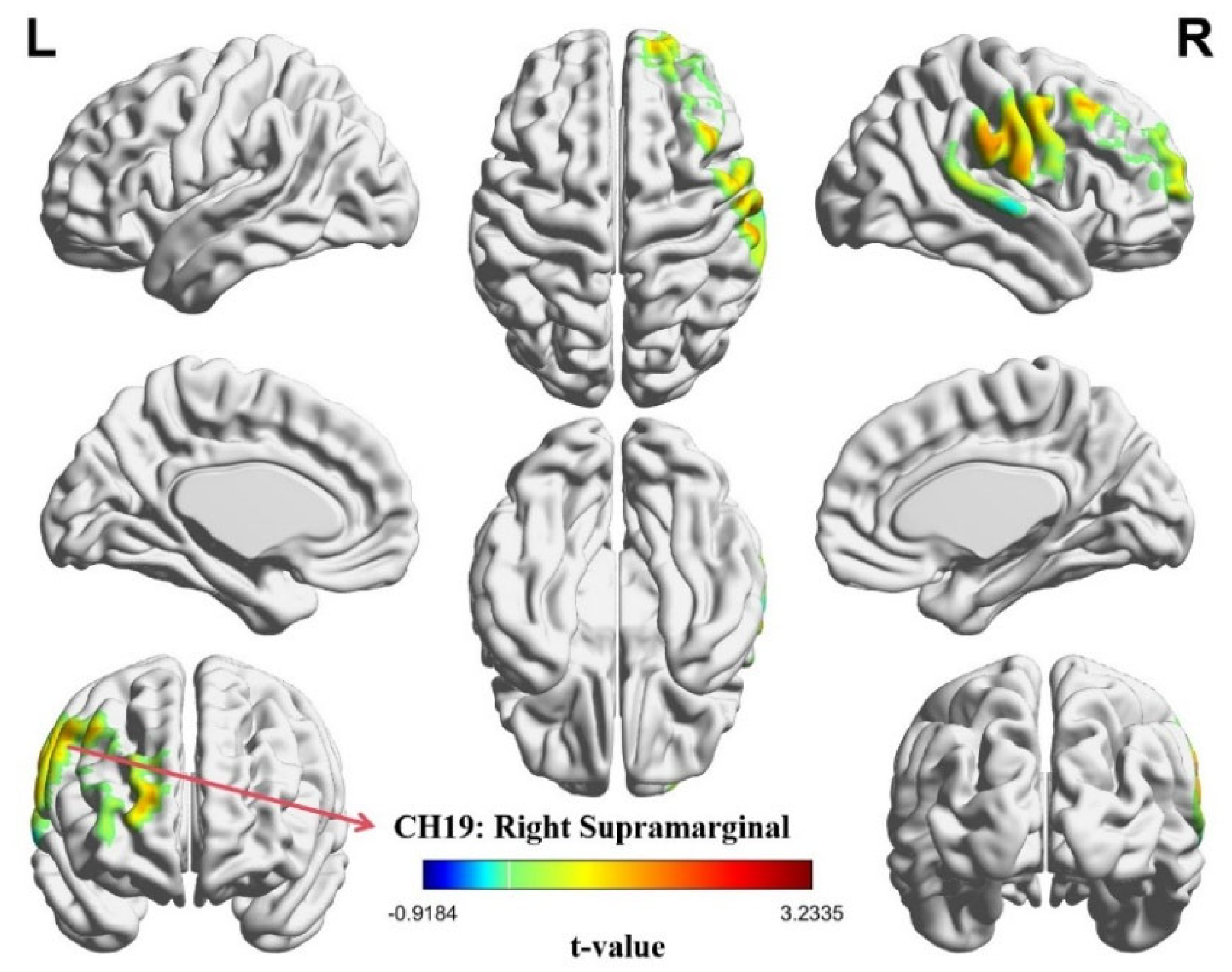
3.2. Intra-Brain Activation Results
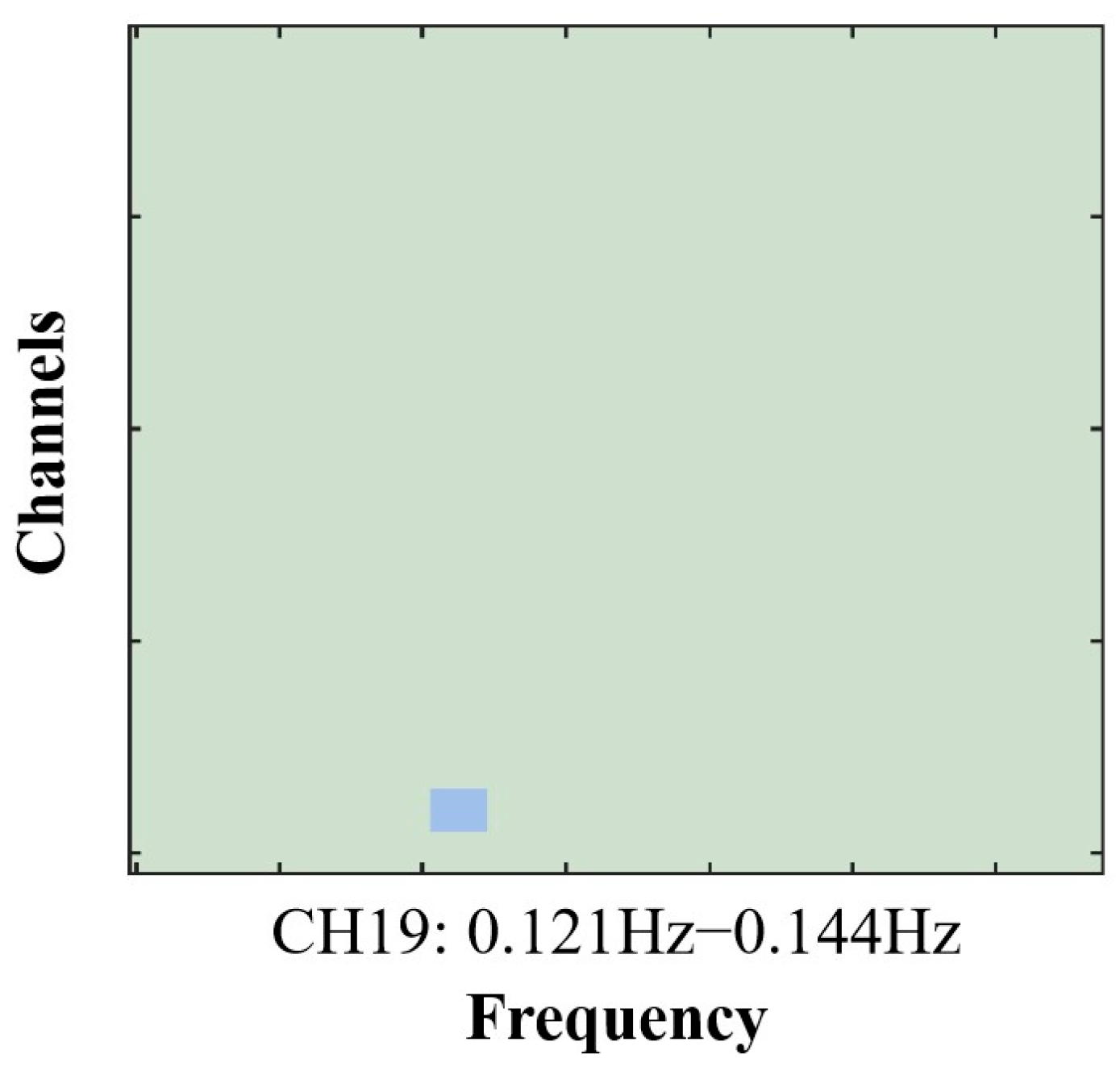
3.3. Inter-Brain Synchrony Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Channels | MNI x | MNI y | MNI z | Brodmann’s Area | P |
|---|---|---|---|---|---|
| CH01 | 12 | 59 | 40 | 9—Dorsolateral prefrontal cortex | 0.82731 |
| CH02 | 13 | 73 | 15 | 10—Frontopolar area | 1 |
| CH03 | 42 | 29 | 50 | 8—Includes Frontal eye fields | 0.93886 |
| CH04 | 20 | 46 | 50 | 8—Includes Frontal eye fields | 0.91266 |
| CH05 | 24 | 65 | 26 | 10—Frontopolar area | 0.92015 |
| CH06 | 28 | 69 | 10 | 10—Frontopolar area | 1 |
| CH07 | 52 | 28 | 39 | 9—Dorsolateral prefrontal cortex | 0.67993 |
| CH08 | 34 | 49 | 39 | 9—Dorsolateral prefrontal cortex | 0.891 |
| CH09 | 37 | 65 | 10 | 10—Frontopolar area | 1 |
| CH10 | 45 | 49 | 15 | 10—Frontopolar area | 0.53648 |
| CH11 | 64 | −64 | 6 | 6—Pre-Motor and Supplementary Motor Cortex | 0.97193 |
| CH12 | 66 | 8 | 19 | 6—Pre-Motor and Supplementary Motor Cortex | 0.45307 |
| CH13 | 70 | −35 | 19 | 40—Supramarginal gyrus part of Wernicke’s area | 0.9125 |
| CH14 | 65 | –29 | 50 | 40—Supramarginal gyrus part of Wernicke’s area | 0.45353 |
| CH15 | 69 | –9 | 31 | 6—Pre-Motor and Supplementary Motor Cortex | 0.61489 |
| CH16 | 71 | –10 | 0 | 21—Middle Temporal gyrus | 0.55882 |
| CH17 | 69 | –49 | 13 | 22—Superior Temporal gyrus | 0.82026 |
| CH18 | 61 | –38 | 40 | 40—Supramarginal gyrus part of Wernicke’s area | 0.65116 |
| CH19 | 72 | –22 | 42 | 42—Primary and Auditory Association Cortex | 0.63125 |
| CH20 | 73 | –35 | 21 | 21—Middle Temporal gyrus | 0.59621 |
References
- Sang, Z.Q.; Huang, H.M.; Benko, A.; Wu, Y. The spread and development of psychodrama in mainland China. Front. Psychol. 2018, 9, 1368. [Google Scholar] [CrossRef]
- Landis, H.; Skolnik, S. Periphery to core: Scenes from a psychodrama. Soc. Work. Groups 2024, 47, 208–218. [Google Scholar] [CrossRef]
- Cruz, A.; Sales, C.M.; Alves, P.; Moita, G. The core techniques of Morenian psychodrama: A systematic review of literature. Front. Psychol. 2018, 9, 1263. [Google Scholar] [CrossRef]
- Abeditehrani, H.; Dijk, C.; Neyshabouri, M.D.; Arntz, A. Beneficial effects of role reversal in comparison to role-playing on negative cognitions about other’s judgments for social anxiety disorder. J. Behav. Ther. Exp. Psychiatry 2021, 70, 101599. [Google Scholar] [CrossRef]
- Pellicciari, A.; Rossi, F.; Iero, L.; Di Pietro, E.; Verrotti, A.; Franzoni, E. Drama therapy and eating disorders: A historical perspective and an overview of a Bolognese project for adolescents. J. Altern. Complement. Med. 2013, 19, 607–612. [Google Scholar] [CrossRef]
