The Application of Gamma-Range Auditory Steady-State Responses in Animal Models: A Semi-Structured Literature Review
Abstract
1. Introduction
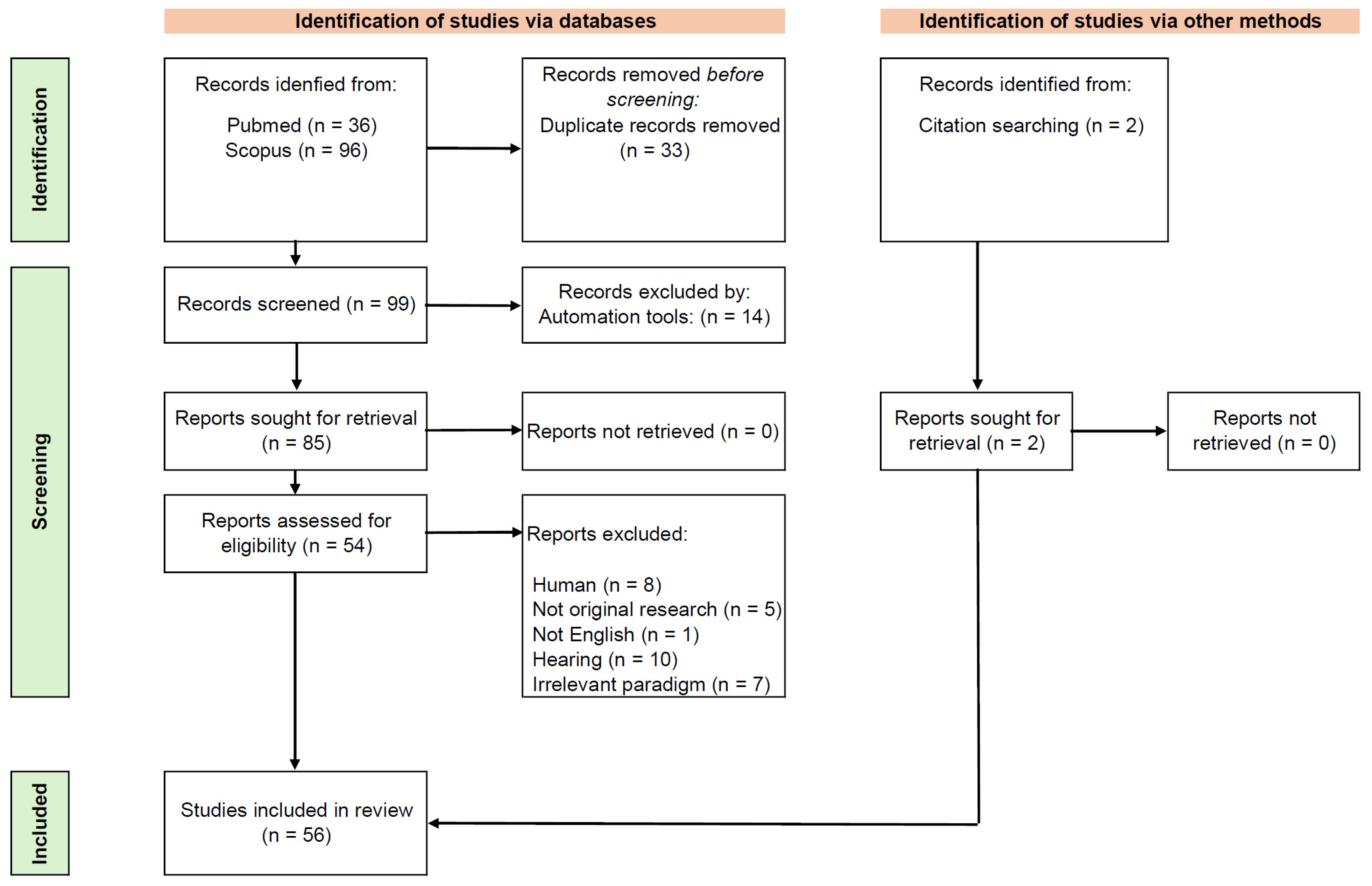
2. Materials and Methods
3. Results
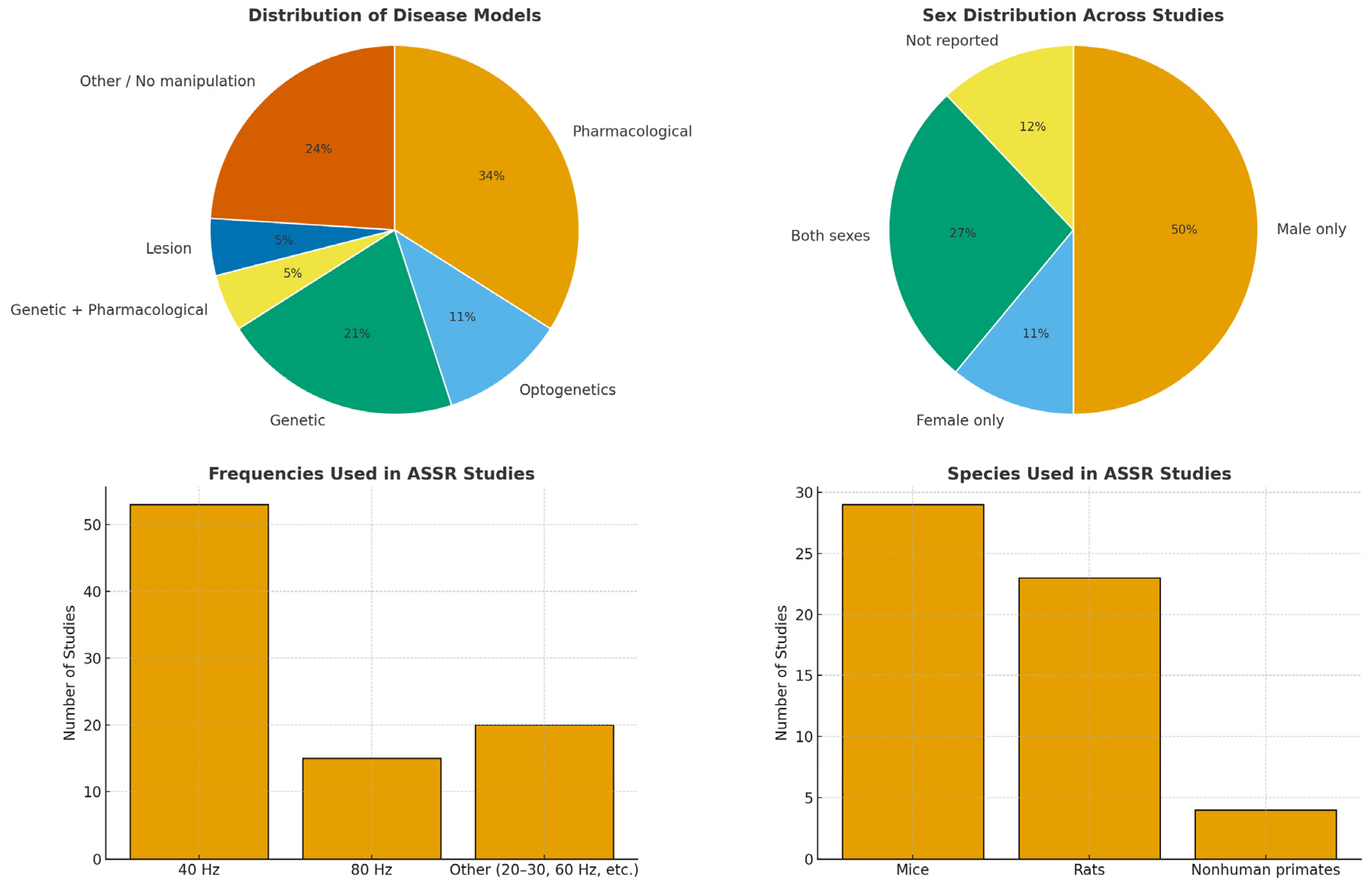
3.1. Disease Models
3.2. Group Characteristics and Experimental Contexts
3.3. Stimulation Protocols
4. Discussion
4.1. Disease Modeling
4.2. Localization of ASSRs and Neural Circuitry
4.3. State and Trait Modulation of ASSRs
4.4. Evidence from Non-Human Primates
4.5. Methodological Settings
4.6. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Auditory cortex |
| AM | Amplitude-modulated |
| ASD | Autism spectrum disorders |
| ASSRs | Auditory steady state responses |
| BF-PV | Basal forebrain parvalbumin |
| ECoG | Electrocorticography |
| EEG | Electroencephalography |
| EGF | Epidermal growth factor |
| FXS | Fragile X Syndrome |
| GABA | Gamma-aminobutyric acid |
| GlyT1 | Glycine transporter 1 |
| GSK3 β | Glycogen synthase kinase-3 |
| IFN-α | Interferon-alpha |
| KO | Knock-out |
| LFP | Local field potentials |
| MGB | Medial geniculate body |
| NMDA | N-methyl D-aspartate |
| NVHL | Neonatal ventral hippocampal lesion |
| Pcdh10 | Protocadherin 10 |
| PCP | Phencyclidine |
| PFC | Prefrontal cortex |
| PV | Parvalbumin |
| SRKO | Serine racemase knock-out |
| SZ | Schizophrenia |
| TRN | Thalamic reticular nucleus |
| WT | Wild-type |
Appendix A
References
- O’Donnell, B.F.; Vohs, J.L.; Krishnan, G.P.; Rass, O.; Hetrick, W.P.; Morzorati, S.L. The Auditory Steady-State Response (ASSR). In Supplements to Clinical Neurophysiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 101–112. ISBN 978-0-7020-5307-8. [Google Scholar]
- Brenner, C.A.; Krishnan, G.P.; Vohs, J.L.; Ahn, W.-Y.; Hetrick, W.P.; Morzorati, S.L.; O’Donnell, B.F. Steady State Responses: Electrophysiological Assessment of Sensory Function in Schizophrenia. Schizophr. Bull. 2009, 35, 1065–1077. [Google Scholar] [CrossRef]
- Kuwada, S.; Anderson, J.S.; Batra, R.; Fitzpatrick, D.C.; Teissier, N.; D’Angelo, W.R. Sources of the Scalp-Recorded Amplitude-Modulation Following Response. J. Am. Acad. Audiol. 2002, 13, 188–204. [Google Scholar] [CrossRef]
- Joris, P.X.; Schreiner, C.E.; Rees, A. Neural Processing of Amplitude-Modulated Sounds. Physiol. Rev. 2004, 84, 541–577. [Google Scholar] [CrossRef]
- Sugiyama, S.; Ohi, K.; Kuramitsu, A.; Takai, K.; Muto, Y.; Taniguchi, T.; Kinukawa, T.; Takeuchi, N.; Motomura, E.; Nishihara, M.; et al. The Auditory Steady-State Response: Electrophysiological Index for Sensory Processing Dysfunction in Psychiatric Disorders. Front. Psychiatry 2021, 12, 644541. [Google Scholar] [CrossRef]
- Grent, T.; Gajwani, R.; Gross, J.; Gumley, A.I.; Krishnadas, R.; Lawrie, S.M.; Schwannauer, M.; Schultze-Lutter, F.; Uhlhaas, P.J. 40-Hz Auditory Steady-State Responses Characterize Circuit Dysfunctions and Predict Clinical Outcomes in Clinical High-Risk for Psychosis Participants: A Magnetoencephalography Study. Biol. Psychiatry 2021, 90, 419–429. [Google Scholar] [CrossRef]
- Tada, M.; Nagai, T.; Kirihara, K.; Koike, S.; Suga, M.; Araki, T.; Kobayashi, T.; Kasai, K. Differential Alterations of Auditory Gamma Oscillatory Responses Between Pre-Onset High-Risk Individuals and First-Episode Schizophrenia. Cereb. Cortex 2016, 26, 1027–1035. [Google Scholar] [CrossRef]
