Effects and Mechanisms of Exercise on Brain-Derived Neurotrophic Factor (BDNF) Levels and Clinical Outcomes in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Method
2.1. Data Sources and Search Strategy
2.2. Criteria for Inclusion
2.3. Data Extraction
2.4. Effect Size Analysis
2.5. Quality Assessment
3. Results
3.1. Selection of Studies
3.2. Quality Assessment
3.3. Summary of the Literature
3.4. BDNF Changes
3.5. Clinical Outcomes
3.6. BDNF–Clinical Outcomes Correlation
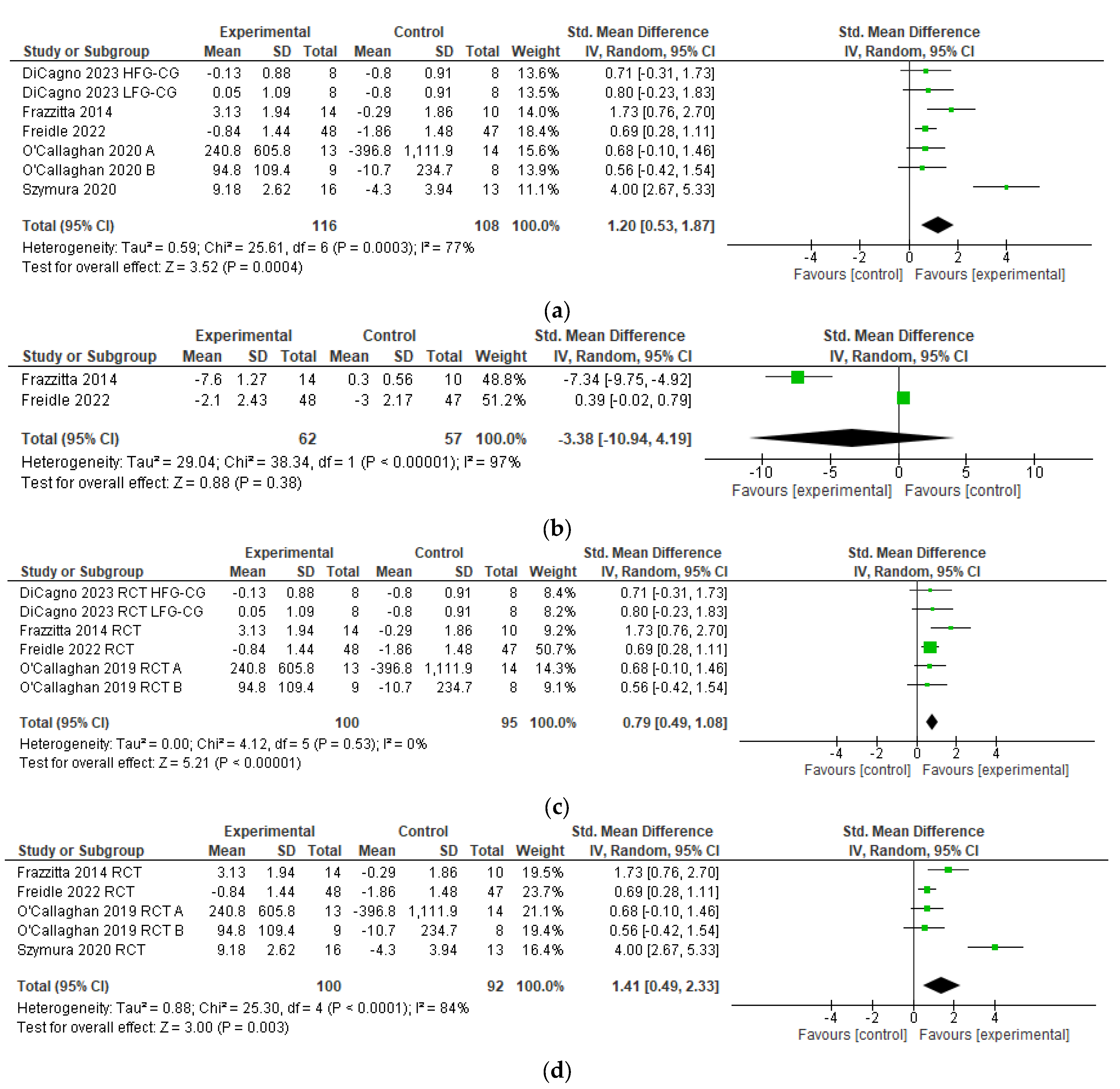
3.7. Meta-Analysis
4. Discussion
Exercise Neuroplastic Mechanisms
5. Limitations
6. Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.; Ritz, B.; Gong, Y.; Cockburn, M.; Duarte Folle, A.; Del Rosario, I.; Yu, Y.; Zhang, K.; Castro, E.; Keener, A.M.; et al. Proximity to residential and workplace pesticides application and the risk of progression of Parkinson’s diseases in Central California. Sci. Total Environ. 2023, 864, 160851. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, S.M.; Schwarzschild, M.A.; Hernán, M.A.; Ascherio, A. Physical activity and the risk of Parkinson disease. Neurology 2005, 64, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hamilton, J.L.; Kopil, C.; Beck, J.C.; Tanner, C.M.; Albin, R.L.; Dorsey, E.R.; Dahodwala, N.; Cintina, I.; Hogan, P.; et al. Current and Projected Future Economic Burden of Parkinson’s Disease in the U.S. NPJ Parkinson’s Dis. 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C. Neuroprotection and pharmacotherapy for motor symptoms in Parkinson’s disease. Lancet Neurol. 2004, 3, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Van Wamelen, D.J.; Rukavina, K.; Podlewska, A.M.; Chaudhuri, K.R. Advances in the Pharmacological and Non-pharmacological Management of Non-motor Symptoms in Parkinson’s Disease: An Update Since 2017. Curr. Neuropharmacol. 2023, 21, 1786–1805. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Mariani, L.-L.; Mangone, G.; Nailly, D.L.F.d.; Charbonnier-Beaupel, F.; Corvol, J.-C. Molecular basis of dopamine replacement therapy and its side effects in Parkinson’s disease. Cell Tissue Res. 2018, 373, 111–135. [Google Scholar] [CrossRef] [PubMed]
- Schiess, N.I.; Cataldi, R.; Okun, M.S.; Fothergill-Misbah, N.; Dorsey, E.R.; Bloem, B.R.; Barretto, M.; Bhidayasiri, R.; Brown, R.; Chishimba, L.; et al. Six action steps to address global disparities in Parkinson disease: A world health organization priority. JAMA Neurol. 2022, 79, 929–936. [Google Scholar] [CrossRef]
- Osborne, J.A.; Botkin, R.; Colon-Semenza, C.; DeAngelis, T.R.; Gallardo, O.G.; Kosakowski, H.; Mar-tello, J.; Pradhan, S.; Rafferty, M.; Readinger, J.L.; et al. Physical therapist management of Parkinson disease: A clinical practice guideline from the American physical therapy association. Phys. Ther. 2022, 102, pzab302. [Google Scholar] [CrossRef]
- Foster, E.R.; Carson, L.G.; Archer, J.; Hunter, E.G. Occupational therapy interventions for in-strumental activities of daily living for adults with Parkinson’s disease: A systematic review. Am. J. Occup. Ther. 2021, 75, p7503190024–p7503190031. [Google Scholar] [CrossRef]