- Somov, P.G. A psychodrama group for substance use relapse prevention training. Arts Psychother. 2008, 35, 151–161. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, F.; Chen, D.; Zhang, X.; Shen, K.; Fan, Y.; Li, L. Intervention effect of psychodrama on depression and anxiety: A meta-analysis based on Chinese samples. Arts Psychother. 2020, 69, 101661. [Google Scholar] [CrossRef]
- Blatner, A. Psychodrama: The state of the art. Arts Psychother. 1997, 24, 23–30. [Google Scholar] [CrossRef]
- Moreno, J.J. Acting Your Inner Music: Music Therapy and Psychodrama; Barcelona Publishers LLC: New Braunfels, TX, USA, 1999. [Google Scholar]
- Moreno, J.J. Musical psychodrama: A new direction in music therapy. J. Music Ther. 1980, 17, 34–42. [Google Scholar] [CrossRef]
- Krøier, J.K.; Stige, B.; Ridder, H.M. Non-verbal interactions between music therapists and persons with dementia. A qualitative phenomenological and arts-based inquiry. Music Ther. Perspect. 2021, 39, 162–171. [Google Scholar] [CrossRef]
- Bergamin, J.A. Habitually breaking habits: Agency, awareness, and decision-making in musical improvisation. Phenomenol. Cogn. Sci. 2024; 1–29, advance online publication. [Google Scholar] [CrossRef]
- Biasutti, M.; Frezza, L. Dimensions of music improvisation. Creat. Res. J. 2009, 21, 232–242. [Google Scholar] [CrossRef]
- Barrett, K.C.; Barrett, F.S.; Jiradejvong, P.; Rankin, S.K.; Landau, A.T.; Limb, C.J. Classical creativity: A functional magnetic resonance imaging (fMRI) investigation of pianist and improviser Gabriela Montero. NeuroImage 2020, 209, 116496. [Google Scholar] [CrossRef]
- Barrett, K.C.; Jiradejvong, P.; Jacobs, L.; Limb, C.J. Children engage neural reward structures for creative musical improvisation. Sci. Rep. 2025, 15, 11346. [Google Scholar] [CrossRef]
- Diaz Abrahan, V.; Bossio, M.; Benítez, M.; Justel, N. Musical strategies to improve children’s memory in an educational context. Psychol. Music 2022, 50, 727–741. [Google Scholar] [CrossRef]
- Müller, V.; Lindenberger, U. Dynamic orchestration of brains and instruments during free guitar improvisation. Front. Integr. Neurosci. 2019, 13, 50. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Ni, J.; De Dreu, C.K.; Ma, Y. Leader–follower behavioural coordination and neural synchronization during intergroup conflict. Nat. Hum. Behav. 2023, 7, 2169–2181. [Google Scholar] [CrossRef]
- Cui, X.; Bryant, D.M.; Reiss, A.L. NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage 2012, 59, 2430–2437. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, H.; Huang, B.; Huang, Y.; Sun, H.; Ru, X.; Zhang, M.; Chen, W. Interbrain neural mechanism and influencing factors underlying different cooperative behaviors: A hyperscanning study. Brain Struct. Funct. 2023, 229, 75–95. [Google Scholar] [CrossRef]