- Koshiyama, D.; Kirihara, K.; Tada, M.; Nagai, T.; Fujioka, M.; Ichikawa, E.; Ohta, K.; Tani, M.; Tsuchiya, M.; Kanehara, A.; et al. Auditory Gamma Oscillations Predict Global Symptomatic Outcome in the Early Stages of Psychosis: A Longitudinal Investigation. Clin. Neurophysiol. 2018, 129, 2268–2275. [Google Scholar] [CrossRef]
- Seymour, R.A.; Rippon, G.; Gooding-Williams, G.; Sowman, P.F.; Kessler, K. Reduced Auditory Steady State Responses in Autism Spectrum Disorder. Mol. Autism 2020, 11, 56. [Google Scholar] [CrossRef]
- Mäkelä, J.P.; Karmos, G.; Molnár, M.; Csépe, V.; Winkler, I. Steady-State Responses from the Cat Auditory Cortex. Hear. Res. 1990, 45, 41–50. [Google Scholar] [CrossRef]
- Jeng, F.-C.; Abbas, P.J.; Brown, C.J.; Miller, C.A.; Nourski, K.V.; Robinson, B.K. Electrically Evoked Auditory Steady-State Responses in Guinea Pigs. Audiol. Neurotol. 2007, 12, 101–112. [Google Scholar] [CrossRef]
- Dolphin, W.F.; Chertoff, M.E.; Burkard, R. Comparison of the Envelope Following Response in the Mongolian Gerbil Using Two-Tone and Sinusoidally Amplitude-Modulated Tones. J. Acoust. Soc. Am. 1994, 96, 2225–2234. [Google Scholar] [CrossRef]
- Azkona, G.; Sanchez-Pernaute, R. Mice in Translational Neuroscience: What R We Doing? Prog. Neurobiol. 2022, 217, 102330. [Google Scholar] [CrossRef]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.M.; Meijboom, F.L.B. Animal to Human Translation: A Systematic Scoping Review of Reported Concordance Rates. J. Transl. Med. 2019, 17, 223. [Google Scholar] [CrossRef]
- Granzotto, A.; Vissel, B.; Sensi, S.L. Lost in Translation: Inconvenient Truths on the Utility of Mouse Models in Alzheimer’s Disease Research. eLife 2024, 13, e90633. [Google Scholar] [CrossRef]
- Brynildsen, J.K.; Rajan, K.; Henderson, M.X.; Bassett, D.S. Network Models to Enhance the Translational Impact of Cross-Species Studies. Nat. Rev. Neurosci. 2023, 24, 575–588. [Google Scholar] [CrossRef]
- Schuelert, N.; Dorner-Ciossek, C.; Brendel, M.; Rosenbrock, H. A Comprehensive Analysis of Auditory Event-Related Potentials and Network Oscillations in an NMDA Receptor Antagonist Mouse Model Using a Novel Wireless Recording Technology. Physiol. Rep. 2018, 6, e13782. [Google Scholar] [CrossRef]
- Rosenbrock, H.; Dorner-Ciossek, C.; Giovannini, R.; Schmid, B.; Schuelert, N. Effects of the Glycine Transporter-1 Inhibitor Iclepertin (BI 425809) on Sensory Processing, Neural Network Function, and Cognition in Animal Models Related to Schizophrenia. J. Pharmacol. Exp. Ther. 2022, 382, 223–232. [Google Scholar] [CrossRef]
- Adraoui, F.W.; Hettak, K.; Viardot, G.; Alix, M.; Guiffard, S.; Meot, B.; L’Hostis, P.; Maurin, A.; Delpy, E.; Drieu La Rochelle, C.; et al. Differential Effects of Aripiprazole on Electroencephalography-Recorded Gamma-Band Auditory Steady-State Response, Spontaneous Gamma Oscillations and Behavior in a Schizophrenia Rat Model. Int. J. Mol. Sci. 2024, 25, 1035. [Google Scholar] [CrossRef]
- Nakao, K.; Nakazawa, K. Brain State-Dependent Abnormal LFP Activity in the Auditory Cortex of a Schizophrenia Mouse Model. Front. Neurosci. 2014, 8, 168. [Google Scholar] [CrossRef]
- Kozono, N.; Okamura, A.; Honda, S.; Matsumoto, M.; Mihara, T. Gamma Power Abnormalities in a Fmr1-Targeted Transgenic Rat Model of Fragile X Syndrome. Sci. Rep. 2020, 10, 18799. [Google Scholar] [CrossRef]
- Herzog, L.E.; Wang, L.; Yu, E.; Choi, S.; Farsi, Z.; Song, B.J.; Pan, J.Q.; Sheng, M. Mouse Mutants in Schizophrenia Risk Genes GRIN2A and AKAP11 Show EEG Abnormalities in Common with Schizophrenia Patients. Transl. Psychiatry 2023, 13, 92. [Google Scholar] [CrossRef]