- Keus, S.H.J.; Munneke, M.; Graziano, M.; Paltamaa, J.; Pelosin, E.; Domingos, J.; Brühlmann, S.; Ra-maswamy, B.; Prins, J.; Struiksma, C.; et al. European Physi-Otherapy Guideline for Parkinson’s Disease; KNGF/ParkinsonNet: Amersfoort, The Netherlands, 2014. [Google Scholar]
- Goodwin, V.A.; Richards, S.H.; Taylor, R.S.; Taylor, A.H.; Campbell, J.L. The effectiveness of exercise interventions for people with Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2008, 23, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Uhrbrand, A.; Stenager, E.; Pedersen, M.S.; Dalgas, U. Parkinson’s disease and intensive exercise therapy—A systematic review and meta-analysis of randomized controlled trials. J. Neurol. Sci. 2015, 353, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Schootemeijer, S.; van der Kolk, N.M.; Bloem, B.R.; de Vries, N.M. Current Perspectives on Aerobic Exercise in People with Parkinson’s Disease. Neurother. J. Am. Soc. Exp. Neurother. 2020, 17, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, C.L.; Herd, C.P.; Clarke, C.E.; Meek, C.; Patel, S.; Stowe, R.; Deane, K.H.; Shah, L.; Sackley, C.M.; Wheatley, K.; et al. Physiotherapy for Parkinson’s disease: A comparison of techniques. Cochrane Database Syst. Rev. 2014, 2014, CD002815. [Google Scholar] [CrossRef]
- Radder, D.L.M.; Lígia Silva de Lima, A.; Domingos, J.; Keus, S.H.J.; van Nimwegen, M.; Bloem, B.R.; de Vries, N.M. Physiotherapy in Parkinson’s disease: A meta-analysis of present treatment modalities. Neurorehabilit. Neural Repair 2020, 34, 871–880. [Google Scholar] [CrossRef]
- Allen, N.E.; Canning, C.G.; Almeida, L.R.S.; Bloem, B.R.; Keus, S.H.; Löfgren, N.; Nieuwboer, A.; Verheyden, G.S.; Yamato, T.P.; Sherrington, C. Interventions for preventing falls in Parkinson’s disease. Cochrane Database Syst. Rev. 2022, 6, CD011574. [Google Scholar] [PubMed]
- Ernst, M.; Folkerts, A.-K.; Gollan, R.; Lieker, E.; Caro-Valenzuela, J.; Adams, A.; Cryns, N.; Monsef, I.; Dresen, A.; Roheger, M.; et al. Physical exercise for people with Parkinson’s disease: A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2023, 2023, CD013856. [Google Scholar] [CrossRef]
- Neeper, S.A.; Góauctemez-Pinilla, F.; Choi, J.; Cotman, C. Exercise and brain neurotrophins. Nature 1995, 373, 109. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef]
- Hirsch, M.A.; Iyer, S.; Sanjak, M. Exercise-induced neuroplasticity in human Parkinson’s disease: What is the evidence telling us? Park. Relat. Disord. 2016, 22 (Suppl. S1), S78–S81. [Google Scholar] [CrossRef]
- Hirsch, M.A.; Farley, B.G. Exercise, neuroplasticity and Parkinson’s disease. Eur. J. Phys. Rehabil. Med. 2009, 45, 215–229. [Google Scholar]
- Fontanesi, C.; Kvint, S.; Frazzitta, G.; Bera, R.; Ferrazzoli, D.; Di Rocco, A.; Rebholz, H.; Friedman, E.; Pezzoli, G.; Quartarone, A.; et al. Intensive Rehabilitation Enhances Lymphocyte BDNF-TrkB Signaling in Patients With Parkinson’s Disease. Neurorehabilit. Neural Repair 2015, 30, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Tillerson, J.L.; Smith, A.D.; Schallert, T.; Zigmond, M.J. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: Possible role of GDNF. J. Neurochem. 2003, 85, 299–305. [Google Scholar] [CrossRef]
