The Effects of a Novel Treatment for Hemianopic Dyslexia on Reading, Symptom Load, and Return to Work
Abstract
1. Introduction
- (1)
- How do individuals with HD perform in basic clinical reading tasks? How are paper-based reading and reading on a computer screen related to each other?
- (2)
- Does the novel reading treatment lead to stable and significant improvements within the reading test battery? Does it transfer to functional reading tasks and reduce symptom load related to reading?
- (3)
- How is vocational reintegration in individuals with HD?
2. Materials and Methods
2.1. Patient Recruitment
- Significant reading impairment in the first 2 reading tests of the test battery (see Table 2, tests 1 and 2).
- No evidence of pure alexia based on 3 tests for differential diagnosis (vertical reading task, letter-by-letter-reading, reading speed > 25 Words per Minute, see Section 2.2.2).
- No evidence of visual neglect (based on conventional neglect screening tests [33]. If it was unclear whether a field defect was caused by neglect we used the trunk-rotation method suggested by Nyffeler et al. [19] to disentangle real from pseudo visual field defects (the latter influenced by neglect).
- No premorbid (i.e., developmental) dyslexia according to medical records, nor dementia according to medical records, and no aphasia (based on the Aachen Aphasia Test AAT, [34]).
- Availability for regular treatments in the outpatient unit over a period of at least several weeks.
2.2. Assessments
2.2.1. Neuro-Visual Screening Tests
2.2.2. Differential Diagnosis of HD from Pure Alexia
2.2.3. Reading Test Battery and Subjective Symptom Load Questionnaire
Detailed Description of Reading Tests
Retest Reliability
2.3. Treatments
2.4. Design and Statistics
Patient Recruitment and Enrollment
3. Results
3.1. Basic Clinical Tests
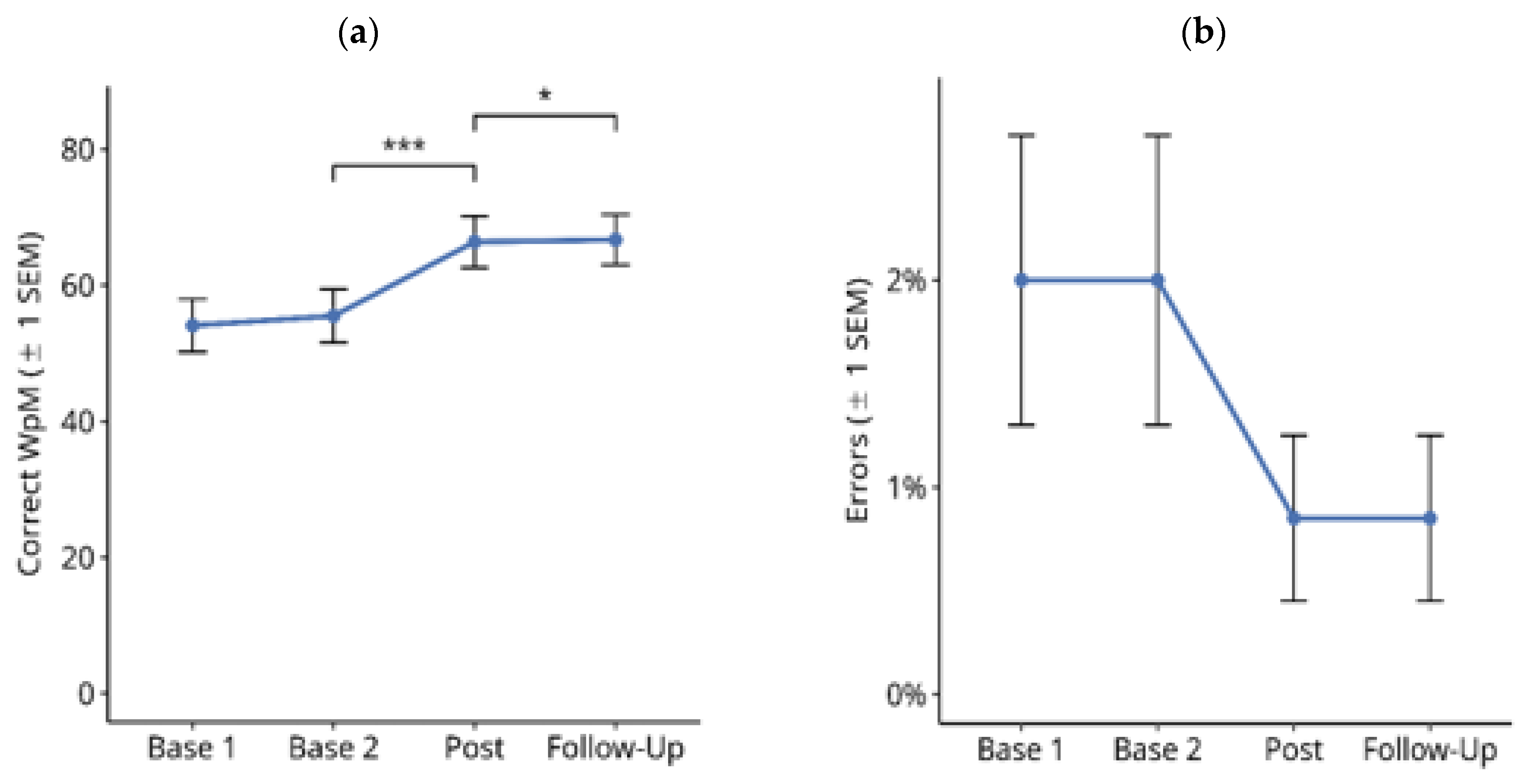
3.1.1. Reading Text on Paper
3.1.2. Reading Text on PC screen
3.1.3. Single-Word Reading on Computer Screen
3.2. Functional Reading Tests
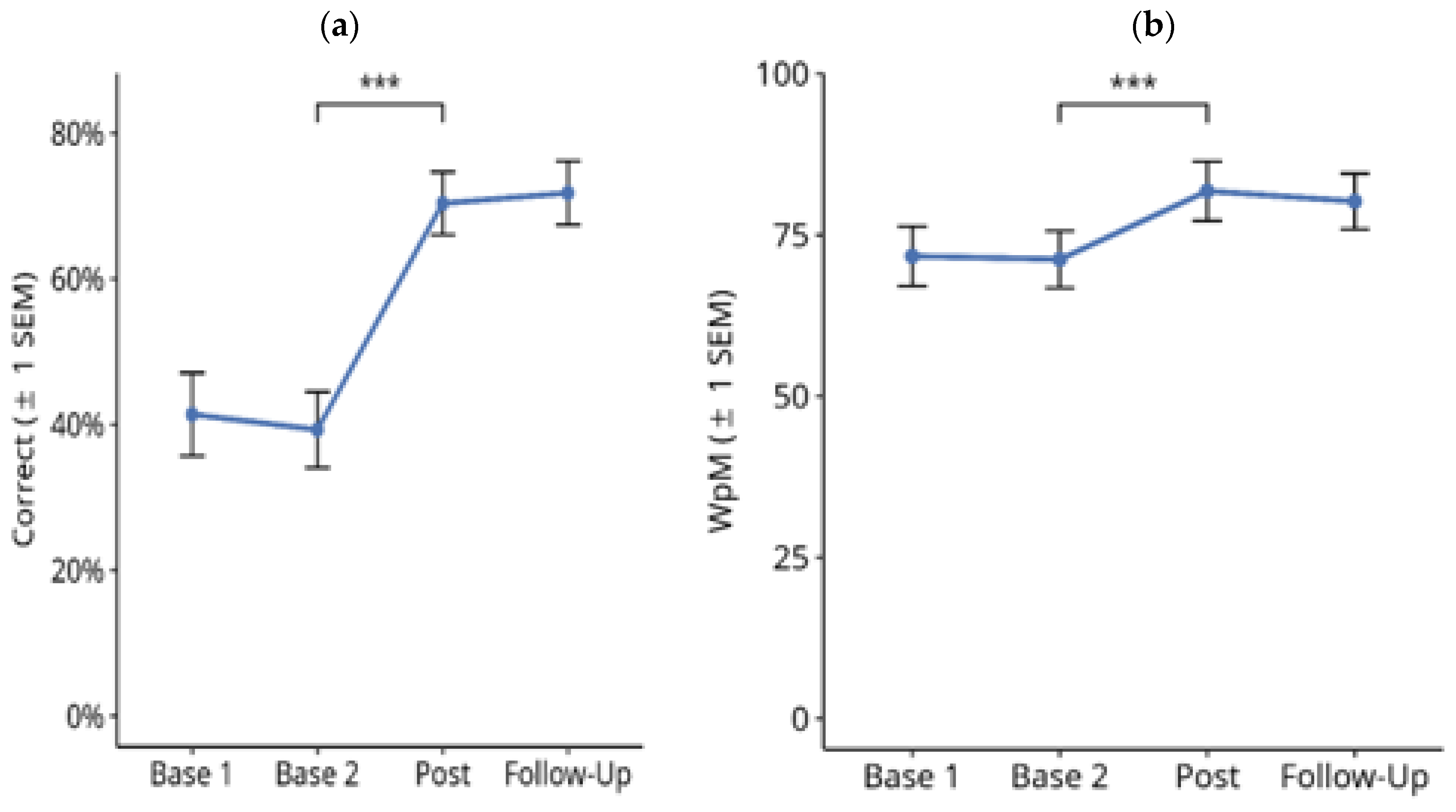
3.2.1. Text Memory