- Vohs, J.L.; Andrew Chambers, R.; Krishnan, G.P.; O’Donnell, B.F.; Berg, S.; Morzorati, S.L. GABAergic Modulation of the 40 Hz Auditory Steady-State Response in a Rat Model of Schizophrenia. Int. J. Neuropsychopharm. 2010, 13, 487. [Google Scholar] [CrossRef]
- Li, S.; Ma, L.; Wang, Y.; Wang, X.; Li, Y.; Qin, L. Auditory Steady-State Responses in Primary and Non-Primary Regions of the Auditory Cortex in Neonatal Ventral Hippocampal Lesion Rats. PLoS ONE 2018, 13, e0192103. [Google Scholar] [CrossRef]
- Croom, K.; Rumschlag, J.A.; Erickson, M.A.; Binder, D.K.; Razak, K.A. Developmental Delays in Cortical Auditory Temporal Processing in a Mouse Model of Fragile X Syndrome. J. Neurodev. Disord. 2023, 15, 23. [Google Scholar] [CrossRef]
- Jonak, C.R.; Assad, S.A.; Garcia, T.A.; Sandhu, M.S.; Rumschlag, J.A.; Razak, K.A.; Binder, D.K. Phenotypic Analysis of Multielectrode Array EEG Biomarkers in Developing and Adult Male Fmr1 KO Mice. Neurobiol. Dis. 2024, 195, 106496. [Google Scholar] [CrossRef] [PubMed]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-Inhibition Balance as a Framework for Investigating Mechanisms in Neuropsychiatric Disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Minzenberg, M.J.; Firl, A.J.; Yoon, J.H.; Gomes, G.C.; Reinking, C.; Carter, C.S. Gamma Oscillatory Power Is Impaired During Cognitive Control Independent of Medication Status in First-Episode Schizophrenia. Neuropsychopharmacology 2010, 35, 2590–2599. [Google Scholar] [CrossRef]
- Chen, C.-M.A.; Stanford, A.D.; Mao, X.; Abi-Dargham, A.; Shungu, D.C.; Lisanby, S.H.; Schroeder, C.E.; Kegeles, L.S. GABA Level, Gamma Oscillation, and Working Memory Performance in Schizophrenia. NeuroImage Clin. 2014, 4, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Port, R.G.; Gajewski, C.; Krizman, E.; Dow, H.C.; Hirano, S.; Brodkin, E.S.; Carlson, G.C.; Robinson, M.B.; Roberts, T.P.L.; Siegel, S.J. Protocadherin 10 Alters γ Oscillations, Amino Acid Levels, and Their Coupling; Baclofen Partially Restores These Oscillatory Deficits. Neurobiol. Dis. 2017, 108, 324–338. [Google Scholar] [CrossRef]
- Croom, K.; Rumschlag, J.A.; Erickson, M.A.; Binder, D.; Razak, K.A. Sex Differences during Development in Cortical Temporal Processing and Event Related Potentials in Wild-Type and Fragile X Syndrome Model Mice. J. Neurodev. Disord. 2024, 16, 24. [Google Scholar] [CrossRef]
- Tao, X.; Croom, K.; Newman-Tancredi, A.; Varney, M.; Razak, K.A. Acute Administration of NLX-101, a Serotonin 1A Receptor Agonist, Improves Auditory Temporal Processing during Development in a Mouse Model of Fragile X Syndrome. J. Neurodev. Disord. 2025, 17, 1. [Google Scholar] [CrossRef]
- Thuné, H.; Recasens, M.; Uhlhaas, P.J. The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-Analysis. JAMA Psychiatry 2016, 73, 1145. [Google Scholar] [CrossRef]
- Raza, M.U.; Sivarao, D.V. Test-Retest Reliability of Tone- and 40 Hz Train-Evoked Gamma Oscillations in Female Rats and Their Sensitivity to Low-Dose NMDA Channel Blockade. Psychopharmacology 2021, 238, 2325–2334. [Google Scholar] [CrossRef]
- Sivarao, D.V.; Frenkel, M.; Chen, P.; Healy, F.L.; Lodge, N.J.; Zaczek, R. MK-801 Disrupts and Nicotine Augments 40 Hz Auditory Steady State Responses in the Auditory Cortex of the Urethane-Anesthetized Rat. Neuropharmacology 2013, 73, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sivarao, D.V.; Chen, P.; Senapati, A.; Yang, Y.; Fernandes, A.; Benitex, Y.; Whiterock, V.; Li, Y.-W.; Ahlijanian, M.K. 40 Hz Auditory Steady-State Response Is a Pharmacodynamic Biomarker for Cortical NMDA Receptors. Neuropsychopharmacology 2016, 41, 2232–2240. [Google Scholar] [CrossRef] [PubMed]