- Steiner, B.; Winter, C.; Hosman, K.; Siebert, E.; Kempermann, G.; Petrus, D.S.; Kupsch, A. Enriched environment induces cellular plasticity in the adult substantia nigra and improves motor behavior function in the 6-OHDA rat model of Parkinson’s disease. Exp. Neurol. 2006, 199, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Wang, T.-F.; Yu, L.; Jen, C.J.; Chuang, J.-I.; Wu, F.-S.; Wu, C.-W.; Kuo, Y.-M. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav. Immun. 2011, 25, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Real, C.; Ferreira, A.; Chaves-Kirsten, G.; Torrão, A.; Pires, R.; Britto, L. BDNF receptor blockade hinders the beneficial effects of exercise in a rat model of Parkinson’s disease. Neuroscience 2013, 237, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Stahl, K.; Mylonakou, M.N.; Skare, Ø.; Amiry-Moghaddam, M.; Torp, R. Cytoprotective effects of growth factors: BDNF more potent than GDNF in an organotypic culture model of Parkinson’s disease. Brain Res. 1378, 2011, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Gash, D.M.; Zhang, Z.; Ai, Y.; Grondin, R.; Coffey, R.; Gerhardt, G.A. Trophic factor distribution predicts functional recovery in parkinsonian monkeys. Ann. Neurol. 2005, 58, 224–233. [Google Scholar] [CrossRef]
- Murer, M.; Boissiere, F.; Yan, Q.; Hunot, S.; Villares, J.; Faucheux, B. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer’s disease. Neuroscience 1999, 88, 1015–1032. [Google Scholar] [CrossRef]
- Fredriksson, A.; Stigsdotter, I.M.; Hurtig, A.; Ewalds-Kvist, B.; Archer, T. Running wheel activity restores MPTP-induced functional deficits. J. Neural Transm. 2010, 118, 407–420. [Google Scholar] [CrossRef]
- Kim, S.-E.; Ko, I.-G.; Shin, M.-S.; Kim, C.-J.; Jin, B.-K.; Hong, H.-P.; Jee, Y.-S. Treadmill exercise and wheel exercise enhance expressions of neutrophic factors in the hippocampus of lipopolysaccharide-injected rats. Neurosci. Lett. 2013, 538, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Segura, C.; Eraso, M.; Bonilla, J.; Mendivil, C.O.; Santiago, G.; Useche, N.; Bernal-Pacheco, O.; Monsalve, G.; Sanchez, L.; Hernández, E.; et al. Effect of a High-Intensity Tandem Bicycle Exercise Program on Clinical Severity, Functional Magnetic Resonance Imaging, and Plasma Biomarkers in Parkinson’s Disease. Front. Neurol. 2020, 11, 656. [Google Scholar] [CrossRef]
- Schaeffer, E.; Roeben, B.; Granert, O.; Hanert, A.; Liepelt-Scarfone, I.; Leks, E.; Otterbein, S.; Saraykin, P.; Busch, J.; Synofzik, M.; et al. Effects of exergaming on hippocampal volume and brain-derived neurotrophic factor levels in Parkinson’s disease. Eur. J. Neurol. 2021, 29, 441–449. [Google Scholar] [CrossRef]
- Ferreira, R.N.; de Miranda, A.S.; Rocha, N.P.; Simoes da Silva, A.C.; Teixeira, A.L.; da Silva Camargos, E.R. Neurotrophic factors in Parkinson’s disease: What have we learned from pre-clinical and clinical studies? Curr. Med. Chem. 2018, 25, 3682–3702. [Google Scholar] [CrossRef]
