MAM-Mediated Mitochondrial Ca2+ Overload and Endoplasmic Reticulum Stress Aggravates Synaptic Plasticity Impairment in Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Drug Administration
2.2. Behavioral Tests
2.3. Golgi Staining and Spine Analysis
2.4. Nissl Staining
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Cell Culture and Treatments
2.7. Transmission Electron Microscopy (TEM)
2.8. Western Blot
2.9. Fluorescent Colocalization Analysis
2.10. Flow Cytometry
2.11. Statistical Analysis
3. Results
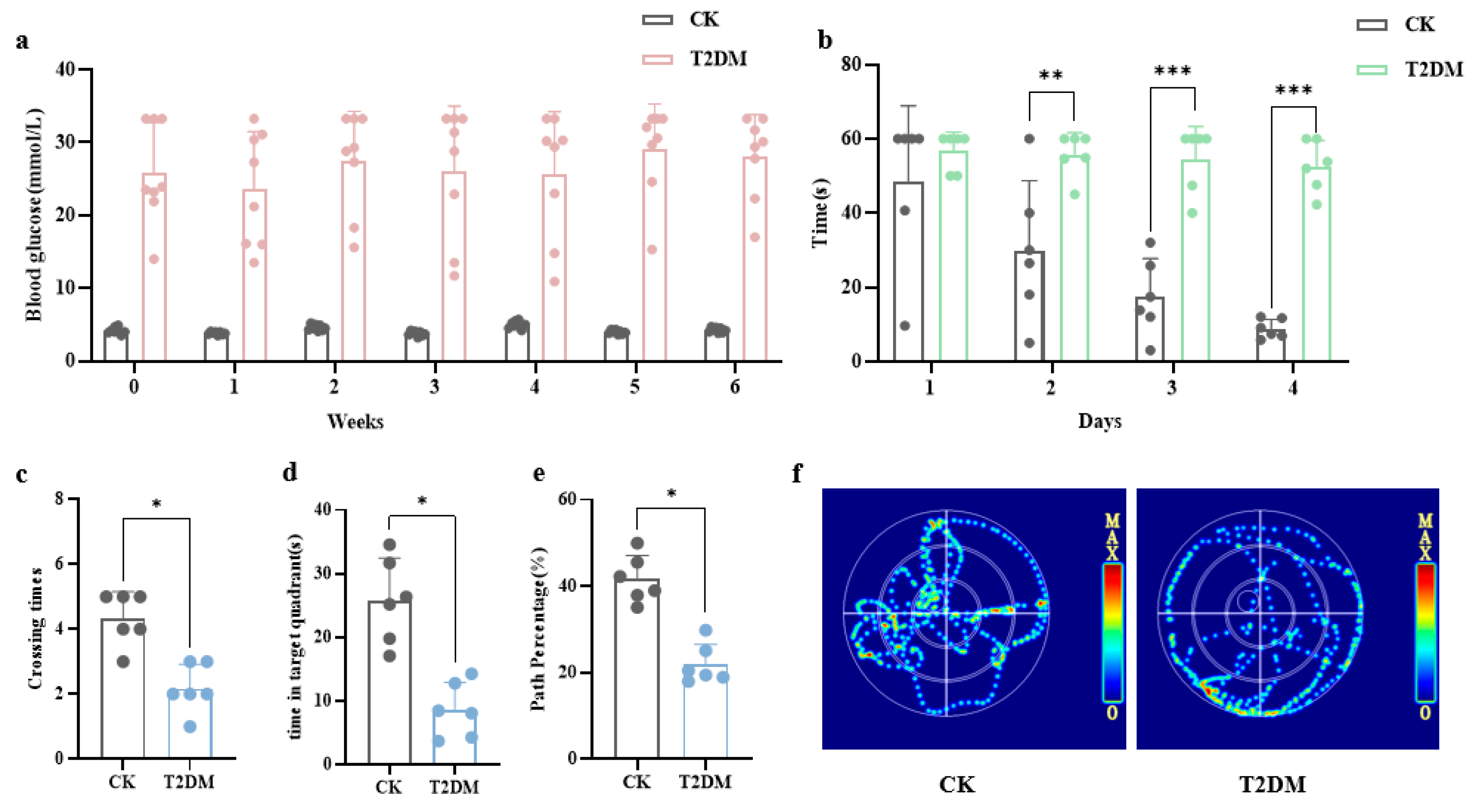
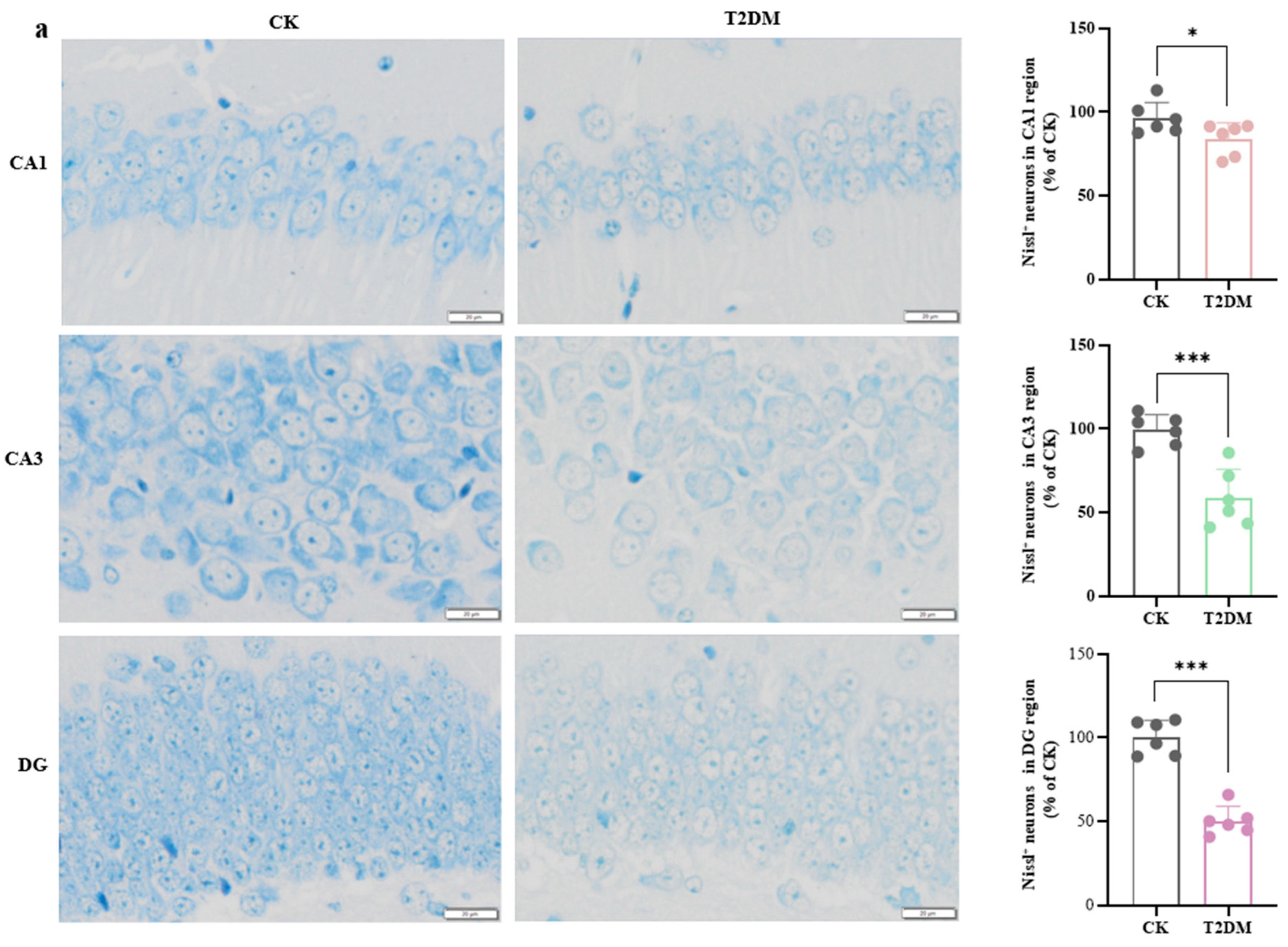
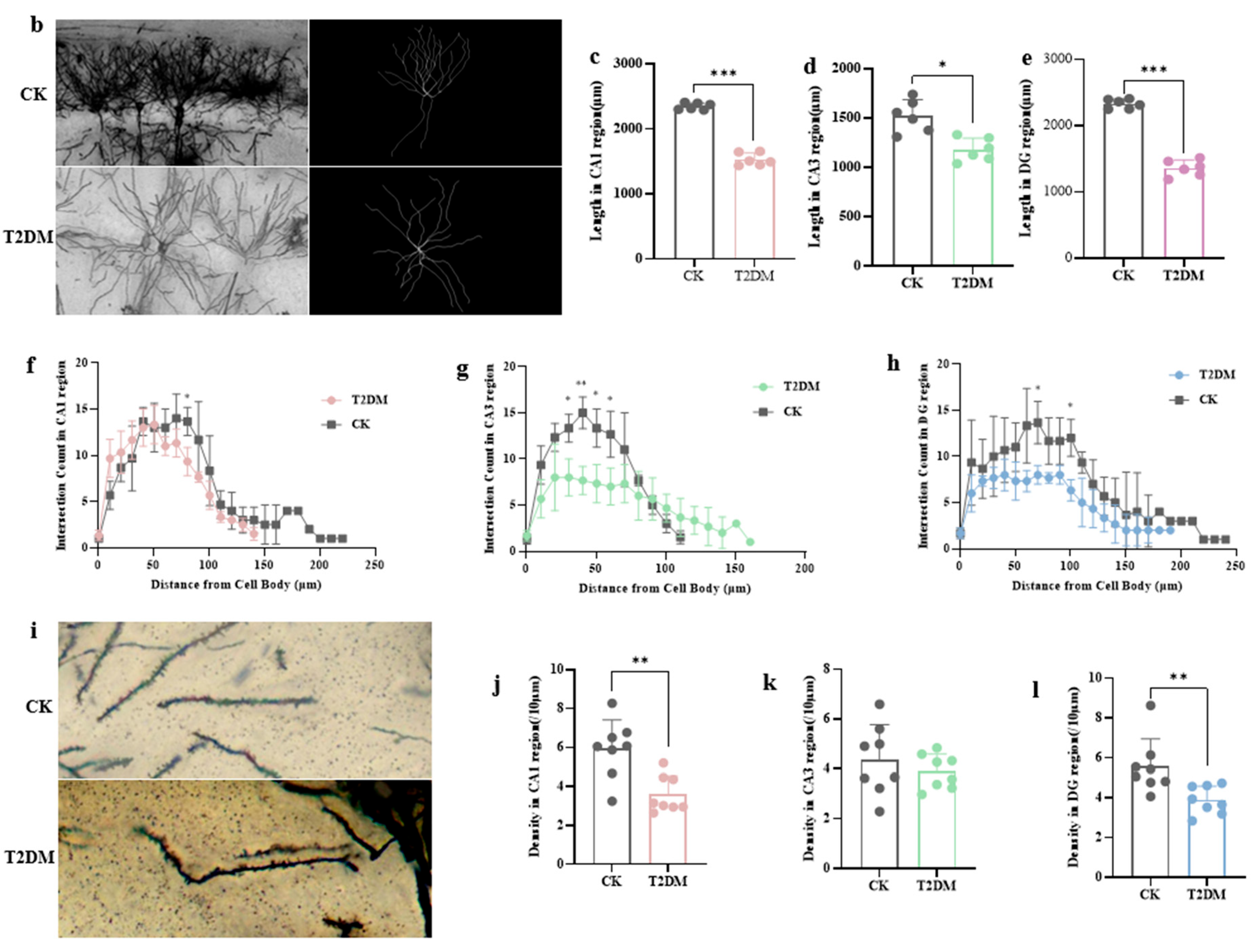
3.1. Hyperglycemia-Induced Neuronal and Synaptic Damage Contribute to Cognitive Dysfunction in Diabetic Mice and Hippocampal Neurons
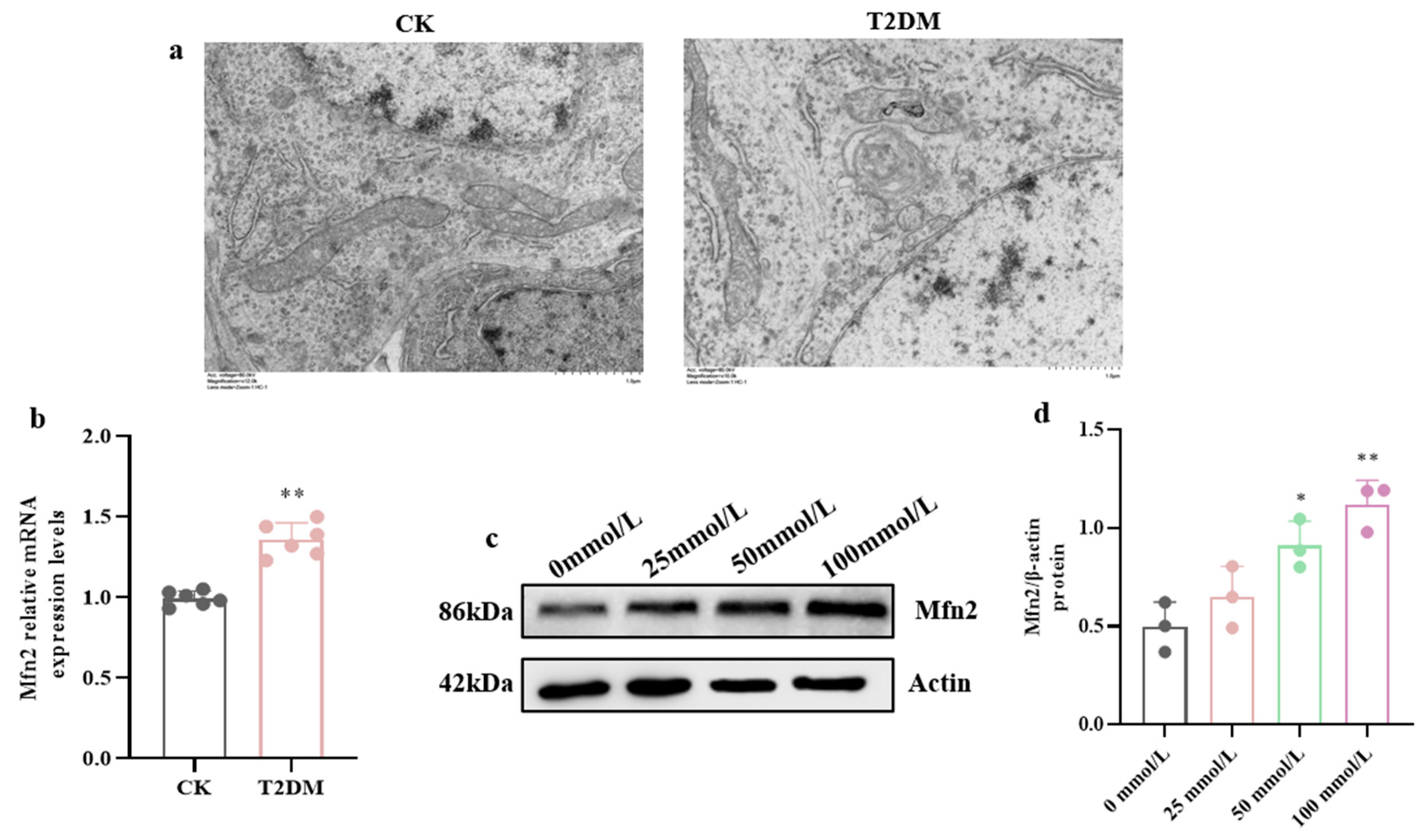
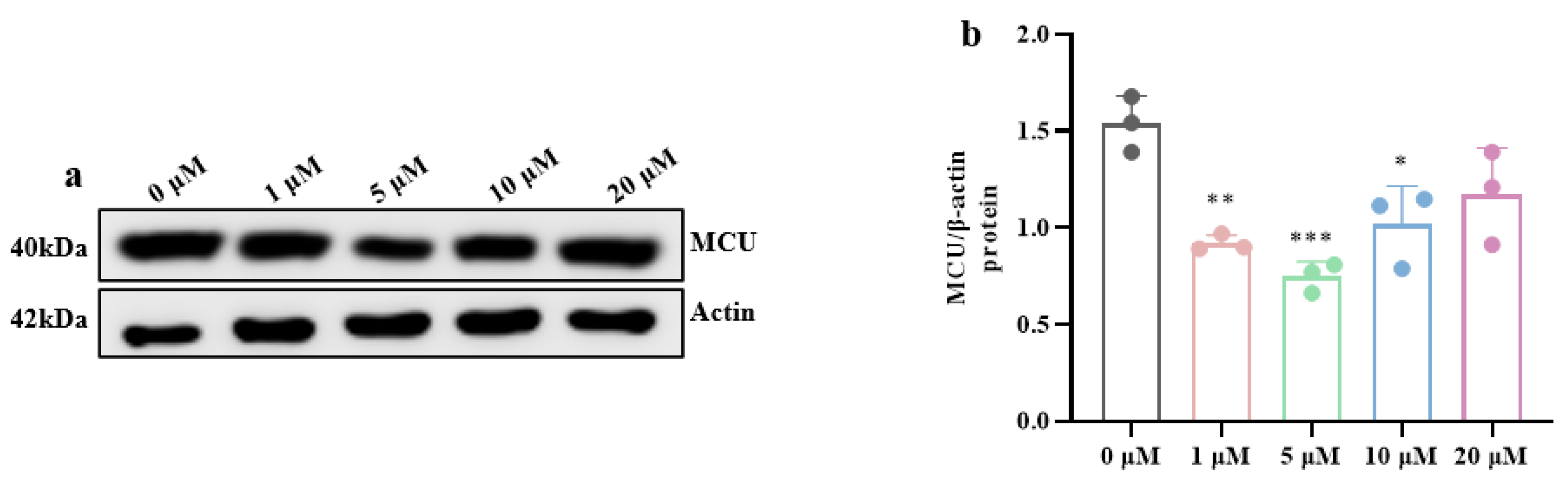
3.2. High-Glucose-Induced MAM Dysfunction, Mitochondrial Ca2+ Overload, and ER Stress in Hippocampal Cells
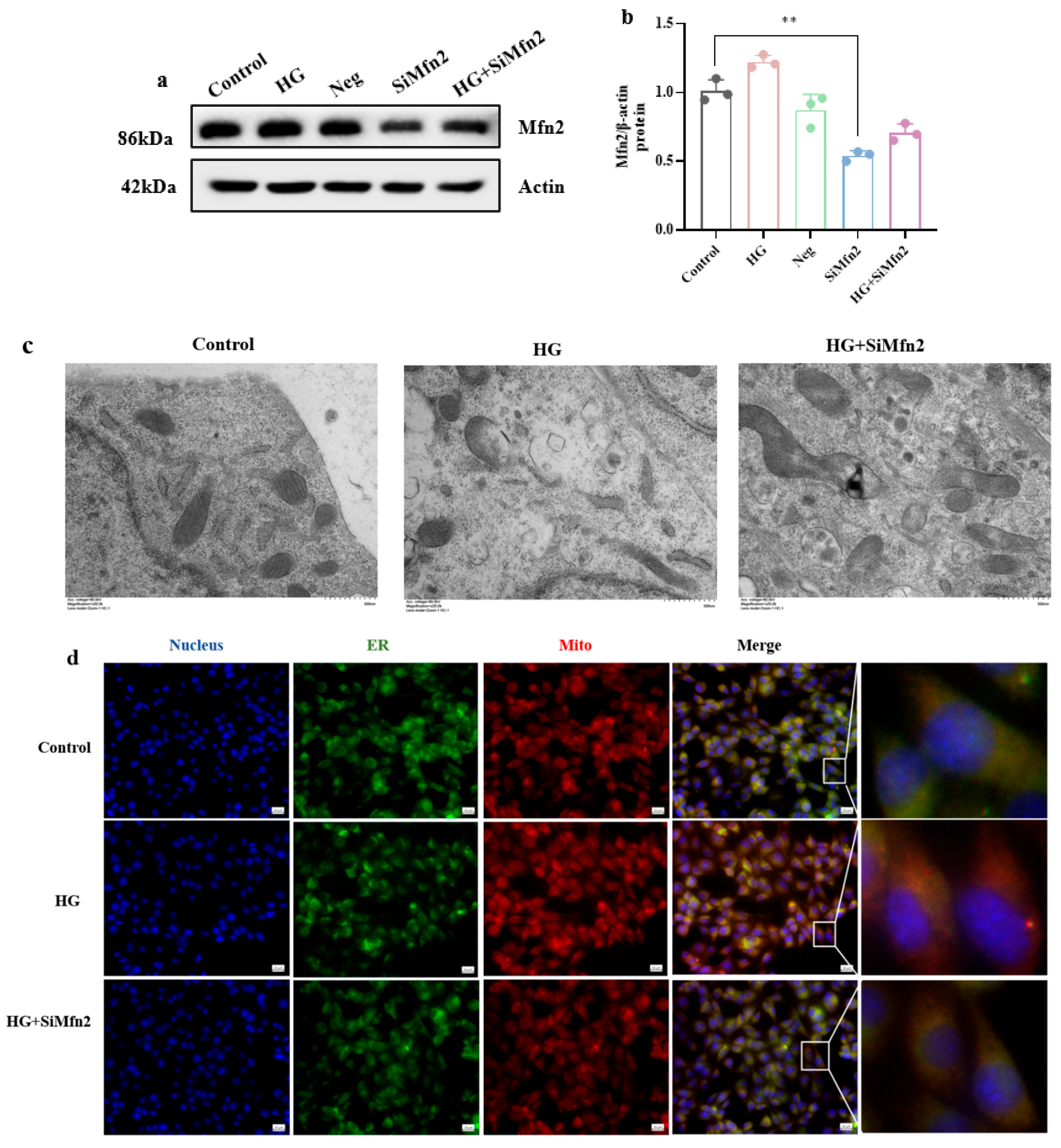
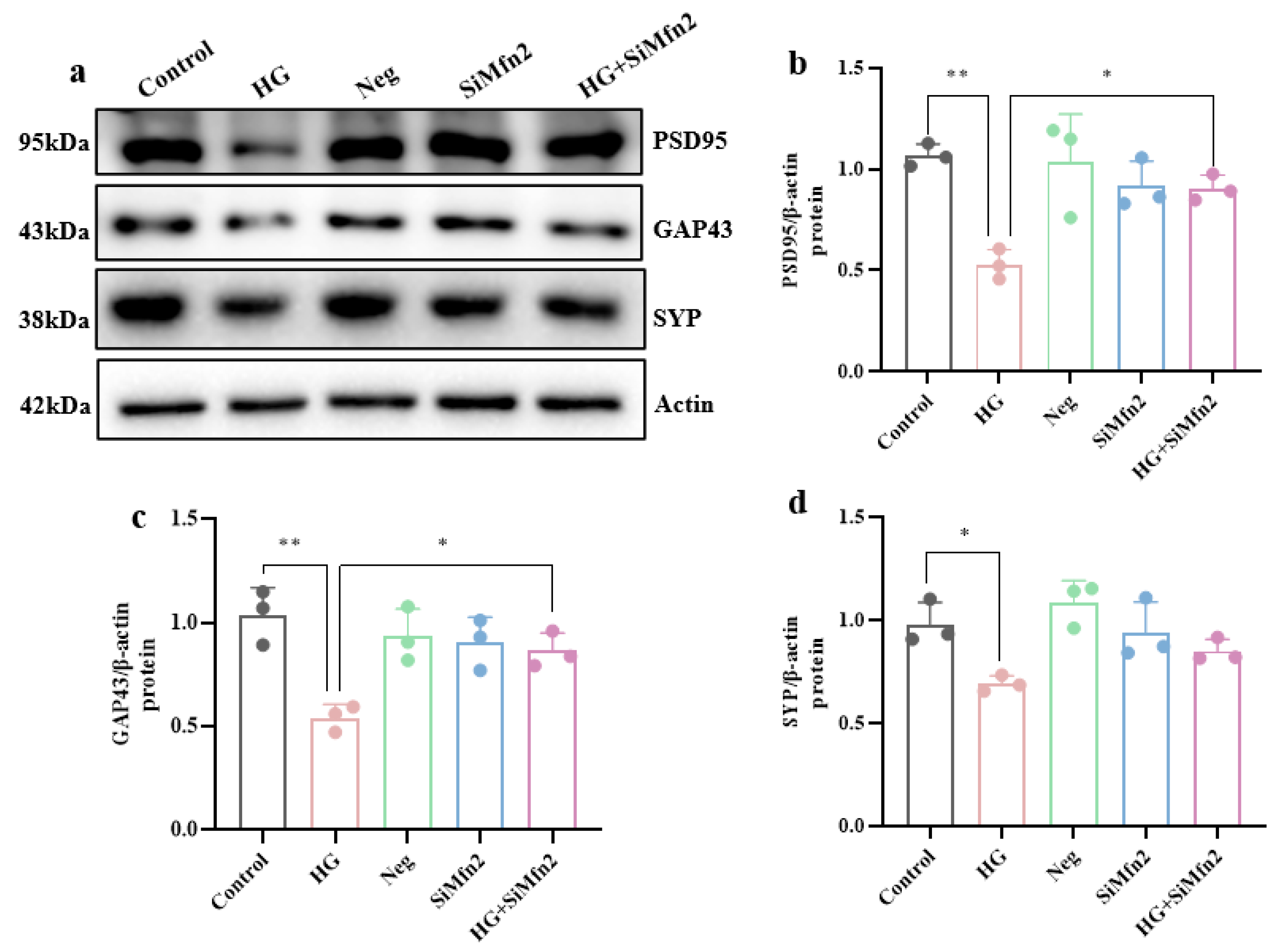
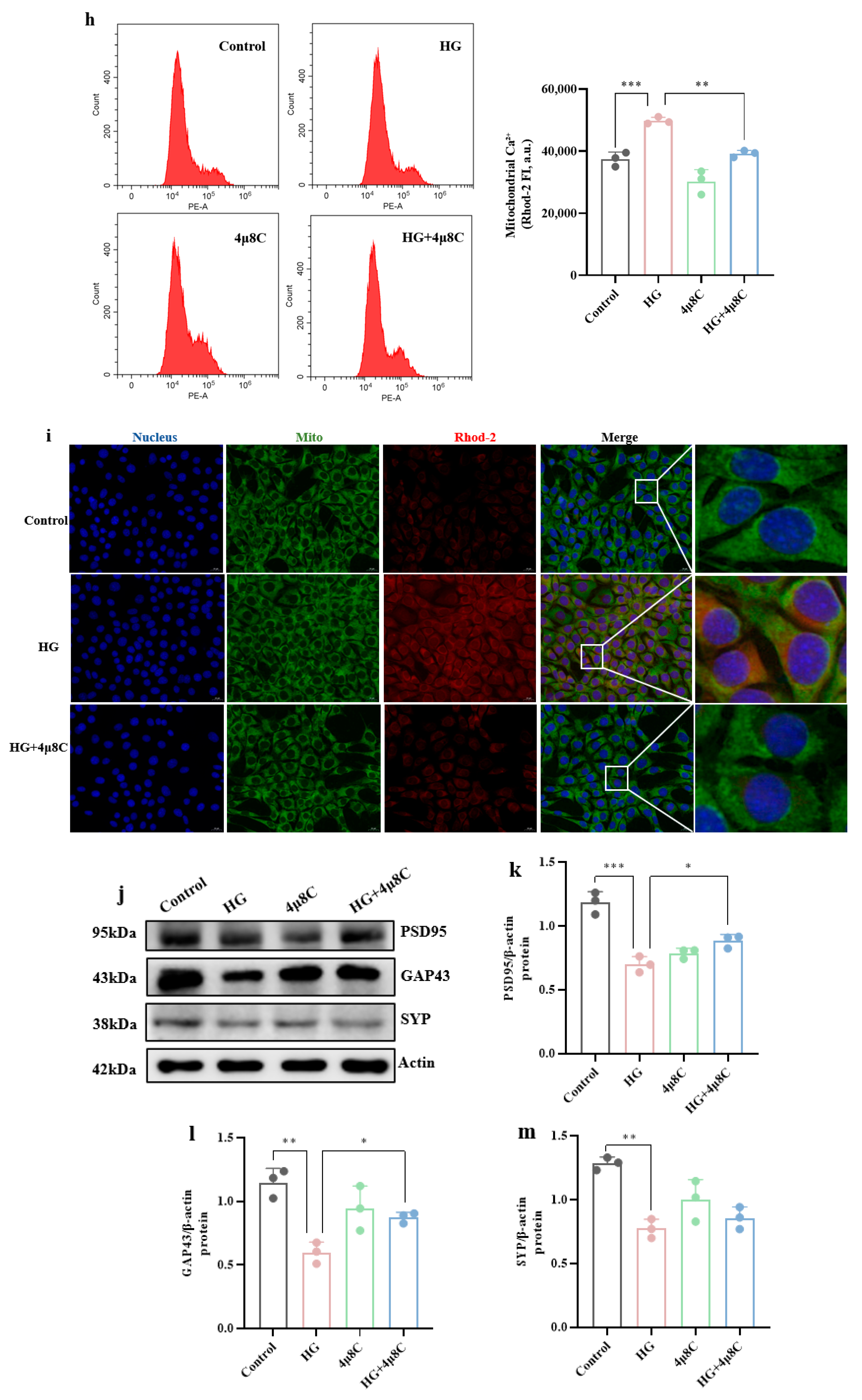
3.3. MAM-Mediated Mitochondrial Ca2+ Overload and ERS Drive High-Glucose-Induced Synaptic Plasticity Injury in Hippocampal Cells
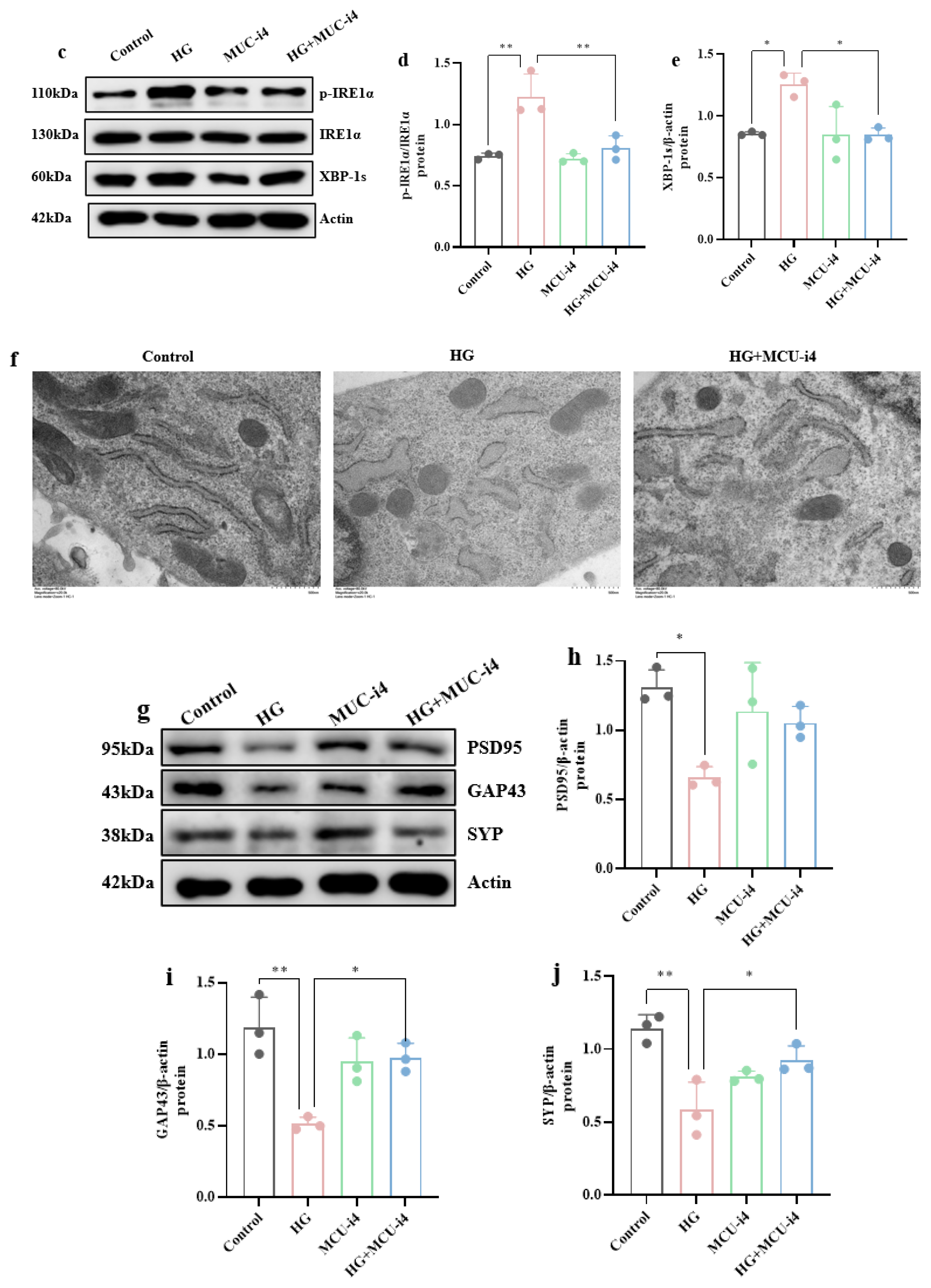
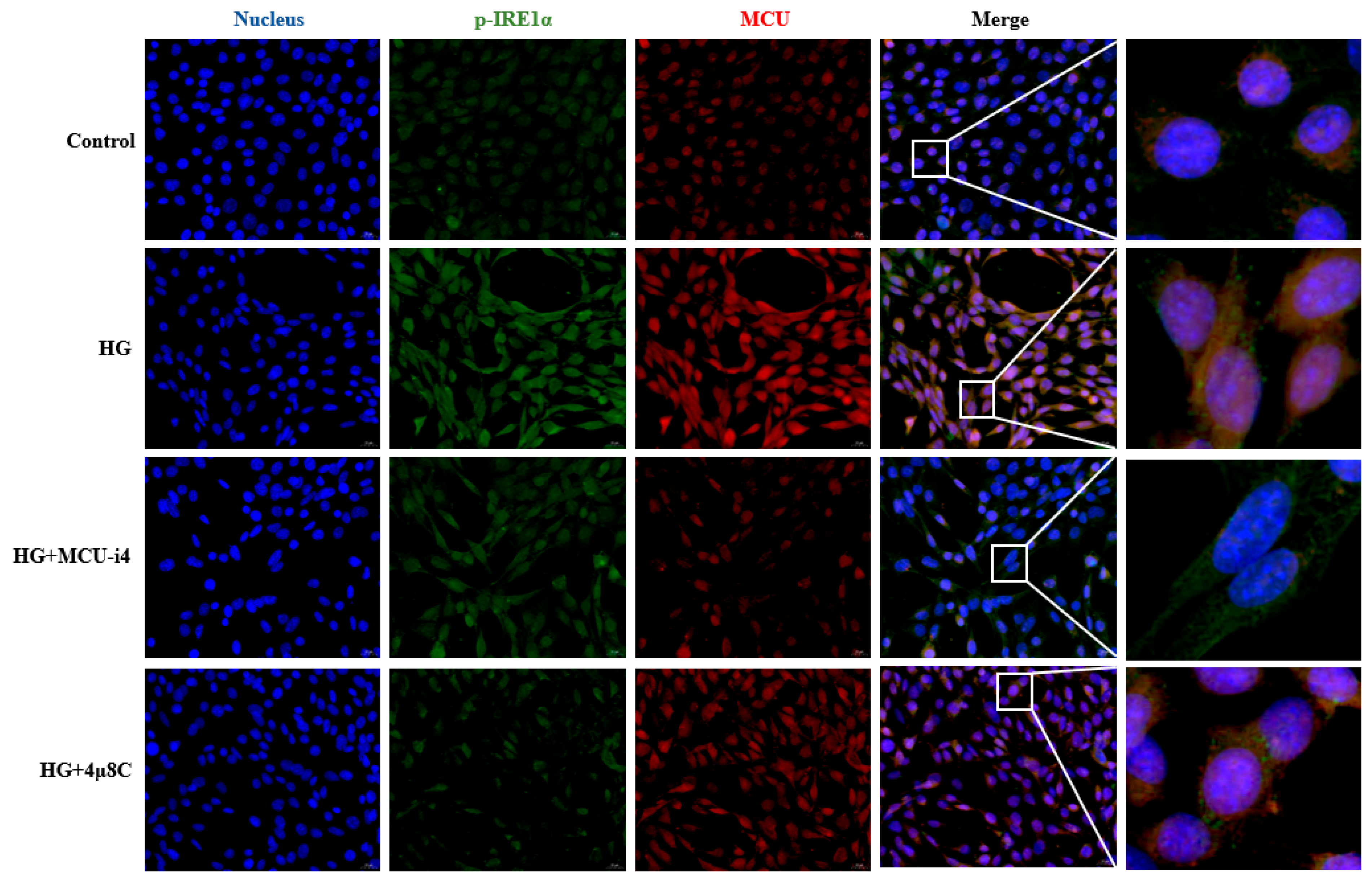
3.4. High-Glucose-Induced ERS and Mitochondrial Ca2+ Overload Synergistically Impair Hippocampal Synaptic Plasticity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAM | Mitochondrial-associated endoplasmic reticulum membranes |
| T2DM | Type 2 diabetes mellitus |
| MWM | Morris water maze |
| TEM | Transmission electron microscopy |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Meng, L.; Geng, L.; Guo, X.; Li, M.; Luo, G.; Huang, X. Relationship between Microvascular Lesions and Serum Metabolic Parameters in Patients with Type 2 Diabetes Mellitus. Prog. Mod. Biomed. 2017, 17, 1713–1715. [Google Scholar] [CrossRef]
- Xiang, Q.; Tao, J.S.; Dong, S.; Liu, X.L.; Yang, L.; Liu, L.N.; Deng, J.; Li, X.H. Heterogeneity and synaptic plasticity analysis of hippocampus based on db−/− mice induced diabetic encephalopathy. Psychoneuroendocrinology 2024, 159, 106412. [Google Scholar] [CrossRef]