- Light, G.A.; Zhang, W.; Joshi, Y.B.; Bhakta, S.; Talledo, J.A.; Swerdlow, N.R. Single-Dose Memantine Improves Cortical Oscillatory Response Dynamics in Patients with Schizophrenia. Neuropsychopharmacology 2017, 42, 2633–2639. [Google Scholar] [CrossRef]
- Murphy, N.; Ramakrishnan, N.; Vo-Le, B.; Vo-Le, B.; Smith, M.A.; Iqbal, T.; Swann, A.C.; Mathew, S.J.; Lijffijt, M. A Randomized Cross-over Trial to Define Neurophysiological Correlates of AV-101 N-Methyl-d-Aspartate Receptor Blockade in Healthy Veterans. Neuropsychopharmacology 2021, 46, 820–827. [Google Scholar] [CrossRef]
- Vohs, J.L.; Chambers, R.A.; O’Donnell, B.F.; Krishnan, G.P.; Morzorati, S.L. Auditory Steady State Responses in a Schizophrenia Rat Model Probed by Excitatory/Inhibitory Receptor Manipulation. Int. J. Psychophysiol. 2012, 86, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, Y.; Krusienski, D.; Dadi, Y.S.; Seo, M.; Shin, H.-S.; Choi, J.H. Impaired Auditory Evoked Potentials and Oscillations in Frontal and Auditory Cortex of a Schizophrenia Mouse Model. World J. Biol. Psychiatry 2016, 17, 439–448. [Google Scholar] [CrossRef]
- Nakao, K.; Singh, M.; Sapkota, K.; Hagler, B.C.; Hunter, R.N.; Raman, C.; Hablitz, J.J.; Nakazawa, K. GSK3β Inhibition Restores Cortical Gamma Oscillation and Cognitive Behavior in a Mouse Model of NMDA Receptor Hypofunction Relevant to Schizophrenia. Neuropsychopharmacology 2020, 45, 2207–2218. [Google Scholar] [CrossRef]
- Balla, A.; Ginsberg, S.D.; Abbas, A.I.; Sershen, H.; Javitt, D.C. Translational Neurophysiological Biomarkers of N-Methyl-d-Aspartate Receptor Dysfunction in Serine Racemase Knockout Mice. Biomark. Neuropsychiatry 2020, 2, 100019. [Google Scholar] [CrossRef]
- Croom, K.; Rumschlag, J.A.; Molinaro, G.; Erickson, M.A.; Binder, D.K.; Huber, K.M.; Razak, K.A. Developmental Trajectory and Sex Differences in Auditory Processing in a PTEN-Deletion Model of Autism Spectrum Disorders. Neurobiol. Dis. 2024, 200, 106628. [Google Scholar] [CrossRef]
- Lovelace, J.W.; Ethell, I.M.; Binder, D.K.; Razak, K.A. Minocycline Treatment Reverses Sound Evoked EEG Abnormalities in a Mouse Model of Fragile X Syndrome. Front. Neurosci. 2020, 14, 771. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, Z.; Li, J.; Xu, J.; Yang, P.; Qin, L. Neuroprotective Effect of Microglia against Impairments of Auditory Steady-State Response Induced by Anti-P IgG from SLE Patients in Naïve Mice. J. Neuroinflammation 2020, 17, 31. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Chen, J.; Li, Z.; Yang, P.; Qin, L. Aberrant Auditory Steady-State Response of Awake Mice Induced by Chronic Interferon-α Treatment. Front. Pharmacol. 2021, 11, 584425. [Google Scholar] [CrossRef]
- Gautam, D.; Shields, A.; Krepps, E.; Ummear Raza, M.; Sivarao, D.V. Click Train Elicited Local Gamma Synchrony: Differing Performance and Pharmacological Responsivity of Primary Auditory and Prefrontal Cortices. Brain Res. 2024, 1841, 149091. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Thankachan, S.; McKenna, J.T.; McNally, J.M.; Yang, C.; Choi, J.H.; Chen, L.; Kocsis, B.; Deisseroth, K.; Strecker, R.E.; et al. Cortically Projecting Basal Forebrain Parvalbumin Neurons Regulate Cortical Gamma Band Oscillations. Proc. Natl. Acad. Sci. USA 2015, 112, 3535–3540. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Wang, X.; Qin, L. Differential Modulation of the Auditory Steady State Response and Inhibitory Gating by Chloral Hydrate Anesthesia. Sci. Rep. 2018, 8, 3683. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Wang, S.; Wang, X.; Chen, J.; Qin, L. Laminar Profile of Auditory Steady-State Response in the Auditory Cortex of Awake Mice. Front. Syst. Neurosci. 2021, 15, 636395. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.D.; Gallagher, A.J.; Coulson, S.; Rangel, L.M. Network Resonance and the Auditory Steady State Response. Sci. Rep. 2024, 14, 16799. [Google Scholar] [CrossRef]
