Tractography-Based Asymmetries in Acquired Brain Injury: Contributions to the Neuropsychological Profile and Rehabilitation in a Case-Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Neuropsychological and Emotional Assessment
- Montreal Cognitive Assessment (MoCA; Spanish validation [29]) for global cognition.
- WAIS-III Digit Span (forward/backward) [30] for attention and working memory.
- Trail Making Test A and B [31] for processing speed and cognitive flexibility.
- Rey–Osterrieth Complex Figure, copy condition, interpreted with NEURONORMA norms [32] for visuoconstruction and planning.
- Hospital Anxiety and Depression Scale (HADS; Spanish validation [33]) for anxiety and depression.
2.4. Neuroimaging Acquisition and Tractography
- Diffusion-weighted imaging (DTI): single-shot echo-planar imaging, b = 1000 s/mm2, 32 non-collinear diffusion directions, voxel size = 2.0 × 2.0 × 2.0 mm3, and one b0 image.
- Structural imaging: 3D T1-weighted isotropic sequence, voxel size = 1 mm3.
2.5. Structural Asymmetry Analysis
2.6. Quality Control
3. Results
3.1. Neuropsychological and Emotional Performance
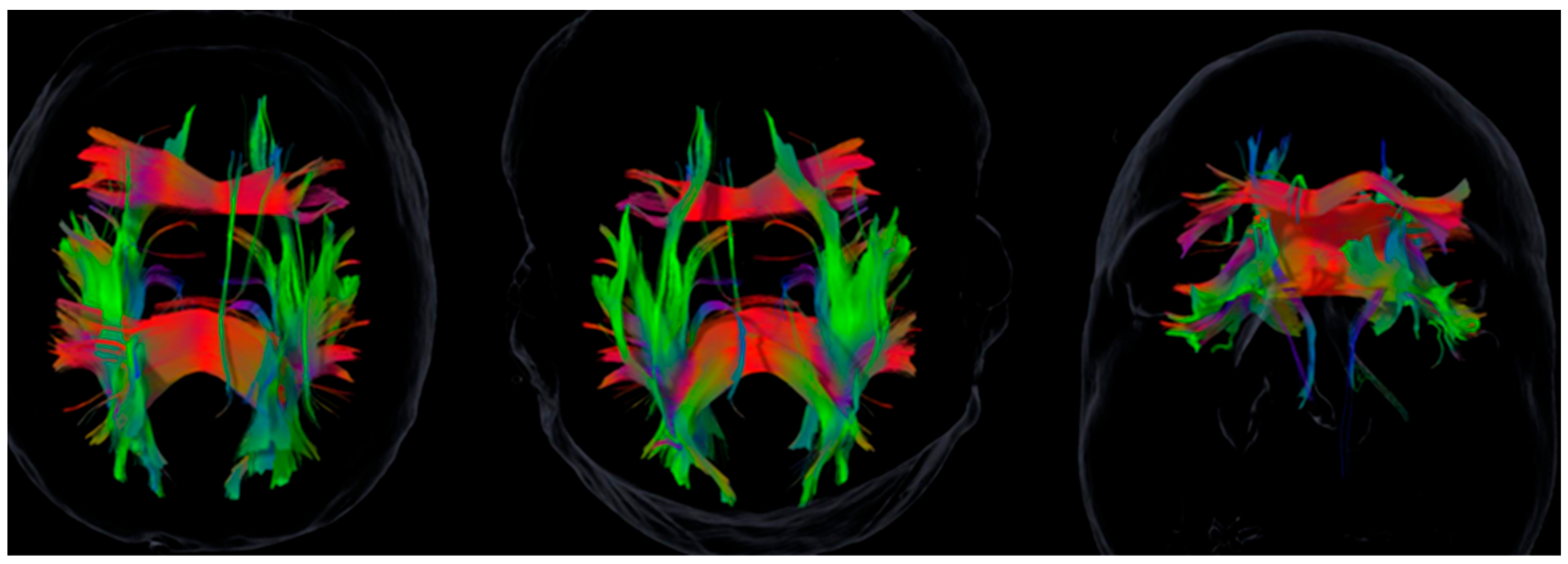
3.2. White Matter Tract Asymmetries
3.3. Integrated Profiles
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABI | Acquired Brain Injury |
| AD | Axial Diffusivity |
| CEIm | Comité de Ética de la Investigación con Medicamentos |
| DTI | Diffusion Tensor Imaging |
| HADS | Hospital Anxiety and Depression Scale |
| HADS-A | Hospital Anxiety and Depression Scale, Anxiety Subscale |
| HADS-D | Hospital Anxiety and Depression Scale, Depression Subscale |
| IFOF/SFOF | Fronto-Occipital Fasciculi |
| ILF | Inferior Longitudinal Fasciculus |
| MD | Mean Diffusivity |
| MoCA | Montreal Cognitive Assessment |
| MRI | Magnetic Resonance Imaging |
| RD | Radial Diffusivity |
| ROI | Region of Interest |
| SAH | Subarachnoid Hemorrhage |
| SAI | Structural Asymmetry Index |
| SD | Standard Deviation |
| SLF | Superior Longitudinal Fasciculus |
| SFOF | Superior Front-Occipital Fasciculus |
| TBI | Traumatic Brain Injury |
| TMT | Trail Making Test |
| TMT-A | Trail Making Test, Part A |
| TMT-B | Trail Making Test, Part B |
| WAIS-III | Wechsler Adult Intelligence Scale. Third Edition |
References
- Lu, Q.; Lu, S.; Wang, X.; Huang, Y.; Liu, J.; Liang, Z. Structural and functional changes of post-stroke depression: A multimodal magnetic resonance imaging study. Neuroimage Clin. 2025, 45, 103743. [Google Scholar] [CrossRef] [PubMed]
- Stubberud, J.; Løvstad, M.; Solbakk, A.-K.; Schanke, A.-K.; Tornås, S. Emotional regulation following acquired brain injury: Associations with executive functioning in daily life and symptoms of anxiety and depression. Front. Neurol. 2020, 11, 1011. [Google Scholar] [CrossRef]