- Do Nascimento Amorim, M.D.S.; Rates, E.R.D.; de Araujo Costa, M.I.V.; Diniz Filho, J.F.S.; dos Santos, C.C. Diabetes and Cognitive Decline: An Innovative Approach to Analyzing the Biophysical and Vibrational Properties of the Hippocampus. ACS Omega 2024, 9, 40870–40881. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Dziobek, I.; Sweat, V.; Tirsi, A.; Rogers, K.; Bruehl, H.; Tsui, W.; Richardson, S.; Javier, E.; Convit, A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 2007, 50, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.; Sommers, M.S.; Lucki, I. Effects of diabetes on hippocampal neurogenesis: Links to cognition and depression. Neurosci. Biobehav. Rev. 2013, 37, 1346–1362. [Google Scholar] [CrossRef]
- Xu, W.; Caracciolo, B.; Wang, H.-X.; Winblad, B.; Bäckman, L.; Qiu, C.; Fratiglioni, L. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes 2010, 59, 2928–2935. [Google Scholar] [CrossRef]
- Kloppenborg, R.P.; Van Den Berg, E.; Kappelle, L.J.; Biessels, G.J. Diabetes and other vascular risk factors for dementia: Which factor matters most? A systematic review. Eur. J. Pharmacol. 2008, 585, 97–108. [Google Scholar] [CrossRef]
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; Sheffer, K.E.; Carrillo-Larco, R.M.; Bennett, J.E. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef]
- Zilliox, L.A.; Chadrasekaran, K.; Kwan, J.Y.; Russell, J.W. Diabetes and Cognitive Impairment. Curr. Diabetes Rep. 2016, 16, 87. [Google Scholar] [CrossRef]
- Freeman, L.R.; Haley-Zitlin, V.; Stevens, C.; Granholm, A.-C. Diet-induced effects on neuronal and glial elements in the middle-aged rat hippocampus. Nutr. Neurosci. 2011, 14, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.-C.; Bimonte-Nelson, H.A.; Moore, A.B.; Nelson, M.E.; Freeman, L.R.; Sambamurti, K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J. Alzheimers Dis. 2008, 14, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Biessels, G.J.; Gispen, W.H.; Ramakers, G.M. Synaptic transmission changes in the pyramidal cells of the hippocampus in streptozotocin-induced diabetes mellitus in rats. Brain Res. 2006, 1073–1074, 276–280. [Google Scholar] [CrossRef]
- Kamal, A.; Ramakers, G.M.J.; Gispen, W.H.; Biessels, G.J.; Al Ansari, A. Hyperinsulinemia in rats causes impairment of spatial memory and learning with defects in hippocampal synaptic plasticity by involvement of postsynaptic mechanisms. Exp. Brain Res. 2013, 226, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Aou, S.; Oomura, Y.; Hori, N.; Fukunaga, K.; Hori, T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 2002, 113, 607–615. [Google Scholar] [CrossRef]