- Hwang, E.; Brown, R.E.; Kocsis, B.; Kim, T.; McKenna, J.T.; McNally, J.M.; Han, H.-B.; Choi, J.H. Optogenetic Stimulation of Basal Forebrain Parvalbumin Neurons Modulates the Cortical Topography of Auditory Steady-State Responses. Brain Struct. Funct. 2019, 224, 1505–1518. [Google Scholar] [CrossRef]
- Toader, O.; von Heimendahl, M.; Schuelert, N.; Nissen, W.; Rosenbrock, H. Suppression of Parvalbumin Interneuron Activity in the Prefrontal Cortex Recapitulates Features of Impaired Excitatory/Inhibitory Balance and Sensory Processing in Schizophrenia. Schizophr. Bull. 2020, 46, 981–989. [Google Scholar] [CrossRef]
- Muller, L.; Chavane, F.; Reynolds, J.; Sejnowski, T.J. Cortical Travelling Waves: Mechanisms and Computational Principles. Nat. Rev. Neurosci. 2018, 19, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Inui, K.; Ohi, K.; Shioiri, T. The Influence of Novelty Detection on the 40-Hz Auditory Steady-State Response in Schizophrenia: A Novel Hypothesis from Meta-Analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 135, 111096. [Google Scholar] [CrossRef] [PubMed]
- Farahani, E.D.; Wouters, J.; van Wieringen, A. Improving Source Modeling of Auditory Steady-State Responses with Frequency-Specific Brain Maps. Neuroscience, 2019; in press. [Google Scholar]
- Jiricek, S.; Koudelka, V.; Lacik, J.; Vejmola, C.; Kuratko, D.; Wójcik, D.K.; Raida, Z.; Hlinka, J.; Palenicek, T. Electrical Source Imaging in Freely Moving Rats: Evaluation of a 12-Electrode Cortical Electroencephalography System. Front. Neuroinform. 2021, 14, 589228. [Google Scholar] [CrossRef]
- Price, J.L.; Drevets, W.C. Neurocircuitry of Mood Disorders. Neuropsychopharmacology 2010, 35, 192–216. [Google Scholar] [CrossRef]
- Paul, T.; See, J.W.; Vijayakumar, V.; Njideaka-Kevin, T.; Loh, H.; Lee, V.J.Q.; Dogrul, B.N. Neurostructural Changes in Schizophrenia and Treatment-Resistance: A Narrative Review. Psychoradiology 2024, 4, kkae015. [Google Scholar] [CrossRef]
- Eilam-Stock, T.; Wu, T.; Spagna, A.; Egan, L.J.; Fan, J. Neuroanatomical Alterations in High-Functioning Adults with Autism Spectrum Disorder. Front. Neurosci. 2016, 10, 237. [Google Scholar] [CrossRef]
- Griskova, I.; Morup, M.; Parnas, J.; Ruksenas, O.; Arnfred, S.M. The Amplitude and Phase Precision of 40 Hz Auditory Steady-State Response Depend on the Level of Arousal. Exp. Brain Res. 2007, 183, 133–138. [Google Scholar] [CrossRef]
- Binder, M.; Górska, U.; Griskova-Bulanova, I. 40 Hz Auditory Steady-State Responses in Patients with Disorders of Consciousness: Correlation between Phase-Locking Index and Coma Recovery Scale-Revised Score. Clin. Neurophysiol. 2017, 128, 799–806. [Google Scholar] [CrossRef]
- Melynyte, S.; Pipinis, E.; Genyte, V.; Voicikas, A.; Rihs, T.; Griskova-Bulanova, I. 40 Hz Auditory Steady-State Response: The Impact of Handedness and Gender. Brain Topogr. 2018, 31, 419–429. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Li, Z.; Chen, J.; Qin, L. Effect of Locomotion on the Auditory Steady State Response of Head-Fixed Mice. World J. Biol. Psychiatry 2020, 22, 362–372. [Google Scholar] [CrossRef]
