Virtual Reality in the Neurorehabilitation of Patients with Idiopathic Parkinson’s Disease: Pilot Study
Abstract
1. Introduction
2. Materials and Methods
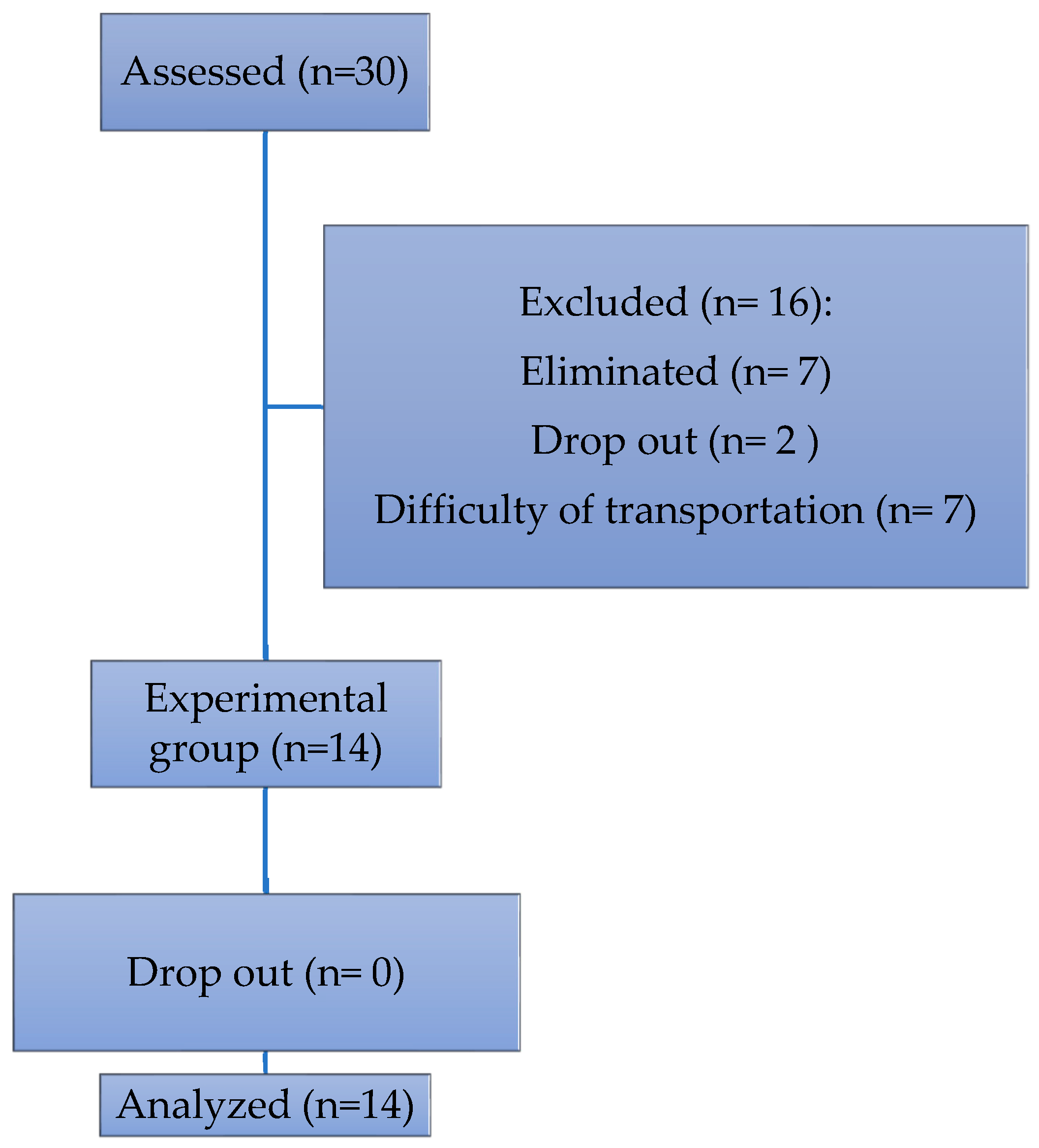
2.1. Sample
2.2. Procedure
2.3. Instruments and Materials
- -
- Axial index: Language, getting up from a chair, gait, posture, retropulsion test (postural stability);
- -
- Bradykinesia index: Facial expressions, index finger-thumb tapping, hand opening, pronosupination, lower limb agility, bradykinesia;
- -
- Stiffness index: Stiffness (neck, upper limbs (MMSS), lower limbs (MMII);
- -
- Tremor index: Rest tremors (MMII, MMSS), action tremors (MMSS).