- Artola, A.; Kamal, A.; Ramakers, G.M.J.; Biessels, G.J.; Gispen, W.H. Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus. Eur. J. Neurosci. 2005, 22, 169–178. [Google Scholar] [CrossRef]
- Cholewa-Waclaw, J.; Bird, A.; von Schimmelmann, M.; Schaefer, A.; Yu, H.; Song, H.; Madabhushi, R.; Tsai, L.-H. The Role of Epigenetic Mechanisms in the Regulation of Gene Expression in the Nervous System. J. Neurosci. 2016, 36, 11427–11434. [Google Scholar] [CrossRef]
- Karimi, S.A.; Salehi, I.; Komaki, A.; Sarihi, A.; Zarei, M.; Shahidi, S. Effect of high-fat diet and antioxidants on hippocampal long-term potentiation in rats: An in vivo study. Brain Res. 2013, 1539, 1–6. [Google Scholar] [CrossRef]
- Siyu, W.; Jiahui, C.; Lu, L. Effect of Early Intervention of Fluoxetine lmproves Cognitive lmpairmentand Hippocampal Synaptic Plasticity in APP/PS1 Mice. J. Henan Med. Coll. 2024, 36, 289–294. [Google Scholar]
- Qi, M.-Y.; Yan, L.; Liu, S.-H. Artemisinin Ameliorates Diabetic Cognitive lmpairment by lmprovingSynaptic Plasticity via Pl3K/Akt Pathway in Mice. Prog. Biochem. Biophys. 2022, 49, 1530–1542. [Google Scholar]
- Filadi, R.; Theurey, P.; Pizzo, P. The endoplasmic reticulum-mitochondria coupling in health and disease: Molecules, functions and significance. Cell Calcium 2017, 62, 1–15. [Google Scholar] [CrossRef]
- Hayashi, T.; Rizzuto, R.; Hajnoczky, G.; Su, T.-P. MAM: More than just a housekeeper. Trends Cell Biol. 2009, 19, 81–88. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, S.; Li, M.; Xu, Z.; Zhu, L.; Deng, J.; Deng, H. Copper-mediated MAM regulation of the NF-κB signalling pathway enhances Seneca Valley virus replication in PK-15 cells. Vet. Res. 2025, 56, 175. [Google Scholar] [CrossRef]
- de Brito, O.M.; Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456, 605–610. [Google Scholar] [CrossRef]
- Han, S.; Zhao, F.; Hsia, J.; Ma, X.; Liu, Y.; Torres, S.; Fujioka, H.; Zhu, X. The role of Mfn2 in the structure and function of endoplasmic reticulum-mitochondrial tethering in vivo. J. Cell Sci. 2021, 134, jcs253443. [Google Scholar] [CrossRef]
- Mendes, C.C.P.; Gomes, D.A.; Thompson, M.; Souto, N.C.; Goes, T.S.; Goes, A.M.; Rodrigues, M.A.; Gomez, M.V.; Nathanson, M.H.; Leite, M.F. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J. Biol. Chem. 2005, 280, 40892–40900. [Google Scholar] [CrossRef]