- Liu, H.-H.; Liu, C.-M.; Hsieh, M.H.; Chien, Y.-L.; Hsu, Y.-F.; Lai, W.-S. Dysregulated Affective Arousal Regulates Reward-Based Decision Making in Patients with Schizophrenia: An Integrated Study. Schizophrenia 2022, 8, 26. [Google Scholar] [CrossRef]
- Hegerl, U.; Wilk, K.; Olbrich, S.; Schoenknecht, P.; Sander, C. Hyperstable Regulation of Vigilance in Patients with Major Depressive Disorder. World J. Biol. Psychiatry 2012, 13, 436–446. [Google Scholar] [CrossRef]
- Palmisano, A.; Pandit, S.; Smeralda, C.L.; Demchenko, I.; Rossi, S.; Battelli, L.; Rivolta, D.; Bhat, V.; Santarnecchi, E. The Pathophysiological Underpinnings of Gamma-Band Alterations in Psychiatric Disorders. Life 2024, 14, 578. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, S.M.; Kim, H.J.; Han, D.H. Comparison of Attention and Brain Functional Connectivity between Patient Groups with Schizophrenia and Attention Deficit Hyperactivity Disorder. Psychiatry Res. 2025, 345, 116376. [Google Scholar] [CrossRef]
- McNally, J.M.; Aguilar, D.D.; Katsuki, F.; Radzik, L.K.; Schiffino, F.L.; Uygun, D.S.; McKenna, J.T.; Strecker, R.E.; Deisseroth, K.; Spencer, K.M.; et al. Optogenetic Manipulation of an Ascending Arousal System Tunes Cortical Broadband Gamma Power and Reveals Functional Deficits Relevant to Schizophrenia. Mol. Psychiatry 2020, 26, 3461–3475. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Nakamura, I.; Tamura, S.; Onitsuka, T. Long-Term Test-Retest Reliability of Auditory Gamma Oscillations Between Different Clinical EEG Systems. Front. Psychiatry 2020, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Jasinskyte, U.; Buisas, R.; Griskova-Bulanova, I.; Guzulaitis, R. Auditory Steady-State Responses in the Auditory Cortex of Mice during Estrus Cycle. Brain Res. 2023, 1810, 148376. [Google Scholar] [CrossRef]
- Griskova-Bulanova, I.; Griksiene, R.; Korostenskaja, M.; Ruksenas, O. 40 Hz Auditory Steady-State Response in Females: When Is It Better to Entrain? Acta Neurobiol. Exp. 2014, 74, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Moniem, I.; Kafetzopoulos, V. Sex Differences in Schizophrenia: Symptomatology, Treatment Efficacy and Adverse Effects. Front. Psychiatry 2025, 16, 1594334. [Google Scholar] [CrossRef]
- Napolitano, A.; Schiavi, S.; La Rosa, P.; Rossi-Espagnet, M.C.; Petrillo, S.; Bottino, F.; Tagliente, E.; Longo, D.; Lupi, E.; Casula, L.; et al. Sex Differences in Autism Spectrum Disorder: Diagnostic, Neurobiological, and Behavioral Features. Front. Psychiatry 2022, 13, 889636. [Google Scholar] [CrossRef]
- Sloan, D.M.; Sandt, A.R. Gender Differences in Depression. Womens Health 2006, 2, 425–434. [Google Scholar] [CrossRef]
- Parker, G.; Fletcher, K.; Paterson, A.; Anderson, J.; Hong, M. Gender Differences in Depression Severity and Symptoms Across Depressive Sub-Types. J. Affect. Disord. 2014, 167, 351–357. [Google Scholar] [CrossRef]
- Handy, A.B.; Greenfield, S.F.; Yonkers, K.A.; Payne, L.A. Psychiatric Symptoms Across the Menstrual Cycle in Adult Women: A Comprehensive Review. Harv. Rev. Psychiatry 2022, 30, 100–117. [Google Scholar] [CrossRef]
- Mazza, M.; Marano, G. Unmasking the Cycle: Premenstrual and Menstrual Exacerbation of Psychiatric Disorders and Impact on Female Mental Health. World J. Psychiatry 2025, 15, 107132. [Google Scholar] [CrossRef] [PubMed]
- Lear, A.; Baker, S.N.; Clarke, H.F.; Roberts, A.C.; Schmid, M.C.; Jarrett, W. Understanding Them to Understand Ourselves: The Importance of NHP Research for Translational Neuroscience. Curr. Res. Neurobiol. 2022, 3, 100049. [Google Scholar] [CrossRef]