2.4. Statistical Analysis
3. Results
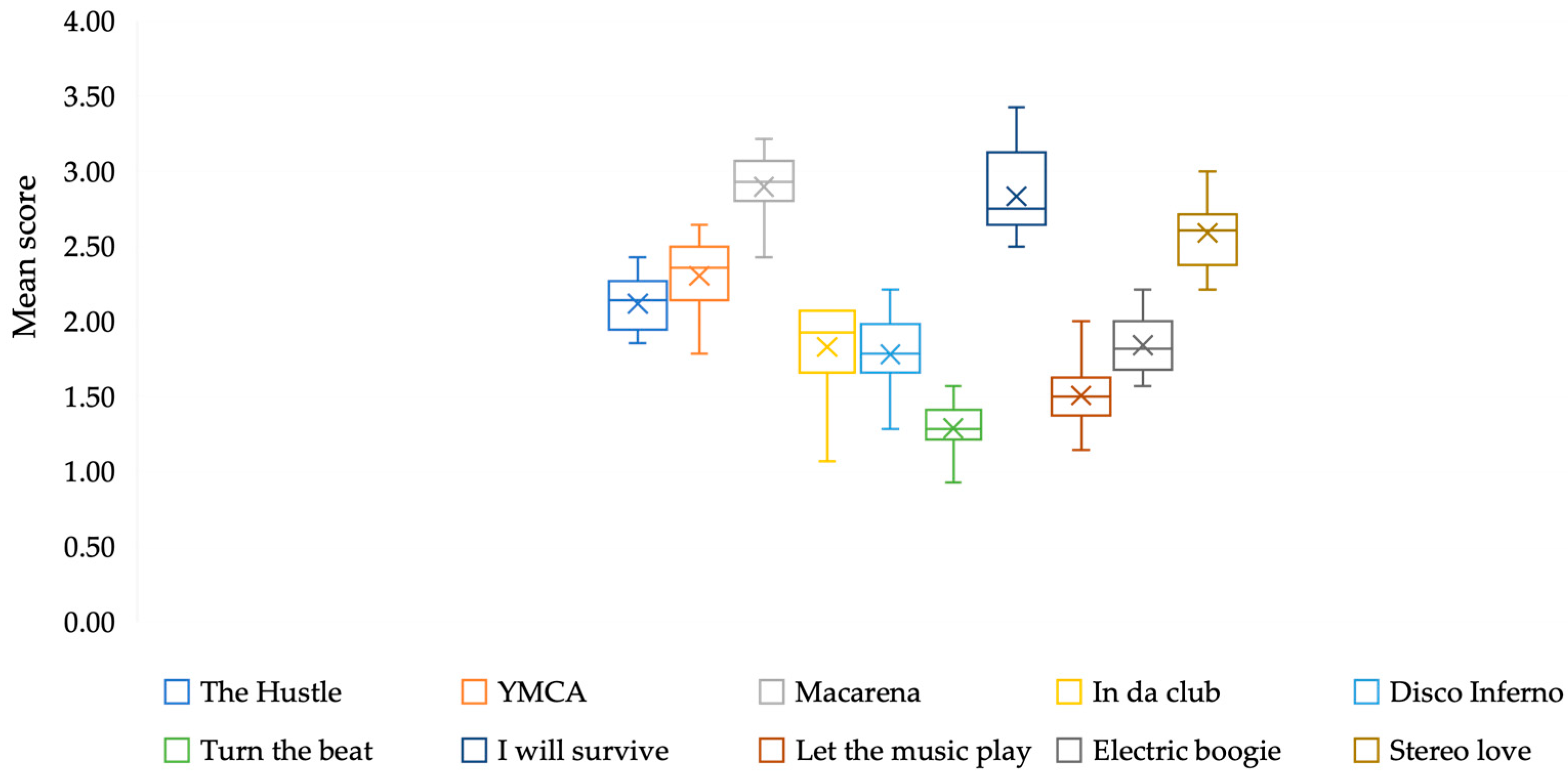
3.1. Sessions
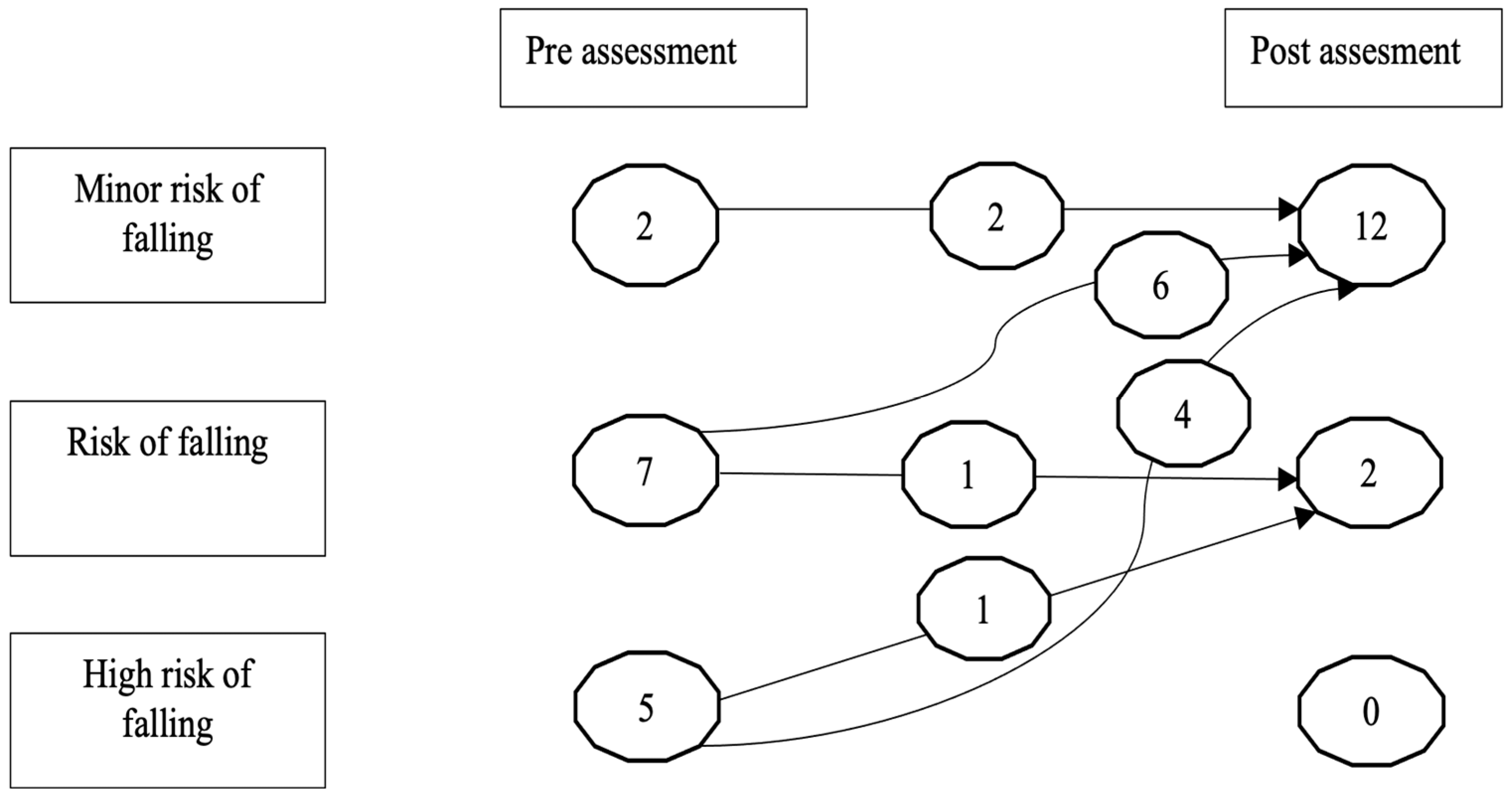
3.2. Gait
4. Discussion
5. Conclusions
Limitations of the Study and Suggestions for Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| RV | Virtual reality |
| UPDRS-III | Unified Parkinson’s Disease Scale |
| TUG | Timed Up and Go Test |
References
- WHO. Neurological Disorders: Public Health Challenges; WHO: Geneva, Switzerland, 2006.
- Kasten, M.; Chade, A.; Tanner, C.M. Epidemiology of Parkinson’s disease. Handb. Clin. Neurol. 2007, 83, 129–151. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016, 22 (Suppl. S1), S119–S122. [Google Scholar] [CrossRef]
- Dunalska, A.; Pikul, J.; Schok, K.; Wiejak, K.A.; Alster, P. The Significance of Vascular Pathogenesis in the Examination of Corticobasal Syndrome. Front. Aging Neurosci. 2021, 13, 668614. [Google Scholar] [CrossRef]
- Sonne, J.; Reddy, V.; Beato, M.R. Neuroanatomy, Substantia Nigra. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK536995/ (accessed on 10 October 2025).