3.2.2. Finding Typing Errors
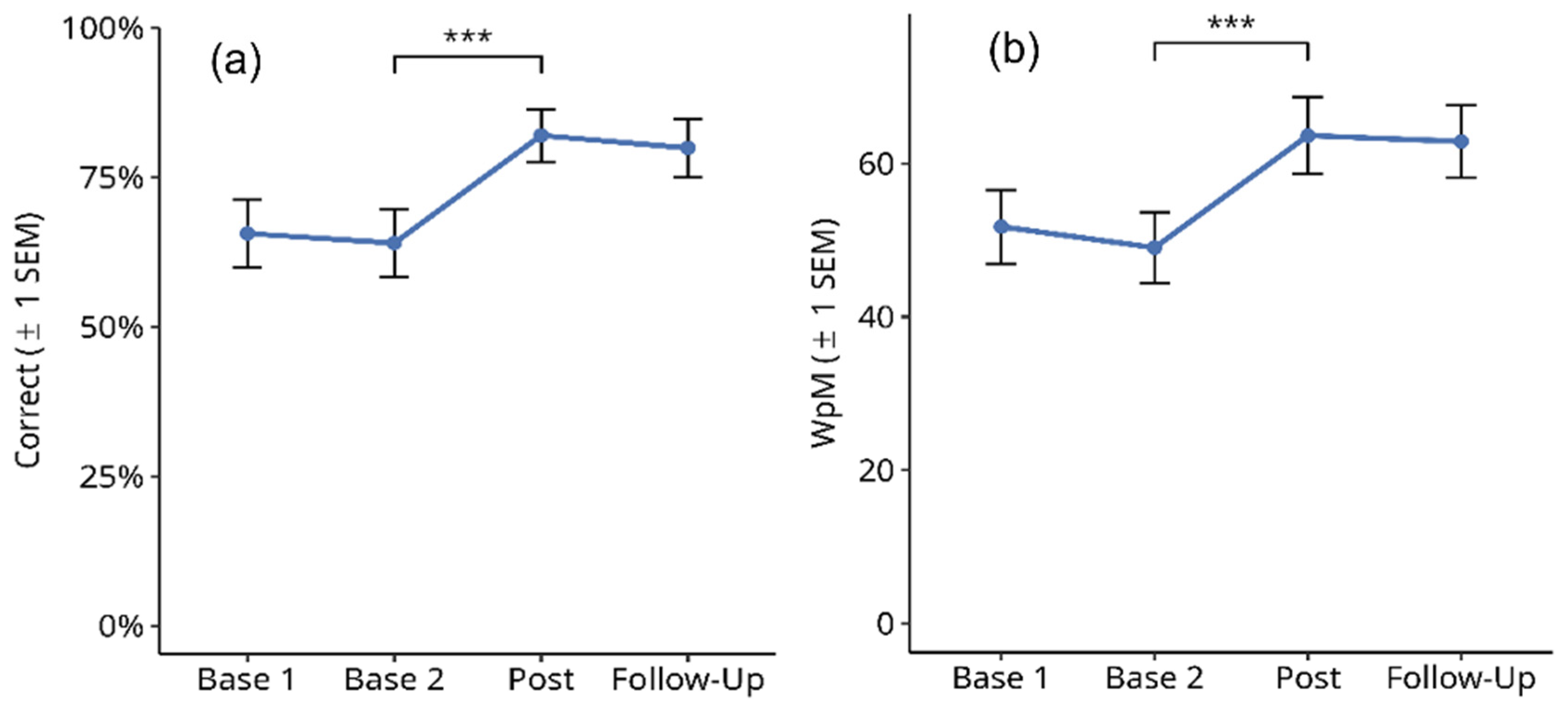
3.2.3. Phone Numbers
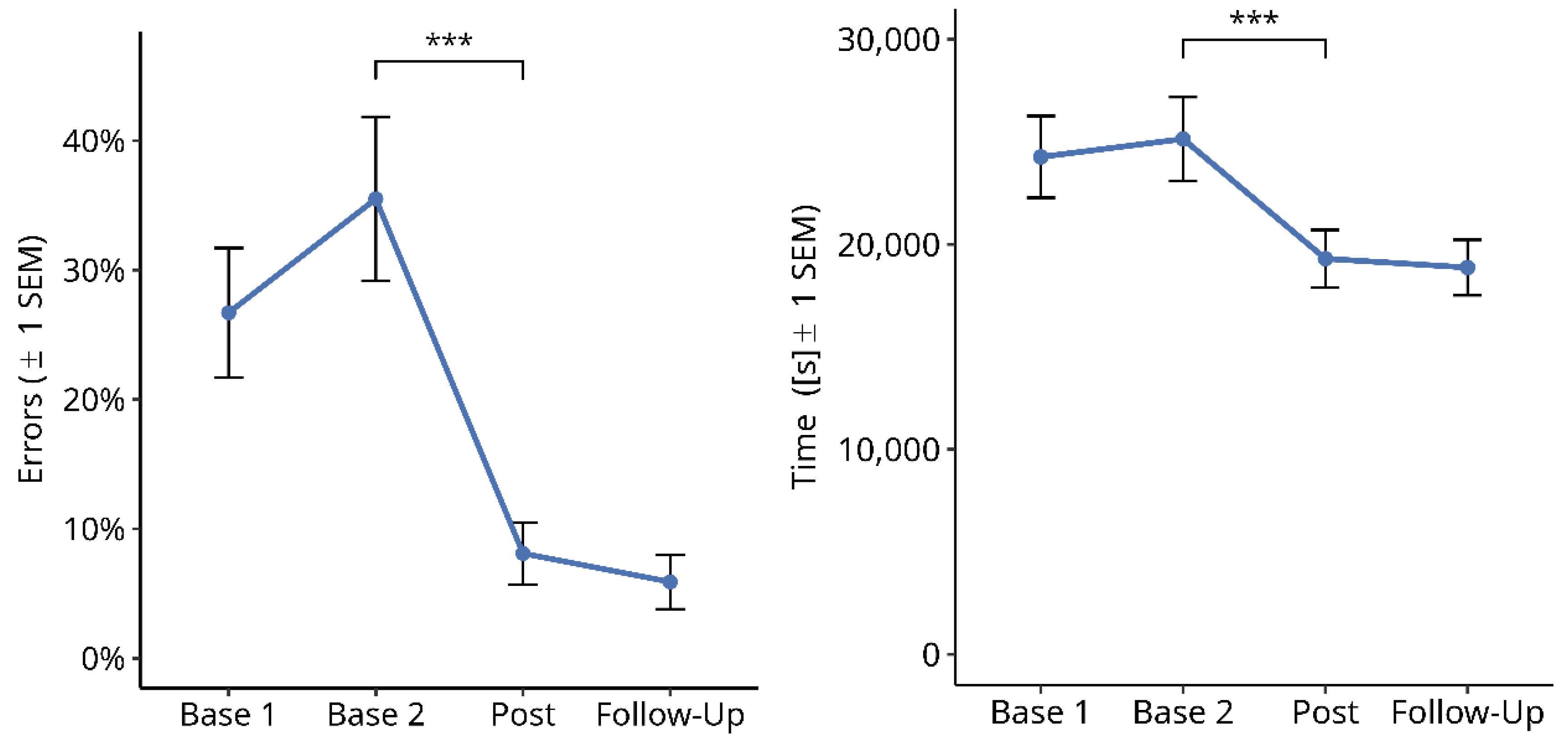
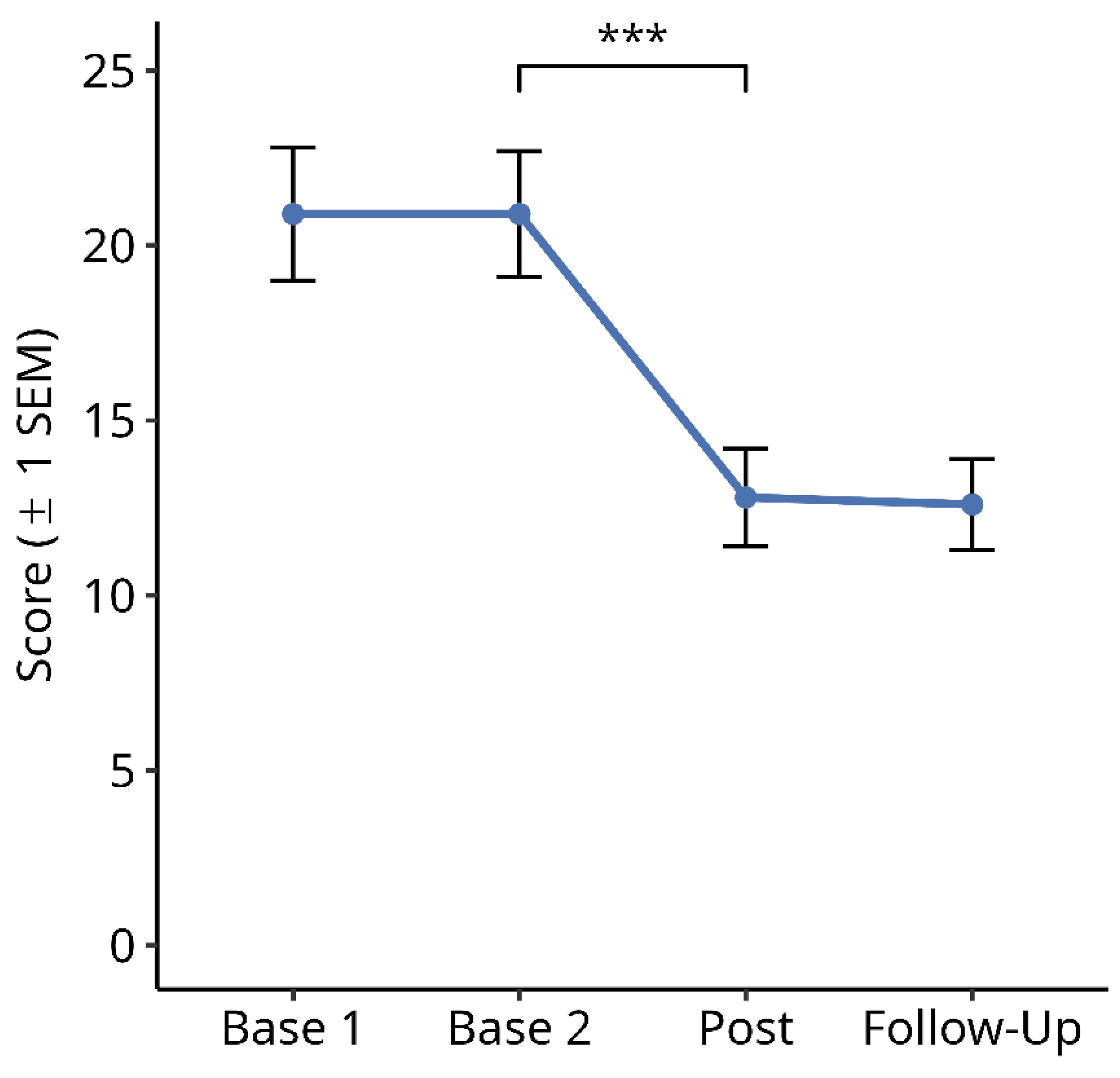
3.2.4. Subjective Symptom Load Questionnaire
3.2.5. Return to Work
3.3. Further Analyses
4. Discussion
4.1. Basic Clinical Reading Tests and Relationship between Paper and Screen Reading Tasks
4.2. Effects of Reading Therapy on Reading Tests and Possible Mechanisms
4.3. Effects on Subjective Symptom Load, Treatment Intensity and Return to Work
5. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- de Haan, G.A.; Heutink, J.; Melis-Dankers, B.J.M.; Tucha, O.; Brouwer, W.H. Spontaneous recovery and treatment effects in patients with homonymous visual field defects: A meta-analysis of existing literature in terms of the ICF framework. Surv. Ophthalmol. 2014, 59, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, G. Restorative and compensatory therapy approaches in cerebral blindness—A review. Restor. Neurol. Neurosci. 1999, 15, 255–271. [Google Scholar] [PubMed]
- Rowe, F.; Brand, D.; Jackson, C.A.; Price, A.; Walker, L. Visual impairment following stroke: Do stroke patients require vision assessment? Age Ageing 2009, 38, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kedar, S.; Lynn, M.J.; Newman, N.J.; Biousse, V. Homonymous hemianopia in stroke. J. Neuroophthalmol. 2006, 26, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kedar, S.; Lynn, M.J.; Newman, N.J.; Biousse, V. Homonymous hemianopias: Clinical-anatomic correlations in 904 cases. Neurology 2006, 66, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, M.B.; Johansson, J.; Ygge, J.; Borg, K. Vision-Related Symptoms after Acquired Brain Injury and the Association with Mental Fatigue, Anxiety and Depression. J. Rehabil. Med. 2019, 51, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Bruce, B.B.; Zhang, X.; Kedar, S.; Newman, N.J.; Biousse, V. Traumatic homonymous hemianopia. J. Neurol. Neurosurg. Psychiatry 2006, 77, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kedar, S.; Lynn, M.J.; Newman, N.J.; Biousse, V. Natural history of homonymous hemianopia. Neurology 2006, 66, 901–905. [Google Scholar] [CrossRef]