- Green, S.L.; Gignac, G.E.; Watson, P.A.; Brosnan, N.; Becerra, R.; Pestell, C.; Weinborn, M. Apathy and depression as predictors of activities of daily living following stroke and traumatic brain injuries in adults: A meta-analysis. Neuropsychol. Rev. 2022, 32, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Ru, X.; Gao, L.; Zhou, J.; Wang, S.; Yang, J.; Chen, J. Secondary white matter injury and therapeutic targets after subarachnoid hemorrhage. Front. Neurol. 2021, 12, 659740. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J. Effectiveness of robot-assisted gait training in stroke rehabilitation: A systematic review and meta-analysis. J. Clin. Med. 2025, 14, 4809. [Google Scholar] [CrossRef]
- Boardsworth, K.; Rashid, U.; Olsen, S.; Rodríguez-Ramírez, E.; Browne, W.; Alder, G.; Signal, N. Upper limb robotic rehabilitation following stroke: Systematic review and meta-analysis investigating efficacy and the influence of device features and program parameters. J. Neuroeng. Rehabil. 2025, 22, 164. [Google Scholar] [CrossRef]
- Soule, A.C.; Fish, T.J.; Thomas, K.G.F.; Schrieff-Brown, L. Attention training after moderate-to-severe traumatic brain injury in adults: A systematic review. Arch. Phys. Med. Rehabil. 2025, 106, 433–443. [Google Scholar] [CrossRef]
- van der Veen, R.; Königs, M.; Bakker, S.; van Iperen, A.; Peerdeman, S.; Bet, P.M.; Oosterlaan, J. Pharmacotherapy to improve cognitive functioning after Acquired brain injury: A meta-analysis and meta-regression. Clin. Pharmacol. Ther. 2024, 115, 971–987. [Google Scholar] [CrossRef]
- Maggio, M.; Baglio, F.; Maione, R.; Calapai, R.; Di Iulio, F.; Dos Santos, P.C.R.; Maldonado-Díaz, M.; Pistorino, G.; Cerasa, A.; Quartarone, A.; et al. The overlooked role of exergames in cognitive-motor neurorehabilitation. NPJ Digit. Med. 2025, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Ayerbe, L.; Ayis, S.; Wolfe, C.D.A.; Rudd, A.G. Natural history, predictors and outcomes of depression after stroke: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 14–21. [Google Scholar] [CrossRef]
- Ganesh, A.; Al-Shamli, S.; Mahadevan, S.; Chan, M.F.; Burke, D.T.; Al Rasadi, K.; Al Saadoon, M.; Al-Adawi, S. The frequency of neuropsychiatric sequelae after traumatic brain injury in the Global South: A systematic review and meta-analysis. Sultan Qaboos Univ. Med. J. 2024, 24, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Nothdurfter, C.; Jawinski, P.; Markett, S. White matter tract integrity is reduced in depression: Findings from the UK Biobank. Biol. Psychiatry 2024, 95, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.M.; Nguyen, L.; Leibenluft, E.; Stange, J.P.; Linke, J.O. A meta-analysis on the uncinate fasciculus in depression. Psychol. Med. 2023, 53, 2721–2731. [Google Scholar] [CrossRef]
- Parsaei, M.; Hahsemi, S.M.; Seyedmirzaei, H.; Cattarinussi, G.; Sambataro, F.; Brambilla, P.; Delvecchio, G. Microstructural white matter alterations associated with social anxiety disorders: A systematic review. J. Affect. Disord. 2024, 350, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Forkel, S.J.; Friedrich, P.; Thiebaut de Schotten, M.; Howells, H. White matter variability, cognition, and disorders: A systematic review. Brain Struct. Funct. 2022, 227, 529–544. [Google Scholar] [CrossRef]