- Montague, P.R.; Berns, G.S.; Cohen, J.D.; McClure, S.M.; Pagnoni, G.; Dhamala, M.; Fisher, R.E. Hyperscanning: Simultaneous fMRI during linked social interactions. Neuroimage 2002, 16, 1159–1164. [Google Scholar] [CrossRef]
- Yu, X.; Liu, T.; He, L.; Li, Y. Micro-foundations of strategic decision-making in family business organisations: A cognitive neuroscience perspective. Long Range Plan. 2023, 56, 102198. [Google Scholar] [CrossRef]
- Hakim, U.; De Felice, S.; Pinti, P.; Zhang, X.; Noah, J.; Ono, Y.; Burgess, P.W.; Hamilton, A.; Hirsch, J.; Tachtsidis, I. Quantification of inter-brain coupling: A review of current methods used in haemodynamic and electrophysiological hyperscanning studies. NeuroImage 2023, 280, 120354. [Google Scholar] [CrossRef]
- Clark, A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 2013, 36, 181–204. [Google Scholar] [CrossRef]
- Kube, T.; Schwarting, R.; Rozenkrantz, L.; Glombiewski, J.A.; Rief, W. Distorted cognitive processes in major depression: A predictive processing perspective. Biol. Psychiatry 2020, 87, 388–398. [Google Scholar] [CrossRef]
- Lim, M.; Carollo, A.; Bizzego, A.; Chen, S.A.; Esposito, G. Decreased activation in left prefrontal cortex during role-play: An fNIRS study of the psychodrama sociocognitive model. Arts Psychother. 2024, 87, 102098. [Google Scholar] [CrossRef]
- Fávero, M.; Sousa, R.; Budal-Oliveira, L.; Sousa-Gomes, V. Psychodrama: Comprehensive review of the effectiveness of psychodrama in sexual abuse trauma. Eur. Psychol. 2024, 29, 17. [Google Scholar] [CrossRef]
- Kipper, D.; Ritchie, T. The effectiveness of psychodramatic techniques: A meta analysis. Group Dyn. Theory Res. Pract. 2003, 7, 13–25. [Google Scholar] [CrossRef]
- Yaniv, D. Dynamics of creativity and empathy in role reversal: Contributions from neuroscience. Rev. Gen. Psychol. 2012, 16, 70–77. [Google Scholar] [CrossRef]
- Wu, M.; Cameirao, J.; Brown, S. Role reversal enhances an understanding of the other, but not of the self. Arts Psychother. 2025, 93, 102288. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, J.; Luo, R.; Hao, N. Brain to brain musical interaction: A systematic review of neural synchrony in musical activities. Neurosci. Biobehav. Rev. 2024, 164, 105812.53. [Google Scholar] [CrossRef]
- Moreno, J.J. Musical psychodrama in Naples. Arts Psychother. 1991, 18, 331–339. [Google Scholar] [CrossRef]
- Moreno, J.J. Musical psychodrama in Paris. Music Ther. Perspect. 1984, 1, 2–6. [Google Scholar] [CrossRef]