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelonsin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Cardoso Suárez, T.; Álvarez González, C.R.; Díaz de la Fe, A.; Méndez Alonso, C.M.; Sabater Hernández, H.; Álvarez González, L.M. Gait disorders in Parkinson disease: Clinical, physiopathologic and therapeutical features. RCMFR 2017, 1, 131–146. [Google Scholar]
- Kearney, F.C.; Harwood, R.H.; Gladman, J.R.F.; Lincoln, N.; Masud, T. The relationship between executive function and falls and gait abnormalities in older adults: A systematic review. Dement. Geriatr. Cogn. Disord. 2013, 36, 20–35. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Marchese, R.; Avanzino, L.; Pelosin, E. Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Park. Relat. Disord. 2016, 22 (Suppl. S1), S60–S64. [Google Scholar] [CrossRef]
- da Silva, F.C.; Iop, R.D.R.; de Oliveira, L.C.; Boll, A.M.; de Alvarenga, J.G.S.; Gutierres Filho, P.J.B.; de Melo, L.M.A.B.; Xavier, A.J.; da Silva, R. Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: A systematic review of randomized controlled trials of the last 10 years. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef]
- Ventura, M.I.; Barnes, D.E.; Ross, J.M.; Lanni, K.E.; Sigvardt, K.A.; Disbrow, E.A. A pilot study to evaluate multi-dimensional effects of dance for people with Parkinson’s disease. Contemp. Clin. Trials 2016, 51, 50–55. [Google Scholar] [CrossRef]
- Lee, N.Y.; Lee, D.K.; Song, H.S. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J. Phys. Ther. Sci. 2015, 27, 145–147. [Google Scholar] [CrossRef]
- Hazamy, A.A.; Horne, S.A.; Okun, M.S.; Hass, C.J.; Altmann, L.J. Effects of a Cycling Dual Task on Emotional Word Choice in Parkinson’s Disease. J. Speech Lang. Hear. Res. 2019, 62, 1951–1958. [Google Scholar] [CrossRef]
- Mancilla, E.; Valenzuela, J.H.; Escobar, M. Performance in the Timed Up and Go and Unipodal Station tests in Chilean adults aged between 60 and 89. Rev. Med. Chile 2015, 143, 39–46. [Google Scholar]
- Nocera, J.R.; Stegemoller, E.L.; Malaty, I.A.; Okun, M.S.; Marsiske, M.; Hass, C.J. National Parkinson Foundation Quality Improvement Initiative Investigators. Using the Timed Up with Go Test in a Clinical Setting to predict falling in Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2013, 94, 1300–1305. [Google Scholar] [CrossRef]
- Vega Elguézabal, J.F.; Díaz de León González, E.; Barragán Berlanga, A.J.; Méndez Lozano, D.H. A Tinetti score of 24 points or less is a factor associated with falls in geriatric patients. Avances 2010, 7, 31–40. [Google Scholar]
- Kegelmeyer, D.A.; Kloos, A.D.; Thomas, K.M.; Kostyk, S.K. Reliability and Validity of the Tinetti Mobility Test for Individuals with Parkinson Disease. Phys. Ther. 2007, 87, 1369–1378. [Google Scholar] [CrossRef]
- Evans, J.R.; Mason, S.L.; Williams-Gray, C.H.; Foltynie, T.; Trotter, M.; Barker, R.A. The factor structure of the UPDRS as an index of disease progression in Parkinson’s disease. J. Park. Dis. 2011, 1, 75–82. [Google Scholar] [CrossRef]
- Rodríguez-Violante, M.; Cervantes-Arriaga, A. The Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Clinical application and research. Arch. Neurocien. 2014, 19, 157–163. [Google Scholar] [CrossRef]