- Levinson, C.A.; Christian, C.; Becker, C.B. How idiographic methodologies can move the clinical-science field forward to integrate personalized treatment into everyday clinical care and improve treatment outcomes. Clin. Psychol. Sci. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Paolini, M.; Marrone, S.; Scalia, G.; Gerardi, R.; Bonosi, L.; Benigno, U.; Musso, S.; Scerrati, A.; Gerardo, D.; Signorelli, F.; et al. Diffusion tensor imaging as a prognostic tool in traumatic brain injury. Brain Sci. 2025, 15, 70. [Google Scholar] [CrossRef]
- Geschwind, N. Disconnexion Syndromes in Animals and Man. Brain 1965, 88, 237–294. [Google Scholar] [CrossRef]
- Catani, M.; Ffytche, D.H. The Rises and Falls of Disconnection Syndromes. Brain 2005, 128, 2224–2239. [Google Scholar] [CrossRef]
- Thiebaut de Schotten, M.; Foulon, C.; Nachev, P. Brain Disconnections Link Structural Connectivity with Function (The Human Disconnectome). Nat. Commun. 2020, 11, 5094. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Crum, G.; Siu, K. Effects of virtual reality on stroke rehabilitation: An umbrella review. Health Sci. Rep. 2024, 7, e70082. [Google Scholar] [CrossRef]
- Olana, D.; Abessa, T.; Lamba, D.; Tedesco, L.; Bonnechere, B. Effect of virtual reality-based upper limb training on activity of daily living and quality of life among stroke survivors. J. Neuroeng. Rehabil. 2025, 22, 92. [Google Scholar] [CrossRef]
- Medina, R.; Dave, A.; Keogh, C.; Bartfield, J.; Estenssoro, F.; Fraga, M.; Lucke-Wold, B. Integrating neuroimaging, biomarkers, and rehabilitation strategies for optimized diagnosis and recovery in traumatic brain injury. OBM Neurobiol. 2025, 9, 292. [Google Scholar] [CrossRef]
- Sarubbo, S.; Vavassori, L.; Zigiotto, L.; Corsini, F.; Annicchiarico, L.; Rozzanigo, U.; Avesani, P. Changing the paradigm for tractography segmentation in neurosurgery: Validation of a streamline-based approach. Brain Sci. 2024, 14, 1232. [Google Scholar] [CrossRef] [PubMed]
- Fahlström, M.; Karlsson, P.; Pemberton, H.; Sundgren, P.C.; Nilsson, D.; Orädd, G.; Andersson, M. Qualitative and visual along-tract analysis of diffusion-based parameters in patients with diffuse gliomas. Brain Sci. 2024, 14, 213. [Google Scholar] [CrossRef]
- Catani, M.; Dell’Acqua, F.; Thiebaut de Schotten, M. A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev. 2013, 37, 1724–1737. [Google Scholar] [CrossRef]
- Bartolomeo, P.; Liu, J.; Seidel Malkinson, T. Frontoparietal asymmetries leading to conscious perception. Trends Cogn. Sci. 2025, 29, 222–225. [Google Scholar] [CrossRef]
- Pepping, N.; Weinborn, M.; Pestell, C.F.; Preece, D.A.; Malkani, M.; Moore, S.; Gross, J.J.; Becerra, R. Improving emotion regulation ability after brain injury: A systematic review of targeted interventions. Neuropsychol. Rehabil. 2025, 35, 1283–1323. [Google Scholar] [CrossRef]