- Feng, K.; Shen, C.Y.; Ma, X.Y.; Chen, G.F.; Zhang, M.L.; Xu, B.; Liu, X.-M.; Sun, J.-J.; Zhang, X.-Q.; Liu, P.-Z.; et al. Effects of music therapy on major depressive disorder: A study of prefrontal hemodynamic functions using fNIRS. Psychiatry Res. 2019, 275, 86–93. [Google Scholar] [CrossRef]
- Wong, P.C.; Chan, A.H.; Roy, A.; Margulis, E.H. The bimusical brain is not two monomusical brains in one: Evidence from musical affective processing. J. Cogn. Neurosci. 2011, 23, 4082–4093. [Google Scholar] [CrossRef]
- Abeditehrani, H.; Dijk, C.; Toghchi, M.S.; Arntz, A. Integrating cognitive behavioral group therapy and psychodrama for social anxiety disorder: An intervention description and an uncontrolled pilot trial. Clin. Psychol. Eur. 2020, 2, e2693. [Google Scholar] [CrossRef]
- MacDonald, R.A.; Wilson, G.B. Musical improvisation and health: A review. Psychol. Well-Being 2014, 4, 20. [Google Scholar] [CrossRef]
- Raglio, A.; Oasi, O.; Gianotti, M.; Rossi, A.; Goulene, K.; Stramba-Badiale, M. Improvement of spontaneous language in stroke patients with chronic aphasia treated with music therapy: A randomized controlled trial. Int. J. Neurosci. 2016, 126, 235–242. [Google Scholar] [CrossRef]
- Kipper, D.A. The cognitive double: Integrating cognitive and action techniques. J. Group Psychother. Psychodrama Sociom. 2002, 55, 93–106. [Google Scholar] [CrossRef]
- Ames, D.L.; Jenkins, A.C.; Banaji, M.R.; Mitchell, J.P. Taking another person’s perspective increases self-referential neural processing. Psychol. Sci. 2008, 19, 642–644. [Google Scholar] [CrossRef]
- Majdandžić, J.; Amashaufer, S.; Hummer, A.; Windischberger, C.; Lamm, C. The selfless mind: How prefrontal involvement in mentalizing with similar and dissimilar others shapes empathy and prosocial behavior. Cognition 2016, 157, 24–38. [Google Scholar] [CrossRef]
- Yaniv, D. Revisiting Morenian psychodramatic encounter in light of contemporary neuroscience: Relationship between empathy and creativity. Arts Psychother. 2011, 38, 52–58. [Google Scholar] [CrossRef]
- Penagos-Corzo, J.C.; Cosio van-Hasselt, M.; Escobar, D.; Vázquez-Roque, R.A.; Flores, G. Mirror neurons and empathy-related regions in psychopathy: Systematic review, meta-analysis, and a working model. Soc. Neurosci. 2022, 17, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Beaty, R.E. The neuroscience of musical improvisation. Neurosci. Biobehav. Rev. 2015, 51, 108–117. [Google Scholar] [CrossRef]