- Szabadkai, G.; Bianchi, K.; Várnai, P.; De Stefani, D.; Wieckowski, M.R.; Cavagna, D.; Nagy, A.I.; Balla, T.; Rizzuto, R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006, 175, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Singhal, K.; Sandhir, R. L-type calcium channel blocker ameliorates diabetic encephalopathy by modulating dysregulated calcium homeostasis. J. Neurosci. Res. 2015, 93, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z. The Mechanism of Store-Operated Ca2+ Influx in High-Glucose-Induced Neuronal Injury. Ph.D. Thesis, Shandong University, Jinan, China, 2016. [Google Scholar]
- Hutchins, B.I.; Li, L.; Kalil, K. Wnt-induced calcium signaling mediates axon growth and guidance in the developing corpus callosum. Sci. Signal. 2012, 5, pt1. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, I.; Kerkhofs, M.; Lemos, F.O.; Loncke, J.; Bultynck, G.; Parys, J.B. The ER-mitochondria interface, where Ca(2+) and cell death meet. Cell Calcium 2023, 112, 102743. [Google Scholar] [CrossRef]
- Chang, Y. The Mechanism of SIRT3 Regulating Mitochondria Associated Reticulum Membranes in Improving Diabetic Cognitive Dysfunction. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2023. [Google Scholar]
- Karagas, N.E.; Venkatachalam, K. Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis. Cells 2019, 8, 1232. [Google Scholar] [CrossRef]
- Hitomi, J.; Katayama, T.; Eguchi, Y.; Kudo, T.; Taniguchi, M.; Koyama, Y.; Manabe, T.; Yamagishi, S.; Bando, Y.; Imaizumi, K.; et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J. Cell Biol. 2004, 165, 347–356. [Google Scholar] [CrossRef]
- Siman, R.; Flood, D.G.; Thinakaran, G.; Neumar, R.W. Endoplasmic reticulum stress-induced cysteine protease activation in cortical neurons: Effect of an Alzheimer’s disease-linked presenilin-1 knock-in mutation. J. Biol. Chem. 2001, 276, 44736–44743. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014, 5, 296. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Chen, Y.; Miao, G.; Zhao, H.; Qu, W.; Li, D.; Wang, Z.; Liu, N.; Li, L.; Chen, S.; et al. The ER-Localized Transmembrane Protein EPG-3/VMP1 Regulates SERCA Activity to Control ER-Isolation Membrane Contacts for Autophagosome Formation. Mol. Cell 2017, 67, 974–989.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, H.; Guo, G.; Xia, S.; Wang, L. VMP1 affects endoplasmic reticulum stress sensitivity via differential modulation of the three unfolded protein response arms. Cell Rep. 2023, 42, 112209. [Google Scholar] [CrossRef] [PubMed]