- Ayon-Olivas, M.; Wolf, D.; Andreska, T.; Granado, N.; Lüningschrör, P.; Ip, C.W.; Moratalla, R.; Sendtner, M. Dopaminergic Input Regulates the Sensitivity of Indirect Pathway Striatal Spiny Neurons to Brain-Derived Neurotrophic Factor. Biology 2023, 12, 1360. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.B.; Siegel, G.J.; Lee, J.M. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J. Chem. Neuroanat. 2001, 21, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Z.; Zhang, H.; Zhang, H. A meta-analysis on the role of brain-derived neurotrophic factor in Parkinson’s disease patients. Adv. Clin. Exp. Med. 2022, 32, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Jones, T.A.; Schallert, T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem. Res. 2003, 28, 1757–1769. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Majerczak, J.; Zeligowska, E.; Mencel, J.; Jaskolski, A.; Jaskolska, A.; Marusiak, J. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and de-creases inflammation in Parkinson’s disease patients. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2014, 65, 441–448. [Google Scholar]
- Marusiak, J.; Żeligowska, E.; Mencel, J.; Kisiel-Sajewicz, K.; Majerczak, J.; Zoladz, J.; Jaskã³Lska, A. Interval training-induced alleviation of rigidity and hypertonia in patients with Parkinson’s disease is accompanied by increased basal serum brain-derived neurotrophic factor. J. Rehabil. Med. 2015, 47, 372–375. [Google Scholar] [CrossRef]
- Angelucci, F.; Piermaria, J.; Gelfo, F.; Shofany, J.; Tramontano, M.; Fiore, M.; Caltagirone, C.; Peppe, A. The effects of motor rehabilitation training on clinical symptoms and serum BDNF levels in Parkinson’s disease subjects. Can. J. Physiol. Pharmacol. 2016, 94, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.A.; van Wegen, E.E.H.; Newman, M.A.; Heyn, P.C. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson’s disease: A systematic review and meta-analysis. Transl. Neurodegener. 2018, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Hagströmer, M.; Grooten, W.J.A.; Franzén, E. Exercise-Induced Neuroplasticity in Parkinson’s Disease: A Metasynthesis of the Literature. Neural Plast. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Loevaas, M.B.; Guan, C.; Goh, L.; Allen, N.E.; Mak, M.K.Y.; Lv, J.; Paul, S.S. Does Exercise Attenuate Disease Progression in People With Parkinson’s Disease? A Systematic Review With Meta-Analyses. Neurorehabilit. Neural Repair 2023, 37, 328–352. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, R.; Proietti, S.; Perluigi, M.; Padua, E.; Stocchi, F.; Fini, M.; Stocchi, V.; Volpe, D.; De Pandis, M.F. Physical activity and neurotrophic factors as potential drivers of neuroplasticity in Parkinson’s Disease: A systematic review and meta-analysis. Ageing Res. Rev. 2023, 92, 102089. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Review Manager. Version 5.4. The Cochrane Collaboration, 20-9-2020. Available online: https://Revman.cochrane.org (accessed on 4 January 2024).