- Bryant, M.S.; Hou, J.G.; Collins, R.L.; Protas, E.J. Contribution of Axial Motor Impairment to Physical Inactivity in Parkinson Disease. Am. J. Phys. Med. Rehabil. 2016, 95, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Navarro, S.G.; Mimenza-Alvarado, A.J.; Palacios-García, A.A.; Samudio-Cruz, A.; Gutiérrez-Gutiérrez, L.A.; Ávila-Funes, J.A. Validity and Reliability of the Spanish Version of the Montreal Cognitive Assessment (MoCA) for the Detection of Cognitive Impairment in Mexico. Rev. Colomb. Psiquiatr. (Engl. Ed.) 2018, 47, 237–243. [Google Scholar] [CrossRef]
- Arrieta, J.; Aguerrebere, M.; Raviola, G.; Flores, H.; Elliott, P.; Espinosa, A.; Reyes, A.; Ortiz-Panozo, E.; Rodríguez-Gutierrez, E.G.; Mukherjee, J.; et al. Validity and Utility of the Patient Health Questionnaire (PHQ)-2 and PHQ-9 for Screening and Diagnosis of Depression in Rural Chiapas, Mexico: A Cross-Sectional Study. J. Clin. Psychol. 2017, 73, 1076–1090. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Elena, P.; Demetris, S.; Christina, M.; Marios, P. Differences Between Exergaming Rehabilitation and Conventional Physiotherapy on Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 683385. [Google Scholar] [CrossRef]
- Carvalho, A.; Barbirato, D.; Araujo, N.; Martins, J.V.; Cavalcanti, J.L.; Santos Tony, M.; Coutinho, E.S.; Laks, J.; Deslandes, A.C. Comparison of strength training, aerobic training, and additional physical therapy as supplementary treatments for Parkinson’s disease: Pilot study. Clin. Interv. Aging 2015, 10, 183–191. [Google Scholar] [CrossRef]
- Tillmann, A.C.; Andrade, A.; Swarowsky, A.; Guimarães, A.C.A. Brazilian Samba Protocol for Individuals with Parkinson’s Disease: A Clinical Non-Randomized Study. JMIR Res. Protoc. 2017, 6, e129. [Google Scholar] [CrossRef]
- de Natale, E.R.; Paulus, K.S.; Aiello, E.; Sanna, B.; Manca, A.; Sotgiu, G.; Leali, P.T.; Deriu, F. Dance therapy improves motor and cognitive functions in patients with Parkinson’s disease. NeuroRehabilitation 2017, 40, 141–144. [Google Scholar] [CrossRef]
- van der Kolk, N.M.; Overeem, S.; de Vries, N.M.; Kessels, R.P.; Donders, R.; Brouwer, M.; Berg, D.; Post, B.; Bloem, B.R. Design of the Park-in-Shape study: A phase II double blind randomized controlled trial evaluating the effects of exercise on motor and non-motor symptoms in Parkinson’s disease. BMC Neurol. 2015, 15, 56. [Google Scholar] [CrossRef]
- Cikajlo, I.; Hukić, A.; Dolinšek, I.; Zajc, D.; Vesel, M.; Krizmanič, T.; Blažica, B.; Biasizzo, A.; Novak, F.; Peterlin Potisk, K. Can telerehabilitation games lead to functional improvement of upper extremities in individuals with Parkinson’s disease? Int. J. Rehabil. Res. 2018, 41, 230–238. [Google Scholar] [CrossRef]
- Fu, A.S.; Gao, K.L.; Tung, A.K.; Tsang, W.W.; Kwan, M.M. Effectiveness of Exergaming Training in Reducing Risk and Incidence of Falls in Frail Older Adults with a History of Falls. Arch. Phys. Med. Rehabil. 2015, 96, 2096–2102. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Postuma, R.B. Subtypes of Parkinson’s Disease: What Do They Tell Us About Disease Progression? Curr. Neurol. Neurosci. Rep. 2017, 17, 34. [Google Scholar] [CrossRef]
- Foster, E.R.; Golden, L.; Duncan, R.P.; Earhart, G.M. A community-based Argentine tango dance program is associated with increased activity participation among individuals with Parkinson’s disease. Arch. Phys. Med. Rehabil. 2013, 94, 240–249. [Google Scholar] [CrossRef]