- Wada, I.; Rindress, D.; Cameron, P.; Ou, W.; Doherty, J.; Louvard, D.; Bell, A.; Dignard, D.; Thomas, D.; Bergeron, J. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 1991, 266, 19599–19610. [Google Scholar] [CrossRef]
- Baksh, S.; Michalak, M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J. Biol. Chem. 1991, 266, 21458–21465. [Google Scholar] [CrossRef]
- Van, P.N.; Peter, F.; Söling, H.D. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J. Biol. Chem. 1989, 264, 17494–17501. [Google Scholar] [CrossRef] [PubMed]
- Sur, C.; Kost, J.; Scott, D.; Adamczuk, K.; Fox, N.C.; Cummings, J.L.; Tariot, P.N.; Aisen, P.S.; Vellas, B.; Voss, T.; et al. BACE inhibition causes rapid, regional, and non-progressive volume reduction in Alzheimer’s disease brain. Brain 2020, 143, 3816–3826. [Google Scholar] [CrossRef]
- Nogalska, A.; Engel, W.K.; Askanas, V. Increased BACE1 mRNA and noncoding BACE1-antisense transcript in sporadic inclusion-body myositis muscle fibers--possibly caused by endoplasmic reticulum stress. Neurosci. Lett. 2010, 474, 140–143. [Google Scholar] [CrossRef]
- Belal, C.; Ameli, N.J.; El Kommos, A.; Bezalel, S.; Al’KHafaji, A.M.; Mughal, M.R.; Mattson, M.P.; Kyriazis, G.A.; Tyrberg, B.; Chan, S.L. The homocysteine-inducible endoplasmic reticulum (ER) stress protein Herp counteracts mutant α-synuclein-induced ER stress via the homeostatic regulation of ER-resident calcium release channel proteins. Hum. Mol. Genet. 2012, 21, 963–977. [Google Scholar] [CrossRef]
- Maity, S.; Komal, P.; Kumar, V.; Saxena, A.; Tungekar, A.; Chandrasekar, V. Impact of ER Stress and ER-Mitochondrial Crosstalk in Huntington’s Disease. Int. J. Mol. Sci. 2022, 23, 780. [Google Scholar] [CrossRef]
- He, Y. Zonisamide Ameliorates Cognitive Dysfunction in Type 2 Diabetic Mice by Alleviating Endoplasmic Reticulum Stress. Master’s Thesis, Guangzhou Medical University, Guangzhou, China, 2020. [Google Scholar]
- Tang, Y.D.; Wang, X.Z.; Zhang, J.J. Research progress in the construction of type II diabetes animal models. Acta Lab. Anim. Sci. Sin. 2020, 28, 870–876. [Google Scholar]
- Zhu, S.; Wu, H.; Cui, H.; Guo, H.; Ouyang, Y.; Ren, Z.; Deng, Y.; Geng, Y.; Ouyang, P.; Wu, A.; et al. Induction of mitophagy via ROS-dependent pathway protects copper-induced hypothalamic nerve cell injury. Food Chem. Toxicol. 2023, 181, 114097. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Ryan, C.; Launer, L.J. Diabetes and Cognitive Impairment. In Diabetes in America; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018. [Google Scholar]