- Machner, B.; Sprenger, A.; Sander, T.; Heide, W.; Kimmig, H.; Helmchen, C.; Kömpf, D. Visual search disorders in acute and chronic homonymous hemianopia. Ann. N. Y. Acad. Sci. 2009, 1164, 419–426. [Google Scholar] [CrossRef]
- Schuett, S. The rehabilitation of hemianopic dyslexia. Nat. Rev. Neurol. 2009, 5, 427–437. [Google Scholar] [CrossRef]
- Baier, B.; Mueller, N.; Fechir, M.; Dieterich, M. Line bisection error and its anatomic correlate. Stroke 2010, 41, 1561–1563. [Google Scholar] [CrossRef][Green Version]
- Savir, H.; Michelson, I.; David, C.; Mendelson, L.; Najenson, T. Homonymous hemianopsia and rehabilitation in fifteen cases of C.C.I. Scand. J. Rehab. Med. 1977, 9, 151–153. [Google Scholar]
- Neumann, G.; Schaadt, A.K.; Reinhart, S.; Kerkhoff, G. Clinical and Psychometric Evaluations of the Cerebral Vision Screening Questionnaire in 461 Nonaphasic Individuals Poststroke. Neurorehabil. Neural Repair 2016, 30, 187–198. [Google Scholar] [CrossRef]
- McConkie, G.W.; Rayner, K. The span of the effective stimulus during a fixation in reading. Percept. Psychophys. 1975, 17, 578–586. [Google Scholar] [CrossRef]
- Pflugshaupt, T.; Gutbrod, K.; Wurtz, P.; von Wartburg, R.; Nyffeler, T.; de Haan, B.; Karnath, H.O.; Mueri, R.M. About the role of visual field defects in pure alexia. Brain 2009, 132, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, S.; Keller, I.; Kerkhoff, G. Effects of head rotation on space- and word-based reading errors in spatial neglect. Neuropsychologia 2010, 48, 3706–3714. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, S.; Schaadt, A.K.; Adams, M.; Leonhardt, E.; Kerkhoff, G. The frequency and significance of the word length effect in neglect dyslexia. Neuropsychologia 2013, 51, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Arduino, L.S.; Vallar, G.; Burani, C. Left neglect dyslexia and the effect of stimulus duration. Neuropsychologia 2005, 44, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Nyffeler, T.; Paladini, R.E.; Hopfner, S.; Job, O.; Nef, T.; Pflugshaupt, T.; Vanbellingen, T.; Bohlhalter, S.; Muri, R.M.; Kerkhoff, G.; et al. Contralesional Trunk Rotation Dissociates Real vs. Pseudo-Visual Field Defects due to Visual Neglect in Stroke Patients. Front. Neurol. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, G.; Munsinger, U.; Eberle-Strauss, G.; Stogerer, E. Rehabilitation of hemianopic alexia in patients with postgeniculate visual field disorders. Neuropsychol. Rehabil. 1992, 2, 21–42. [Google Scholar] [CrossRef]
- Leff, A.P.; Spitzyna, G.A.; Plant, G.T.; Wise, R.J. Structural anatomy of pure and hemianopic alexia. J. Neurosurg. Psychiatry 2006, 77, 1004–1007. [Google Scholar] [CrossRef]
- Aimola, L.; Lane, A.R.; Smith, D.T.; Kerkhoff, G.; Ford, G.A.; Schenk, T. Efficacy and Feasibility of Home-Based Training for Individuals with Homonymous Visual Field Defects. Neurorehabilit. Neural Repair 2014, 28, 207–218. [Google Scholar] [CrossRef]
- Kerkhoff, G.; Rode, G.; Clark, S. Treating neurovisual deficits and spatial neglect. In Clinical Pathways in Neurorehabilitation; Platz, T., Ed.; Springer: Heidelberg, Germany, 2021; pp. 191–217. [Google Scholar]
- Pollatsek, A.; Bolozky, S.; Well, A.D.; Rayner, K. Asymmetries in the Perceptual Span for Israeli Readers. Brain Lang. 1981, 14, 174–180. [Google Scholar] [CrossRef]
- Jordan, T.R.; Almabruk, A.A.A.; Gadalla, E.A.; McGowan, V.A.; White, S.J.; Abedipour, L.; Paterson, K.B. Reading direction and the central perceptual span: Evidence from Arabic and English. Psychon. Bull. Rev. 2014, 21, 505–511. [Google Scholar] [CrossRef]
- Paterson, K.B.; McGowan, V.A.; White, S.J.; Malik, S.; Abedipour, L.; Jordan, T.R. Reading Direction and the Central Perceptual Span in Urdu and English. PLoS ONE 2014, 9, e88358. [Google Scholar] [CrossRef]
- Kuester-Gruber, S.; Kabisch, P.; Cordey, A.; Karnath, H.O.; Trauzettel-Klosinski, S. Training of vertical versus horizontal reading in patients with hemianopia—A randomized and controlled study. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 745–757. [Google Scholar] [CrossRef]