- Pereiro, A.X.; Ramos-Lema, S.; Lojo-Seoane, C.; Guàrdia-Olmos, J.; Facal-Mayo, J.; Juncos-Rabadán, O. Normative Data for the Montreal Cognitive Assessment (MoCA) in a Spanish Sample of Community-dweller Adults. Eur. Geriatr. Med. 2017, 8, 240–244. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS-III: Escala de Inteligencia de Wechsler para Adultos—Manual Técnico; TEA Ediciones: Madrid, Spain, 1999. [Google Scholar]
- Arango-Lasprilla, J.C.; Rivera, D.; Aguayo, A.; Rodríguez, W.; Garza, M.T.; Saracho, C.P.; Rodríguez-Agudelo, Y.; Aliaga, A.; Weiler, G.; Luna, M.; et al. Trail Making Test: Normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation 2015, 37, 639–661. [Google Scholar] [CrossRef] [PubMed]
- Peña-Casanova, J.; Quiñones-Úbeda, S.; Gramunt-Fombuena, N.; Quintana-Aparicio, M.; Aguilar, M.; Badenes, D.; Molinuevo, J.L.; Robles, A.; Barquero, M.S.; Antúnez, C.; et al. NEURONORMA Project: Norms for the Rey-Osterrieth Complex Figure Copy. Arch. Clin. Neuropsychol. 2009, 24, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.J.; Blanch, J.; Peri, J.M.; De Pablo, J.; Pintor, L.; Bulbena, A. A validation study of the Hospital Anxiety and Depression Scale (HADS) in a Spanish population. Gen. Hosp. Psychiatry 2003, 25, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.K.; Knösche, T.R.; Turner, R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage 2013, 73, 239–254. [Google Scholar] [CrossRef]
- Raffelt, D.A.; Tournier, J.-D.; Smith, R.E.; Vaughan, D.N.; Jackson, G.; Ridgway, G.R.; Connelly, A. Investigating white matter fibre density and morphology using fixel-based analysis. NeuroImage 2017, 144 Pt A, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Bennett, J.M.; de la Piedad García, X.; Willis, M.L. Emotion recognition and traumatic brain injury: A systematic review and meta-analysis. Neuropsychol. Rev. 2022, 32, 520–536. [Google Scholar] [CrossRef]
- Narayana, P.A. White matter changes in patients with mild traumatic brain injury: MRI perspective. Concussion 2017, 2, CNC35. [Google Scholar] [CrossRef]
- MacPherson, S.E.; Cox, S.R.; Dickie, D.A.; Karama, S.; Starr, J.M.; Evans, C.J.; Bastin, M.E.; Deary, I.J. Processing speed and the relationship between Trail Making Test-B performance, white matter integrity, and cortical thickness in older adults. Cortex 2017, 95, 92–103. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Alashram, A.R. Repetitive transcranial magnetic stimulation for cognitive rehabilitation in stroke survivors: A systematic review and meta-analysis of randomized controlled trials. Appl. Neuropsychol. Adult, 2025; 1–15, advance online publication. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Cheng, C.H.; Liao, K.K.; Lee, I.H.; Lin, Y.Y. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: A meta-analysis. Stroke 2012, 43, 1849–1857. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.; Chen, R.; Cohen, L.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex Male/Fem | Age | Digit Span Forward | Digit Span Backward | TMT-A (s) | TMT-B (s) | HADS-D | HADS-A | Rey Copy Type | Rey Copy Percentile | MoCA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 50 | 5 | 5 | 30 | 89 | 11 | 10 | 50 | 80 | 25 |