- Harding, J.D. Nonhuman Primates and Translational Research: Progress, Opportunities, and Challenges. ILAR J. 2017, 58, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Konoike, N.; Iwaoki, H.; Miwa, M.; Sakata, H.; Itoh, K.; Nakamura, K. Comparison of Non-Invasive, Scalp-Recorded Auditory Steady-State Responses in Humans, Rhesus Monkeys, and Common Marmosets. Sci. Rep. 2022, 12, 9210. [Google Scholar] [CrossRef]
- Nakamura, T.; Dinh, T.H.; Asai, M.; Nishimaru, H.; Matsumoto, J.; Setogawa, T.; Ichijo, H.; Honda, S.; Yamada, H.; Mihara, T.; et al. Characteristics of Auditory Steady-State Responses to Different Click Frequencies in Awake Intact Macaques. BMC Neurosci. 2022, 23, 57. [Google Scholar] [CrossRef]
- Iwamura, Y.; Nakayama, T.; Matsumoto, A.; Ogi, Y.; Yamaguchi, M.; Kobayashi, A.; Matsumoto, K.; Katsura, Y.; Konoike, N.; Nakamura, K.; et al. Effect of Dopamine Receptor-Related Compounds on Naïve Common Marmosets for Auditory Steady-State Response. J. Neurophysiol. 2022, 128, 229–238. [Google Scholar] [CrossRef]
- Yan, T.; Suzuki, K.; Kameda, S.; Kuratomi, T.; Mihara, M.; Maeda, M.; Hirata, M. Intracranial EEG Recordings of High-Frequency Activity From a Wireless Implantable BMI Device in Awake Nonhuman Primates. IEEE Trans. Biomed. Eng. 2023, 70, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, D.D.; Radzik, L.K.; Schiffino, F.L.; Folorunso, O.O.; Zielinski, M.R.; Coyle, J.T.; Balu, D.T.; McNally, J.M. Altered Neural Oscillations and Behavior in a Genetic Mouse Model of NMDA Receptor Hypofunction. Sci. Rep. 2021, 11, 9031. [Google Scholar] [CrossRef]
- Cizus, E.; Jasinskyte, U.; Guzulaitis, R. Effects of Acute and Chronic Ketamine Administration on Spontaneous and Evoked Brain Activity. Brain Res. 2024, 1846, 149232. [Google Scholar] [CrossRef] [PubMed]
- Dejean, C.; Dupont, T.; Verpy, E.; Gonçalves, N.; Coqueran, S.; Michalski, N.; Pucheu, S.; Bourgeron, T.; Gourévitch, B. Detecting Central Auditory Processing Disorders in Awake Mice. Brain Sci. 2023, 13, 1539. [Google Scholar] [CrossRef]
- Gautam, D.; Raza, M.U.; Miyakoshi, M.; Molina, J.L.; Joshi, Y.B.; Clayson, P.E.; Light, G.A.; Swerdlow, N.R.; Sivarao, D.V. Click-Train Evoked Steady State Harmonic Response as a Novel Pharmacodynamic Biomarker of Cortical Oscillatory Synchrony. Neuropharmacology 2023, 240, 109707. [Google Scholar] [CrossRef]
- Inaba, H.; Kai, R.; Namba, H.; Sotoyama, H.; Jodo, E.; Nin, F.; Hibino, H.; Yabe, H.; Eifuku, S.; Horii, A.; et al. Perinatal Epidermal Growth Factor Signal Perturbation Results in the Series of Abnormal Auditory Oscillations and Responses Relevant to Schizophrenia. Schizophr. Bull. Open 2021, 2, sgaa070. [Google Scholar] [CrossRef]
- Kozono, N.; Honda, S.; Tada, M.; Kirihara, K.; Zhao, Z.; Jinde, S.; Uka, T.; Yamada, H.; Matsumoto, M.; Kasai, K.; et al. Auditory Steady State Response; Nature and Utility as a Translational Science Tool. Sci. Rep. 2019, 9, 8454. [Google Scholar] [CrossRef]
- Leishman, E.; O’Donnell, B.F.; Millward, J.B.; Vohs, J.L.; Rass, O.; Krishnan, G.P.; Bolbecker, A.R.; Morzorati, S.L. Phencyclidine Disrupts the Auditory Steady State Response in Rats. PLoS ONE 2015, 10, e0134979. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Wang, X.; Liu, Y.; Wang, S.; Wang, X.; Li, Y.; Qin, L. The Thalamocortical Mechanism Underlying the Generation and Regulation of the Auditory Steady-State Responses in Awake Mice. J. Neurosci. 2024, 44, e1166232023. [Google Scholar] [CrossRef]