- Hebel, N.; Cramon, D.V. Der Posteriorinfarkt. Fortschritte Neurol. Psychiatr. 1987, 55, 37–53. [Google Scholar] [CrossRef]
- Leff, A.P.; Scott, S.K.; Crewes, H.; Hodgson, T.L.; Cowey, A.; Howard, D.; Wise, R.J.S. Impaired reading in patients with right hemianopia. Ann. Neurol. 2000, 47, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Cramon, D.Y.V.; Hebel, N.; Schuri, U. Verbal memory and learning in unilateral posterior cerebral infarction. Brain 1988, 111, 1061–1077. [Google Scholar] [CrossRef]
- Pollock, A.; Hazelton, C.; Henderson, C.A.; Angilley, J.; Dhillon, B.; Langhorne, P.; Livingstone, K.; Munro, F.A.; Orr, H.; Rowe, F.J.; et al. Interventions for visual field defects in patients with stroke. Cochrane Database Syst. Rev. 2011, 43, e37–e38. [Google Scholar] [CrossRef]
- Pollock, A.; Hazelton, C.; Rowe, F.J.; Jonuscheit, S.; Kernohan, A.; Angilley, J.; Henderson, C.A.; Langhorne, P.; Campbell, P. Interventions for visual field defects in people with stroke. Cochrane Database Syst. Rev. 2019, ARTN CD008388. [Google Scholar] [CrossRef]
- Kerkhoff, G.; Reinhart, S.; Ziegler, W.; Artinger, F.; Marquardt, C.; Keller, I. Smooth pursuit eye movement training promotes recovery from auditory and visual neglect: A randomized controlled study. Neurorehabilit. Neural Repair 2013, 27, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Poeck, K.; Weniger, D.; Willmes, K. Der Aachener Aphasie Test (AAT); Verlag für Psychologie, Hogrefe: Göttingen, Germany, 1983. [Google Scholar]
- Kerkhoff, G.; Marquardt, C. EYEMOVE—Standardisierte Analyse und Therapie visueller Explorationsstörungen. Nervenarzt 2009, 80, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, G.; Marquardt, C. Visually based reading disorders after brain damage: Standardised assessment and treatment with READ. Nervenarzt 2009, 80, 1424–1439. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, S.; Höfer, B.; Kerkhoff, G. Visuell bedingte Lesestörungen nach erworbener Hirnschädigung: Therapie. Sprache-Stimme-Gehör 2013, 37, 105–111. [Google Scholar] [CrossRef]