| 2 | M | 56 | 5 | 3 | 122 | 590 | 5 | 8 | 50 | 60 | 20 |
| 3 | M | 55 | 9 | 4 | 30 | 40 | 6 | 11 | 10 | 40 | 25 |
| 4 | F | 49 | 6 | 3 | 59 | 292 | 0 | 1 | 25 | 40 | 16 |
| 5 | F | 57 | 6 | 3 | 30 | 27 | 4 | 10 | 75 | 90 | 23 |
| 6 | M | 63 | 5 | 3 | 45 | 110 | 17 | 12 | 25 | 40 | 24 |
| 7 | F | 40 | 5 | 4 | 41 | 66 | 9 | 9 | 75 | 90 | 24 |
| 8 | F | 29 | 3 | 4 | 30 | 40 | 11 | 13 | 75 | 90 | 22 |
| 9 | F | 57 | 4 | 3 | 69 | 80 | 11 | 16 | 10 | 50 | 22 |
| Patient | SFOF_SAI | IFOF_SAI | SLF_SAI | ILF_SAI | Unc_SAI | Cing_SAI |
|---|---|---|---|---|---|---|
| 1 | −0.037 | −0.052 | −0.149 | −0.080 | 0.039 | 0.039 |
| 2 | −0.250 | 0.003 | −0.088 | −0.497 | −0.007 | −0.007 |
| 3 | −0.070 | 0.011 | 0.270 | 0.117 | 0.221 | −0.143 |
| 4 | 0.094 | −0.079 | −0.170 | 0.074 | 1.200 | −0.205 |
| 5 | 0.265 | −0.011 | −0.128 | 0.272 | −0.535 | 0.036 |
| 6 | −0.180 | −0.078 | −0.348 | 0.074 | −0.344 | 0.127 |
| 7 | 0.006 | −0.003 | 0.223 | 0.000 | −0.201 | −0.249 |
| 8 | 0.014 | 0.181 | 0.174 | 0.204 | −0.063 | 0.061 |
| 9 | −0.003 | −0.065 | 0.211 | −0.054 | −0.013 | −0.256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordón Guerra, R.; Peñate Castro, W.; Ferreiro Díaz-Velis, E.; Sosa Pérez, C.; Bisshopp Alfonso, S.; Hernández Hernández, M.; Hernández Fleta, J.L.; Morera Molina, J. Tractography-Based Asymmetries in Acquired Brain Injury: Contributions to the Neuropsychological Profile and Rehabilitation in a Case-Series. Brain Sci. 2025, 15, 1155. https://doi.org/10.3390/brainsci15111155
Bordón Guerra R, Peñate Castro W, Ferreiro Díaz-Velis E, Sosa Pérez C, Bisshopp Alfonso S, Hernández Hernández M, Hernández Fleta JL, Morera Molina J. Tractography-Based Asymmetries in Acquired Brain Injury: Contributions to the Neuropsychological Profile and Rehabilitation in a Case-Series. Brain Sciences. 2025; 15(11):1155. https://doi.org/10.3390/brainsci15111155
Chicago/Turabian StyleBordón Guerra, Rosario, Wenceslao Peñate Castro, Eilin Ferreiro Díaz-Velis, Coralia Sosa Pérez, Sara Bisshopp Alfonso, María Hernández Hernández, José Luis Hernández Fleta, and Jesús Morera Molina. 2025. "Tractography-Based Asymmetries in Acquired Brain Injury: Contributions to the Neuropsychological Profile and Rehabilitation in a Case-Series" Brain Sciences 15, no. 11: 1155. https://doi.org/10.3390/brainsci15111155
APA StyleBordón Guerra, R., Peñate Castro, W., Ferreiro Díaz-Velis, E., Sosa Pérez, C., Bisshopp Alfonso, S., Hernández Hernández, M., Hernández Fleta, J. L., & Morera Molina, J. (2025). Tractography-Based Asymmetries in Acquired Brain Injury: Contributions to the Neuropsychological Profile and Rehabilitation in a Case-Series. Brain Sciences, 15(11), 1155. https://doi.org/10.3390/brainsci15111155