- Munch, A.S.; Amat-Foraster, M.; Agerskov, C.; Bastlund, J.F.; Herrik, K.F.; Richter, U. Sub-Anesthetic Doses of Ketamine Increase Single Cell Entrainment in the Rat Auditory Cortex during Auditory Steady-State Response. J. Psychopharmacol. 2023, 37, 822–835. [Google Scholar] [CrossRef]
- Prado-Gutierrez, P.; Martínez-Montes, E.; Weinstein, A.; Zañartu, M. Estimation of Auditory Steady-State Responses Based on the Averaging of Independent EEG Epochs. PLoS ONE 2019, 14, e0206018. [Google Scholar] [CrossRef]
- Ummear Raza, M.; Gautam, D.; Rorie, D.; Sivarao, D.V. Differential Effects of Clozapine and Haloperidol on the 40 Hz Auditory Steady State Response-Mediated Phase Resetting in the Prefrontal Cortex of the Female Sprague Dawley Rat. Schizophr. Bull. 2023, 49, 581–591. [Google Scholar] [CrossRef]
- Sullivan, E.M.; Timi, P.; Hong, L.E.; O’Donnell, P. Effects of NMDA and GABA-A Receptor Antagonism on Auditory Steady-State Synchronization in Awake Behaving Rats. Int. J. Neuropsychopharmacol. 2015, 18, pyu118. [Google Scholar] [CrossRef] [PubMed]
- Thankachan, S.; Katsuki, F.; McKenna, J.T.; Yang, C.; Shukla, C.; Deisseroth, K.; Uygun, D.S.; Strecker, R.E.; Brown, R.E.; McNally, J.M.; et al. Thalamic Reticular Nucleus Parvalbumin Neurons Regulate Sleep Spindles and Electrophysiological Aspects of Schizophrenia in Mice. Sci. Rep. 2019, 9, 3607. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Tian, Z.; Wang, X.; Li, Y.; Qin, L. Emotional Arousal Modifies Auditory Steady State Response in the Auditory Cortex and Prefrontal Cortex of Rats. Stress 2019, 22, 492–500. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, J.; Li, Z.; Li, J.; Qin, L. Aberrant Auditory Steady-State Response of Awake Mice After Single Application of the NMDA Receptor Antagonist MK-801 Into the Medial Geniculate Body. Int. J. Neuropsychopharmacol. 2020, 23, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Honda, S.; Tamaki, K.; Irie, M.; Mihara, T. Effects of (+)-Bicuculline, a GABAa Receptor Antagonist, on Auditory Steady State Response in Free-Moving Rats. PLoS ONE 2020, 15, e0236363. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L.; Li, W.; Yang, P.; Qin, L. Cholinergic Modulation of Auditory Steady-State Response in the Auditory Cortex of the Freely Moving Rat. Neuroscience 2016, 324, 29–39. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasinskyte, U.; Vejmola, C.; Guzulaitis, R.; Griskova-Bulanova, I. The Application of Gamma-Range Auditory Steady-State Responses in Animal Models: A Semi-Structured Literature Review. Brain Sci. 2025, 15, 1159. https://doi.org/10.3390/brainsci15111159
Jasinskyte U, Vejmola C, Guzulaitis R, Griskova-Bulanova I. The Application of Gamma-Range Auditory Steady-State Responses in Animal Models: A Semi-Structured Literature Review. Brain Sciences. 2025; 15(11):1159. https://doi.org/10.3390/brainsci15111159
Chicago/Turabian StyleJasinskyte, Urte, Cestmir Vejmola, Robertas Guzulaitis, and Inga Griskova-Bulanova. 2025. "The Application of Gamma-Range Auditory Steady-State Responses in Animal Models: A Semi-Structured Literature Review" Brain Sciences 15, no. 11: 1159. https://doi.org/10.3390/brainsci15111159
APA StyleJasinskyte, U., Vejmola, C., Guzulaitis, R., & Griskova-Bulanova, I. (2025). The Application of Gamma-Range Auditory Steady-State Responses in Animal Models: A Semi-Structured Literature Review. Brain Sciences, 15(11), 1159. https://doi.org/10.3390/brainsci15111159