- Sasaki, M.; Iversen, J.; Callan, D.E. Music improvisation is characterized by increase EEG spectral power in prefrontal and perceptual motor cortical sources and can be reliably classified from non-improvisatory performance. Front. Hum. Neurosci. 2019, 13, 435. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, T.; Yang, Y.; Hu, Y. Experience-dependent counselor-client brain synchronization during psychological counseling. eNeuro 2020, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Morimoto, S.; Hoshino, E.; Suzuki, K.; Minagawa, Y. Two-in-one system and behavior-specific brain synchrony during goal-free cooperative creation: An analytical approach combining automated behavioral classification and the event-related generalized linear model. Neurophotonics 2023, 10, 013511. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Ma, H. (Eds.) Manual of Mental Health Rating Scales, Revised ed.; Chinese Mental Health Journal Press: Beijing, China, 1999. [Google Scholar]
- Leary, M.R. Social anxiousness: The construct and its measurement. J. Pers. Assess. 1983, 47, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Zimeo Morais, G.A.; Balardin, J.B.; Sato, J.R. fNIRS Optodes’ Location Decider (fOLD): A toolbox for probe arrangement guided by brain regions-of-interest. Sci. Rep. 2018, 8, 3341. [Google Scholar] [CrossRef]
- De Witte, S.; Klooster, D.; Dedoncker, J.; Duprat, R.; Remue, J.; Baeken, C. Left prefrontal neuronavigated electrode localization in tDCS: 10–20 EEG system versus MRI-guided neuronavigation. Psychiat. Res. Neuroim. 2018, 274, 1–6. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Y.; Yuan, J. Speech prosodies of different emotional categories activate different brain regions in adult cortex: An fNIRS study. Sci. Rep. 2018, 8, 218. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, Z.; Zhao, C.; Duan, L.; Gong, Y.; Li, Z.; Zhu, C. NIRS-KIT: A MATLAB toolbox for both resting-state and task fNIRS data analysis. Neurophotonics 2021, 8, 010802. [Google Scholar] [CrossRef]
- Nguyen, T.; Schleihauf, H.; Kayhan, E.; Matthes, D.; Vrtička, P.; Hoehl, S. The effects of interaction quality on neural synchrony during mother-child problem solving. Cortex 2020, 124, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yu, T.; Hao, N. Creating while taking turns, the choice to unlocking group creative potential. NeuroImage 2020, 219, 117025. [Google Scholar] [CrossRef]
- Cui, X.; Bray, S.; Reiss, A.L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage 2010, 49, 3039–3046. [Google Scholar] [CrossRef]
- Emberson, L.L.; Crosswhite, S.L.; Goodwin, J.R.; Berger, A.J.; Aslin, R.N. Isolating the effects of surface vasculature in infant neuroimaging using short-distance optical channels: A combination of local and global effects. Neurophotonics 2016, 3, 031406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yin, Z.; Zhang, X.; Zhang, H.; Bao, M.; Xuan, B. Neural mechanisms distinguishing two types of cooperative problem-solving approaches: An fNIRS hyperscanning study. NeuroImage 2024, 291, 120587. [Google Scholar] [CrossRef]