- Rubin, G.S.; Turano, K. Reading without saccadic eye movements. Vis. Res. 1992, 32, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K. The gaze-contingent moving window in reading: Development and review. Vis. Cogn. 2014, 22, 242–258. [Google Scholar] [CrossRef]
- Yong, K.; Rajdev, K.; Warrington, E.; Nicholas, J.; Warren, J.; Crutch, S. A longitudinal investigation of the relationship between crowding and reading: A neurodegenerative approach. Neuropsychologia 2016, 85, 127–136. [Google Scholar] [CrossRef]
- Berends, H.I.; Nijlant, J.M.; Movig, K.L.; Van Putten, M.J.; Jannink, M.J.; Ijzerman, M.J. The clinical use of drugs influencing neurotransmitters in the brain to promote motor recovery after stroke; a Cochrane systematic review. Eur. J. Phys. Rehabil. Med. 2009, 45, 621–630. [Google Scholar]
- Luvizutto, G.J.; Bazan, R.; Braga, G.P.; Resende, L.A.; Bazan, S.G.; El Dib, R. Pharmacological interventions for unilateral spatial neglect after stroke. Cochrane Database Syst. Rev. 2015, CD010882. [Google Scholar] [CrossRef]
- Spitzyna, G.A.; Wise, R.J.; McDonald, S.A.; Plant, G.T.; Kidd, D.; Crewes, H.; Leff, A.P. Optokinetic therapy improves text reading in patients with hemianopic alexia: A controlled trial. Neurology 2007, 68, 1922–1930. [Google Scholar] [CrossRef]
- Virgili, G.; Acosta, R.; Grover, L.L.; Bentley, S.A.; Giacomelli, G. Reading aids for adults with low vision. Cochrane Database Syst. Rev. 2013, 10, CD003303. [Google Scholar] [CrossRef]
- Kaltenegger, K.; Kuester, S.; Altpeter-Ott, E.; Eschweiler, G.W.; Cordey, A.; Ivanov, I.V.; Martus, P.; Knipp, C.; Trauzettel-Klosinski, S. Effects of home reading training on reading and quality of life in AMDa randomized and controlled study. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1499–1512. [Google Scholar] [CrossRef]
- Weicker, J.; Villringer, A.; Thöne-Otto, A. Can Impaired Working Memory Functioning Be Improved By Training? A Meta-Analysis with a Special Focus on Brain Injured Patients. Neuropsychology 2016, 30, 190–212. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J. Neural bases of recovery after brain injury. J. Commun. Disord. 2011, 44, 515–520. [Google Scholar] [CrossRef]