- Hsieh, C.-F.; Liu, C.-K.; Lee, C.-T.; Yu, L.-E.; Wang, J.-Y. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci. Rep. 2019, 9, 840. [Google Scholar] [CrossRef]
- Cervellati, C.; Trentini, A.; Pecorelli, A.; Valacchi, G. Inflammation in Neurological Disorders: The Thin Boundary Between Brain and Periphery. Antioxid. Redox Signal. 2020, 33, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Farbood, Y.; Ghaderi, S.; Rashno, M.; Khoshnam, S.E.; Khorsandi, L.; Sarkaki, A.; Rashno, M. Sesamin: A promising protective agent against diabetes-associated cognitive decline in rats. Life Sci. 2019, 230, 169–177. [Google Scholar] [CrossRef]
- Logan, S.; Royce, G.H.; Owen, D.; Farley, J.; Ranjo-Bishop, M.; Sonntag, W.E.; Deepa, S.S. Accelerated decline in cognition in a mouse model of increased oxidative stress. Geroscience 2019, 41, 591–607. [Google Scholar] [CrossRef]
- Song, Y.; Du, Y.; Zou, W.; Luo, Y.; Zhang, X.; Fu, J. Involvement of impaired autophagy and mitophagy in Neuro-2a cell damage under hypoxic and/or high-glucose conditions. Sci. Rep. 2018, 8, 3301. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, L.; Yuan, Y.; Jiang, T.; Guo, X.; Wang, Z.; Xu, K.; Xu, Z.; Liu, Y.; Zhong, X.; et al. Autophagy Activation is Associated with Neuroprotection in Diabetes-associated Cognitive Decline. Aging Dis. 2019, 10, 1233–1245. [Google Scholar] [CrossRef]
- Chen, J.L.; Luo, C.; Pu, D.; Zhang, G.Q.; Zhao, Y.X.; Sun, Y.; Zhao, K.X.; Liao, Z.Y.; Lv, A.K.; Zhu, S.Y.; et al. Metformin attenuates diabetes-induced tau hyperphosphorylation in vitro and in vivo by enhancing autophagic clearance. Exp. Neurol. 2019, 311, 44–56. [Google Scholar] [CrossRef]
- Kang, X. Asprosin Negatively Regulates the Hippocampal METTL3-m6A-YTHDF1 Axis: A Novel Mechanism in Diabetic Cognitive Dysfunction. Ph.D. Thesis, University of South China, Hunan, China, 2021. [Google Scholar]
- Gao, X. Protective Effect of Melatonin Against T2DM-Related Glycolipid Metabolic Disorder and Neuropsychiatric Injury and Its Potential Mechanism. Master’s Thesis, Anhui Medical University, Hefei, China, 2022. [Google Scholar]