- Wang, H.Y.; You, H.L.; Song, C.L.; Zhou, L.; Wang, S.Y.; Li, X.L.; Liang, Z.-H.; Zhang, B.-W. Shared and distinct prefrontal cortex alterations of implicit emotion regulation in depression and anxiety: An fNIRS investigation. J. Affect. Disord. 2024, 354, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlin. Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Yin, Z.; Xuan, B.; Liu, C.; Yi, J.; Zheng, X.; Zhang, M. The influence of task and interpersonal interdependence on cooperative behavior and its neural mechanisms. npj Sci. Learn. 2025, 10, 9. [Google Scholar] [CrossRef]
- Nozawa, T.; Sasaki, Y.; Sakaki, K.; Yokoyama, R.; Kawashima, R. Interpersonal frontopolar neural synchronization in group communication: An exploration toward fNIRS hyperscanning of natural interactions. Neuroimage 2016, 133, 484–497. [Google Scholar] [CrossRef]
- Fonzo, G.A.; Goodkind, M.S.; Oathes, D.J.; Zaiko, Y.V.; Harvey, M.; Peng, K.K.; Weiss, M.E.; Thompson, A.L.; Zack, S.E.; Mills-Finnerty, C.E.; et al. Selective effects of psychotherapy on frontopolar cortical function in PTSD. Am. J. Psychiatry 2017, 174, 1175–1184. [Google Scholar] [CrossRef]
- Zhang, Z.; Olszewska-Guizzo, A.; Husain, S.F.; Bose, J.; Choi, J.; Tan, W.; Wang, J.; Tran, B.X.; Wang, B.; Jin, Y.; et al. Brief relaxation practice induces significantly more prefrontal cortex activation during arithmetic tasks comparing to viewing greenery images as revealed by functional near-infrared spectroscopy (fNIRS). Int. J. Environ. Res. Public Health 2020, 17, 8366. [Google Scholar] [CrossRef]
- Wolf, R.C.; Walter, H.; Vasic, N. Increasing contextual demand modulates anterior and lateral prefrontal brain regions associated with proactive interference. Int. J. Neurosci. 2010, 120, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, O.; Miura, N.; Watanabe, J.; Takemoto, A.; Uchida, S.; Sugiura, M.; Horie, K.; Sato, S.; Kawashima, R.; Nakamura, K. Right frontopolar cortex activity correlates with reliability of retrospective rating of confidence in short-term recognition memory performance. Neurosci. Res. 2010, 68, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Macuga, K.L.; Frey, S.H. Selective responses in right inferior frontal and supramarginal gyri differentiate between observed movements of oneself vs. another. Neuropsychologia 2011, 49, 1202–1207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, Z.; Xuan, B.; Zhang, M. How role reversal and interpersonal closeness shape verbal communication cooperation: An fNIRS hyperscanning study. Brain Struct. Funct. 2025, 230, 1–16. [Google Scholar] [CrossRef]