- Delextrat, A.; Bateman, J.; Esser, P.; Targen, N.; Dawes, H. The potential benefits of Zumba Gold® in people with mild-to-moderate Parkinson’s: Feasibility and effects of dance styles and number of sessions. Complement. Ther. Med. 2016, 27, 68–73. [Google Scholar] [CrossRef]
| n | % | |
|---|---|---|
| Gender | ||
| Female | 9 | 64.3 |
| Male | 5 | 35.7 |
| Symptom onset side | ||
| Left | 6 | 42.9 |
| Right | 8 | 57.1 |
| H and Y | ||
| Unilateral symptoms (I) | 1 | 7.1 |
| Bilateral mild symptoms (II) | 3 | 21.4 |
| Mild–moderate bilateral symptoms (III) | 10 | 71.5 |
| M | SD | |
| Age | 68.43 | 8.1 |
| Years of study | 10.92 | 4.74 |
| Disease duration (years) | 9.93 | 7.71 |
| Dyskinesias | ||
| Yes | 5 | 35.7 |
| No | 9 | 64.3 |
| Dyskinesias duration (years) | 1.79 | 3.09 |
| MoCA | 26.31 | 1.97 |
| Results | Pre (N = 14) | Post (N = 14) | |||||
|---|---|---|---|---|---|---|---|
| M (SD) | CI (95%) | M (DS) | CI (95%) | Z | Sig. | Cohen d | |
| Gait | |||||||
| TUG | 13.14 (5.43) | (10.01, 16.28) | 11.21 (2.86) | (9.56, 12.87) | −2.003 | 0.001 | 0.53 |
| Tinetti: balance | 12.14 (2.98) | (10.42, 13.87) | 15.07 (1.44) | (14.24, 15.90) | −2.966 | 0.001 | 0.79 |
| Tinetti: gait | 7.50 (2.18) | (6.24, 8.76) | 11.71 (0.73) | (11.30, 12.13) | −3.316 | 0.001 | 0.89 |
| Total Tinetti Test Score | 19.64 (4.63) | (16.97, 22.32) | 26.78 (1.89) | (25.70, 27.88) | −3.299 | 0.001 | 0.88 |
| UPDRS | |||||||
| Total Score | 26.00 (8.26) | (21.23, 30.77) | 20.00 (7.58) | (15.63, 24.37) | −3.047 | 0.002 | 0.82 |
| Rigidity: neck | 1.57 (1.02) | (0.99, 2.16) | 0.86 (0.66) | (0.47, 1.24) | −2.640 | 0.008 | 0.71 |
| Rigidity: right upper limb | 1.29 (0.83) | (0.81, 1.76) | 0.57 (0.51) | (0.28, 0.87) | −2.640 | 0.008 | 0.71 |
| Rigidity: left lower limb | 1.29 (0.83) | (0.81, 1.76) | 0.64 (0.84) | (0.16, 1.13) | −2.714 | 0.007 | 0.76 |
| Pull test | 1.14 (0.86) | (0.64, 1.64) | 0.79 (0.70) | (0.38, 1.19) | −2.236 | 0.025 | 0.60 |
| Axial index score | 4.86 (2.60) | (3.36, 6.36) | 3.57 (2.38) | (2.20, 4.94) | −1.975 | 0.048 | 0.53 |
| Bradykinesia index score | 13.29 (3.56) | (11.23, 15.34) | 12.14 (3.23) | (10.28, 14.01) | −1.980 | 0.048 | 0.53 |
| Rigidity index score | 6.29 (3.58) | (4.22, 8.35) | 3.29 (2.64) | (1.76, 4.81) | −2.952 | 0.003 | 0.79 |
| Total index score | 26.07 (8.13) | (21.38, 30.77) | 20.14 (7.54) | (15.79, 24.50) | −3.048 | 0.002 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Anguiano, D.A.; Rodríguez-Ortiz, U.; Chávez-Oliveros, M.; Paz-Rodríguez, F. Virtual Reality in the Neurorehabilitation of Patients with Idiopathic Parkinson’s Disease: Pilot Study. Brain Sci. 2025, 15, 1116. https://doi.org/10.3390/brainsci15101116
Delgado-Anguiano DA, Rodríguez-Ortiz U, Chávez-Oliveros M, Paz-Rodríguez F. Virtual Reality in the Neurorehabilitation of Patients with Idiopathic Parkinson’s Disease: Pilot Study. Brain Sciences. 2025; 15(10):1116. https://doi.org/10.3390/brainsci15101116
Chicago/Turabian StyleDelgado-Anguiano, Diana Alejandra, Ulises Rodríguez-Ortiz, Mireya Chávez-Oliveros, and Francisco Paz-Rodríguez. 2025. "Virtual Reality in the Neurorehabilitation of Patients with Idiopathic Parkinson’s Disease: Pilot Study" Brain Sciences 15, no. 10: 1116. https://doi.org/10.3390/brainsci15101116
APA StyleDelgado-Anguiano, D. A., Rodríguez-Ortiz, U., Chávez-Oliveros, M., & Paz-Rodríguez, F. (2025). Virtual Reality in the Neurorehabilitation of Patients with Idiopathic Parkinson’s Disease: Pilot Study. Brain Sciences, 15(10), 1116. https://doi.org/10.3390/brainsci15101116