- Yang, X.; Zhuang, J.; Song, W.; Shen, W.; Wu, W.; Shen, H.; Han, S. Mitochondria-associated endoplasmic reticulum membrane: Overview and inextricable link with cancer. J. Cell. Mol. Med. 2023, 27, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Schon, E.A.; Area-Gomez, E. Mitochondria-associated ER membranes in Alzheimer disease. Mol. Cell. Neurosci. 2013, 55, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bryleva, E.Y.; Rogers, M.A.; Chang, C.C.; Buen, F.; Harris, B.T.; Rousselet, E.; Seidah, N.G.; Oddo, S.; LaFerla, F.M.; Spencer, T.A.; et al. ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc. Natl. Acad. Sci. USA 2010, 107, 3081–3086. [Google Scholar] [CrossRef] [PubMed]
- Mary, A.; Eysert, F.; Checler, F.; Chami, M. Mitophagy in Alzheimer’s disease: Molecular defects and therapeutic approaches. Mol. Psychiatry 2023, 28, 202–216. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Ge, X.; Chen, J.; Zhao, Y.; Fu, J. Parkin overexpression attenuates Aβ-induced mitochondrial dysfunction in HEK293 cells by restoring impaired mitophagy. Life Sci. 2020, 244, 117322. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Y.; Jiang, Y.; Jiang, J.; Chen, S.; Sun, D.; Li, S.; Wei, F.; Zhu, H. IP3R2 regulates apoptosis by Ca2+ transfer through mitochondria-ER contacts in hypoxic photoreceptor injury. Exp. Eye Res. 2024, 245, 109965. [Google Scholar] [CrossRef]
- Mercado, G.; Castillo, V.; Soto, P.; López, N.; Axten, J.M.; Sardi, S.P. Targeting PERK signaling with the small molecule GSK2606414 prevents neurodegeneration in a model of Parkinson’s disease. Neurobiol. Dis. 2018, 112, 136–148. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Z.; Hu, J.; Feng, J.; Zhu, Z.; Fan, Y.; Lin, Q.; Ding, G. Mfn2 Regulates High Glucose-Induced MAMs Dysfunction and Apoptosis in Podocytes via PERK Pathway. Front. Cell Dev. Biol. 2021, 9, 769213. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huo, S.; Du, J.; Zhang, X.; Zhang, J.; Wang, Q.; Song, M.; Li, Y. Dibutyl phthalate causes heart damage by disrupting Ca(2+) transfer from endoplasmic reticulum to mitochondria and triggering subsequent pyroptosis. Sci. Total Environ. 2023, 892, 164620. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wen, X.; Tang, H.; Wating, S.; Xia, Z.; Lei, S. Sodium 4-phenylbutyrate alleviates H9c2 cell apoptosis induced by high glucose by inhibiting the endoplasmic reticulum stress-MAMs-mitochondrial calcium overload axis. Med. J. Wuhan Univ. 2025, 1–8. [Google Scholar] [CrossRef]