- Kwakkel, G.; Wagenaar, R.C.; Twisk, J.W.; Lankhorst, G.J.; Koetsier, J.C. Intensity of leg and arm training after primary middle-cerebral-artery stroke: A randomised trial. Lancet 1999, 354, 191–196. [Google Scholar] [CrossRef]
- Chen, P.; Hreha, K.; Gonzalez-Snyder, C.; Rich, T.J.; Gillen, R.W.; Parrott, D.; Barrett, A.M. Impacts of Prism Adaptation Treatment on Spatial Neglect and Rehabilitation Outcome: Dosage Matters. Neurorehabil. Neural Repair 2022, 36, 500–513. [Google Scholar] [CrossRef]
- Breitenstein, C.; Grewe, T.; Floel, A.; Ziegler, W.; Springer, L.; Martus, P.; Huber, W.; Willmes, K.; Ringelstein, E.B.; Haeusler, K.G.; et al. Intensive speech and language therapy in patients with chronic aphasia after stroke: A randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet 2017, 389, 1528–1538. [Google Scholar] [CrossRef]
- Kerkhoff, G. Successful return to professional work after neglect, extinction, and spatial misperception—Three long-term case studies. Neuropsychol. Rehabil. 2021, 31, 837–862. [Google Scholar] [CrossRef] [PubMed]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ. 2021, 374, n2061. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.H.; Brown, M.M.; Robinson, P.; Plant, G.T.; Husain, M.; Leff, A.P. Read-Right: A “web app” that improves reading speeds in patients with hemianopia. J. Neurol. 2012, 259, 2611–2615. [Google Scholar] [CrossRef] [PubMed]
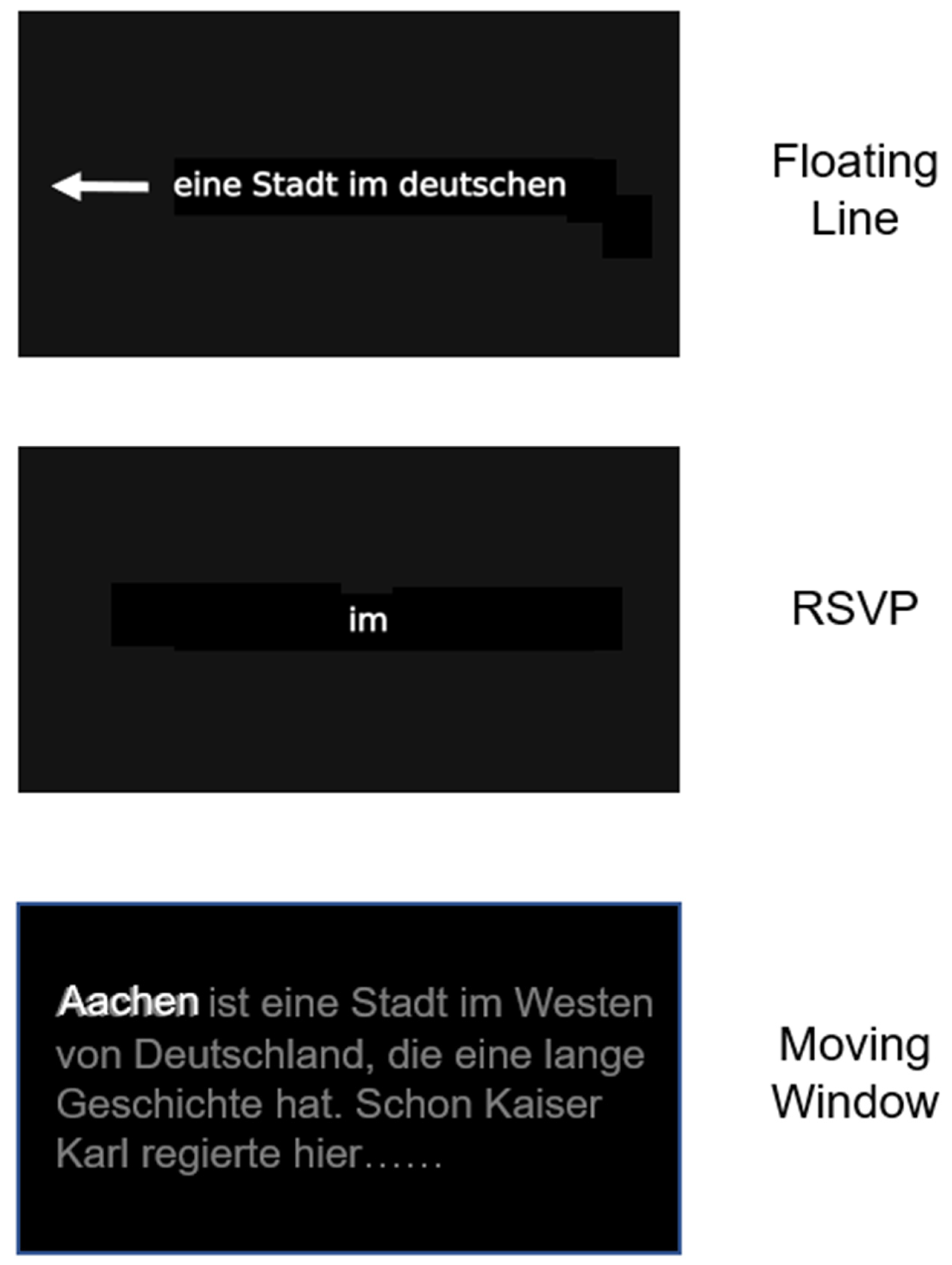
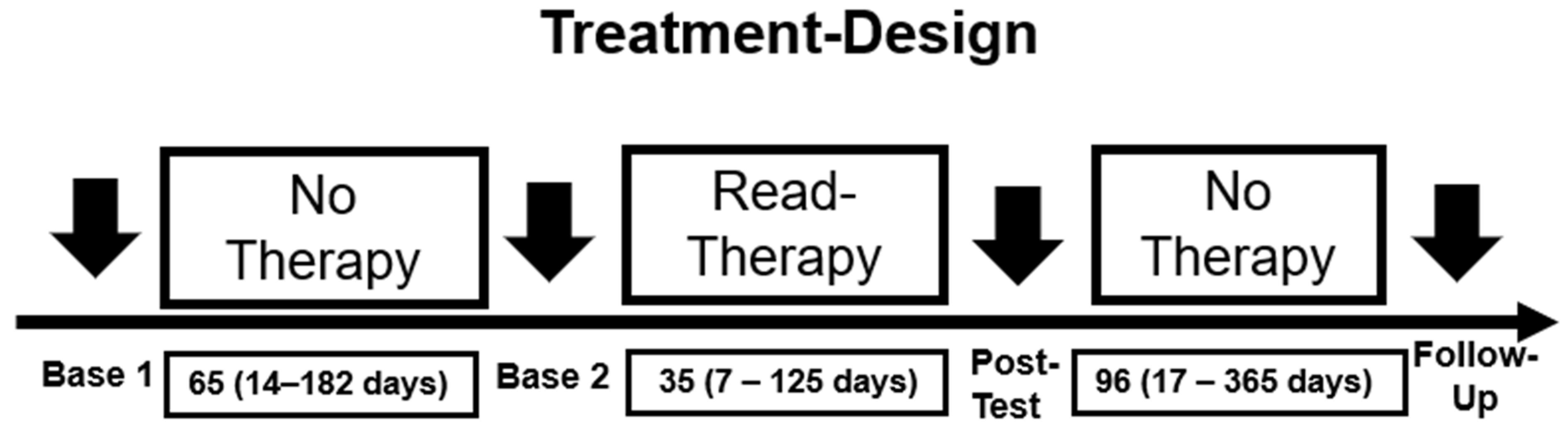
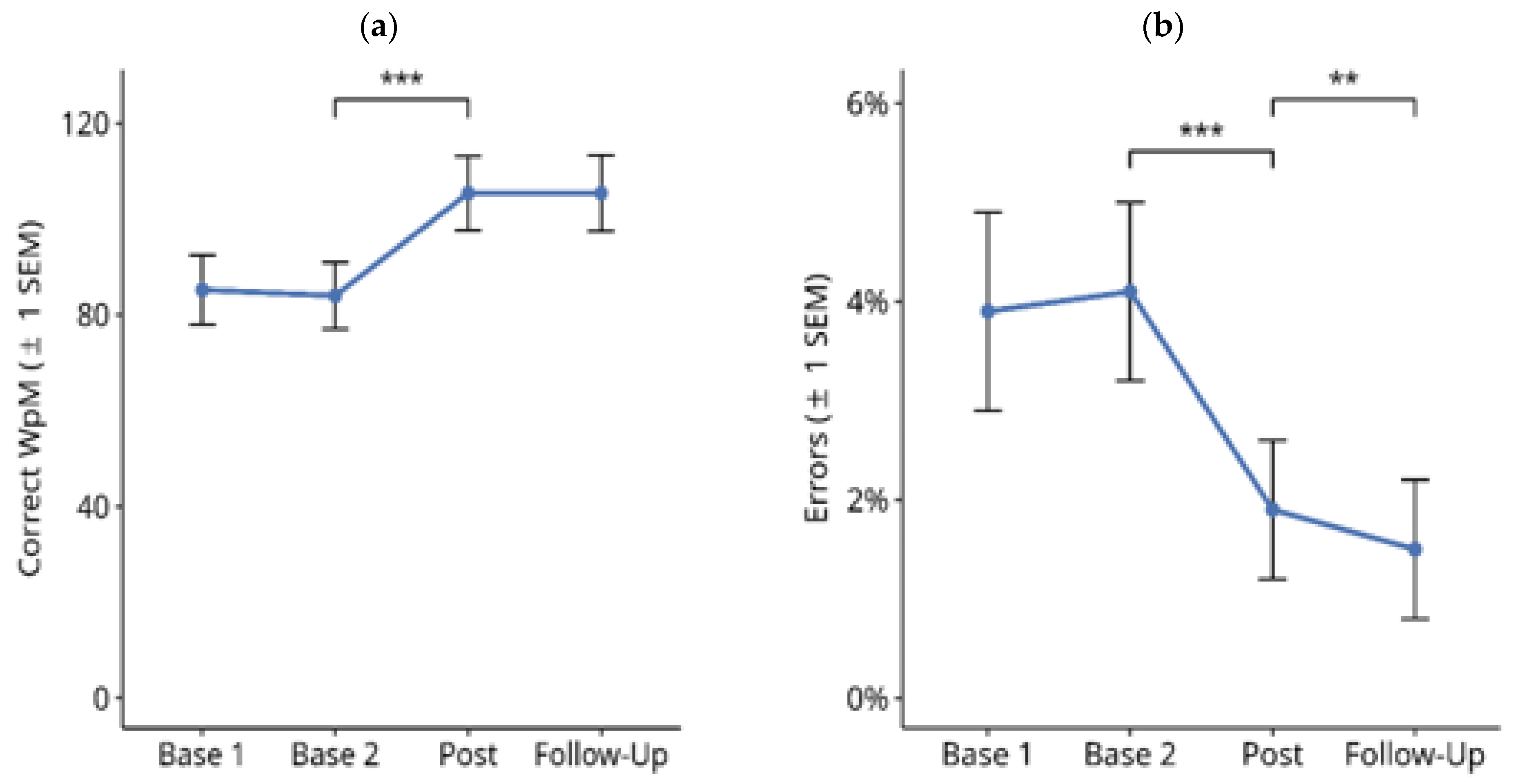
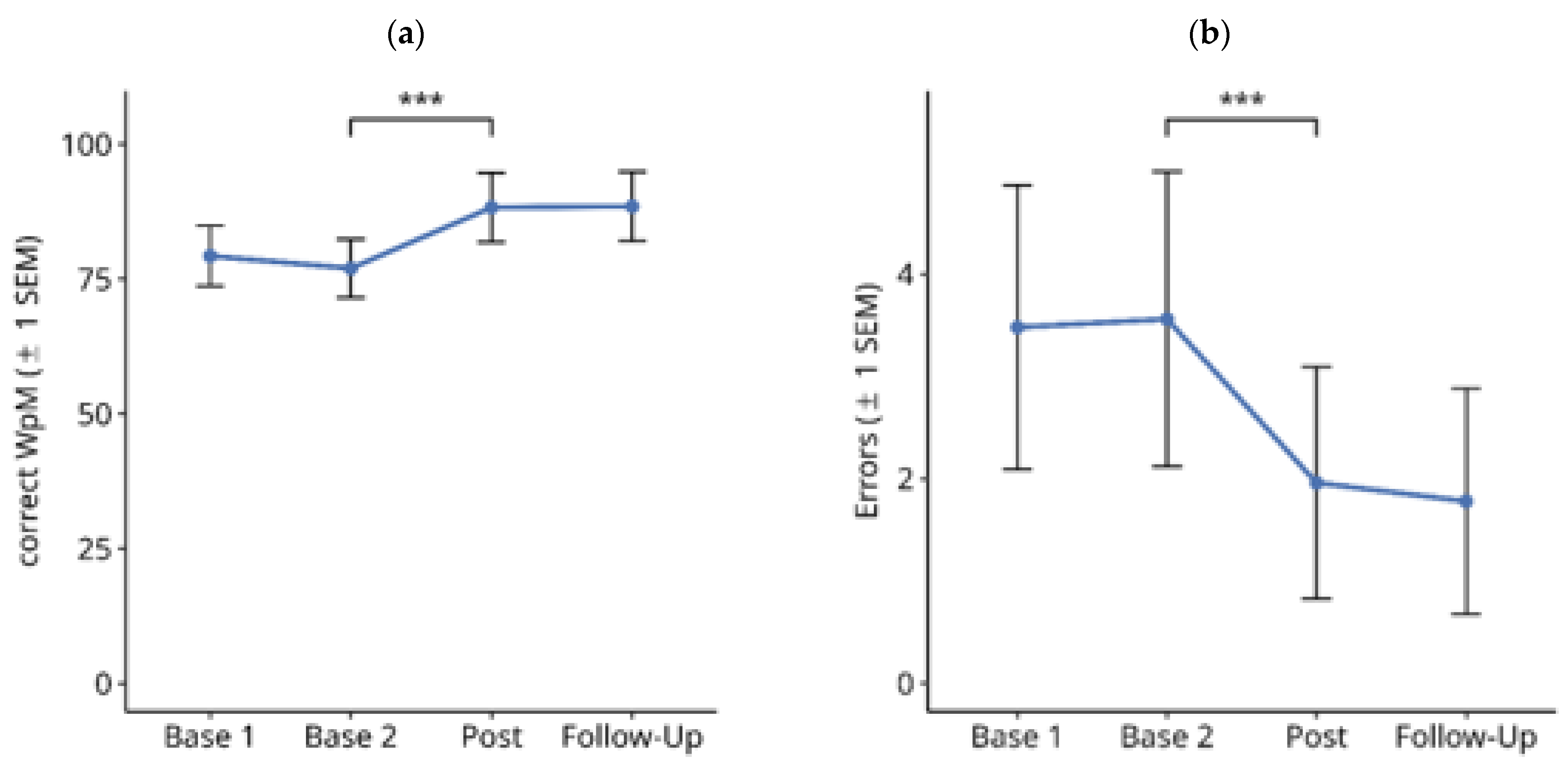
| Variable | Score |
|---|---|
| Age (years) | 50.2 (25–76), Md: 51 |
| Sex (m/f) | 16/11 |
| Time since lesion (days) | 638 (35–3258), Md: 219 |
| Lesion side | |
| L/R/BIL-diffuse | 10/10/7 |
| Corrected visual acuity (%) | |
| Near distance (0.4 m) | 0.97 (0.63–1.25) |
| Far distance (6 m) | 0.93 (0.33–1.25) |
| Lesion location | |
| Frontal/temporal/parietal/occipital | 0/5/8/19 |
| Thalamus | 2 |