- Allen, R. Free improvisation and performance anxiety among piano students. Psychol. Music 2013, 41, 75–88. [Google Scholar] [CrossRef]
- Fachner, J.; Gold, C.; Erkkilã, J. Music therapy modulates fronto-temporal activity in rest-EEG in depressed clients. Brain Topogr. 2013, 26, 338–354. [Google Scholar] [CrossRef]
- Vidanagamage, S.D.; Bhaumik, A.O.; Irugalbandara, A.I. Role reversal in psychodrama: Enhancing empathy and emotional understanding among institutionalized children in Sri Lanka. Int. J. Res. Innov. Soc. Sci. 2024, 8, 1550–1561. [Google Scholar] [CrossRef]
- Usluoglu, F. The effects of psychodrama on relationship between the self and others: A case study. Curr. Psychol. 2023, 42, 30863–30877. [Google Scholar] [CrossRef]
- Toeman, Z. Clinical psychodrama: Auxiliary ego double and mirror techniques. Sociometry 1946, 9, 178–183. [Google Scholar] [CrossRef]
- Belden, A.; Zeng, T.; Przysinda, E.; Anteraper, S.A.; Whitfield-Gabrieli, S.; Loui, P. Improvising at rest: Differentiating jazz and classical music training with resting state functional connectivity. Neuroimage 2020, 207, 116384. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Rüther, M.; Markowitsch, H.J.; Fink, G.R.; Piefke, M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: A functional magnetic resonance imaging approach to empathy. J. Cogn. Neurosci. 2007, 19, 1354–1372. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Peng, K.; Zhang, Y.; Yao, Y.; Zhang, Z.; Zhao, F.; Zheng, M. Neural Mechanisms of Role Reversal in Improvisational Music Psychodrama: An fNIRS Hyperscanning Study. Brain Sci. 2025, 15, 1235. https://doi.org/10.3390/brainsci15111235
Wang Y, Peng K, Zhang Y, Yao Y, Zhang Z, Zhao F, Zheng M. Neural Mechanisms of Role Reversal in Improvisational Music Psychodrama: An fNIRS Hyperscanning Study. Brain Sciences. 2025; 15(11):1235. https://doi.org/10.3390/brainsci15111235
Chicago/Turabian StyleWang, Ying, Kangzhou Peng, Yueqing Zhang, Yuan Yao, Zhen Zhang, Fupei Zhao, and Maoping Zheng. 2025. "Neural Mechanisms of Role Reversal in Improvisational Music Psychodrama: An fNIRS Hyperscanning Study" Brain Sciences 15, no. 11: 1235. https://doi.org/10.3390/brainsci15111235
APA StyleWang, Y., Peng, K., Zhang, Y., Yao, Y., Zhang, Z., Zhao, F., & Zheng, M. (2025). Neural Mechanisms of Role Reversal in Improvisational Music Psychodrama: An fNIRS Hyperscanning Study. Brain Sciences, 15(11), 1235. https://doi.org/10.3390/brainsci15111235