| Target Gene | Accession Number | Primer | Primer Sequence (5’-3’) |
|---|---|---|---|
| Mfn2 | NM_001285920.1 | Forward | GCTTGGACAGGTGGAGTCAA |
| Reverse | GGACTCGAGGTCTCCTCTGT | ||
| PSD95 | NM_001109752.1 | Forward | AGCCCCAGGATATGTGAACG |
| Reverse | ATGGAACCCGCCTCTTTGAG | ||
| SYP | NM_009305.2 | Forward | GACGTTGGTAGTGCCTGTGA |
| Reverse | GCACAGGAAAGTAGGGGGTC | ||
| GAP43 | NM_008083.2 | Forward | GATGGTGTCAAGCCGGAAGA |
| Reverse | CCACGGAAGCTAGCCTGAAT | ||
| β-actin | NM_007393.5 | Forward | TGTACCCAGGCATTGCTGAC |
| Reverse | AACGCAGCTCAGTAACAGTCC |
| Name of Antibody | RRID |
|---|---|
| Actin (ABclonal, Wuhan, China AC026) | AB_2768234 |
| GAP43 (ABclonal, China A19055) | AB_2862548 |
| Mfn2 (Proteintech, Wuhan, China 12186-1-AP) | AB_2266320 |
| PSD95 (ABclonal, China A7889) | AB_2769180 |
| SYP (ABclonal, China A19122) | AB_2862615 |
| IP3R (ABclonal, China A4436) | AB_2314676 |
| GRP75 (Wanleibio, China WL03209) | AB_1661203 |
| MCU (ABclonal, China A22525) | AB_2902658 |
| VDAC1 (ABclonal, China A19707) | AB_10564217 |
| p-IRE1α (Nature Biosciences, Hangzhou, China A24402) | AB_3102214 |
| IRE1α (Nature Biosciences, China A74484) | AB_2927490 |
| XBP-1s (Nature Biosciences, China A58678) | AB_2737816 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jiang, J.; Li, H.; Deng, J.; Dong, W.; Deng, H. MAM-Mediated Mitochondrial Ca2+ Overload and Endoplasmic Reticulum Stress Aggravates Synaptic Plasticity Impairment in Diabetic Mice. Brain Sci. 2025, 15, 1157. https://doi.org/10.3390/brainsci15111157
Zhang J, Jiang J, Li H, Deng J, Dong W, Deng H. MAM-Mediated Mitochondrial Ca2+ Overload and Endoplasmic Reticulum Stress Aggravates Synaptic Plasticity Impairment in Diabetic Mice. Brain Sciences. 2025; 15(11):1157. https://doi.org/10.3390/brainsci15111157
Chicago/Turabian StyleZhang, Jie, Jie Jiang, Haocong Li, Junliang Deng, Wei Dong, and Huidan Deng. 2025. "MAM-Mediated Mitochondrial Ca2+ Overload and Endoplasmic Reticulum Stress Aggravates Synaptic Plasticity Impairment in Diabetic Mice" Brain Sciences 15, no. 11: 1157. https://doi.org/10.3390/brainsci15111157
APA StyleZhang, J., Jiang, J., Li, H., Deng, J., Dong, W., & Deng, H. (2025). MAM-Mediated Mitochondrial Ca2+ Overload and Endoplasmic Reticulum Stress Aggravates Synaptic Plasticity Impairment in Diabetic Mice. Brain Sciences, 15(11), 1157. https://doi.org/10.3390/brainsci15111157