| Diffuse | 7 |
| Etiology | |
| Stroke | 17 |
| Tumor | 4 |
| Closed head trauma | 6 |
| Type of visual field defect | |
| Hemianopia (L, R, BIL) | 4/4/4 |
| Quadranopia (L, R) | 3/3 |
| Paracentral scotoma (L, R) | 3/5 |
| Diffuse small scotomas | 1 |
| Visual field sparing (mean °) | 9.1 (1–35) Md: 4 |
| Treatment hours (h) | 17.6 (5–42) Md: 20 |
| Subtest Description | Parallel Versions | Parameters | Retest Correlation Pearson Coefficient Spearman Coefficient |
|---|---|---|---|
| 6 | Correct words per minute read, errors | cWpM: 0.99 *, Errors: 0.98 * cWpM: 0.99 *, Errors: 0.94 * |
| 3 | Correct words per Minute read, errors | cWpM: 0.99 *, Errors: 0.98 * cWpM: 0.99 *, Errors: 0.86 * |
| 3 | Correct words per Minute read, errors | cWpM:0.97 *, Errors: 0.98 * cWpM:0.95 *, Errors: 0.86 * |
| 3 | % correct remembered, Words per Minute read. | WpM: 0.99 *, correct: 0.92 * Wpm: 0.99 *, correct: 0.95 * |
| 3 | % errors detected, Words per Minute | WpM: 0.87 *, correct: 0.96 * WpM: 0.86 *, correct: 0.93 * |
| 3 | % correct, Total Time (seconds) | Time: 0.98 *, correct: 0.74 * Time: 0.96 *, correct: 0.82 * |
| - | Summed score from 16 questions | Score: 0.99 * Score: 0.99 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerkhoff, G.; Kraft, A. The Effects of a Novel Treatment for Hemianopic Dyslexia on Reading, Symptom Load, and Return to Work. Brain Sci. 2024, 14, 259. https://doi.org/10.3390/brainsci14030259
Kerkhoff G, Kraft A. The Effects of a Novel Treatment for Hemianopic Dyslexia on Reading, Symptom Load, and Return to Work. Brain Sciences. 2024; 14(3):259. https://doi.org/10.3390/brainsci14030259
Chicago/Turabian StyleKerkhoff, Georg, and Antje Kraft. 2024. "The Effects of a Novel Treatment for Hemianopic Dyslexia on Reading, Symptom Load, and Return to Work" Brain Sciences 14, no. 3: 259. https://doi.org/10.3390/brainsci14030259
APA StyleKerkhoff, G., & Kraft, A. (2024). The Effects of a Novel Treatment for Hemianopic Dyslexia on Reading, Symptom Load, and Return to Work. Brain Sciences, 14(3), 259. https://doi.org/10.3390/brainsci14030259