- National Institutes of Health. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 4 January 2024).
- Di Cagno, A.; Buonsenso, A.; Centorbi, M.; Manni, L.; Di Costanzo, A.; Casazza, G.; Parisi, A.; Guerra, G.; Calcagno, G.; Iuliano, E.; et al. Whole body-electromyostimulation effects on serum biomarkers, physical performances and fatigue in Parkinson’s patients: A randomized controlled trial. Front. Aging Neurosci. 2023, 15, 1086487. [Google Scholar] [CrossRef]
- Frazzitta, G.; Maestri, R.; Ghilardi, M.F.; Riboldazzi, G.; Perini, M.; Bertotti, G.; Boveri, N.; Buttini, S.; Lombino, F.L.; Uccellini, D.; et al. Intensive Rehabilitation Increases BDNF Serum Levels in Parkinsonian Patients. Neurorehabilit. Neural Repair 2013, 28, 163–168. [Google Scholar] [CrossRef]
- O’callaghan, A.; Harvey, M.; Houghton, D.; Gray, W.K.; Weston, K.L.; Oates, L.L.; Romano, B.; Walker, R.W. Comparing the influence of exercise intensity on brain-derived neurotrophic factor serum levels in people with Parkinson’s disease: A pilot study. Aging Clin. Exp. Res. 2019, 32, 1731–1738. [Google Scholar] [CrossRef]
- Szymura, J.; Kubica, J.; Wiecek, M.; Pera, J. The Immunomodulary Effects of Systematic Exercise in Older Adults and People with Parkinson’s Disease. J. Clin. Med. 2020, 9, 184. [Google Scholar] [CrossRef]
- Freidle, M.; Johansson, H.; Ekman, U.; Lebedev, A.V.; Schalling, E.; Thompson, W.H.; Svenningsson, P.; Lövdén, M.; Abney, A.; Albrecht, F.; et al. Behavioural and neuroplastic effects of a double-blind randomised controlled balance exercise trial in people with Parkinson’s disease. npj Parkinson’s Dis. 2022, 8, 12. [Google Scholar] [CrossRef]
- Liguori, G. ACSM’s Guidelines for Exercise Testing and Prescription (American College of Sports Medicine); LWW: Philadelphia, PA, USA, 2018. [Google Scholar]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Landers, M.R.; Navalta, J.W.; Murtishaw, A.S.; Kinney, J.W.; Richardson, S.P. A High-Intensity Exercise Boot Camp for Persons With Parkinson Disease: A Phase II, Pragmatic, Randomized Clinical Trial of Feasibility, Safety, Signal of Efficacy, and Disease Mechanisms. J. Neurol. Phys. Ther. 2019, 43, 12–25. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.S.; Iraci, L.; Pinheiro, G.S.; Casal, M.Z.; Haas, A.N.; Pochmann, D.; Martinez, F.G.; Elsner, V.; Dani, C. Effect of exercise and grape juice on epigenetic modulation and functional outcomes in PD: A randomized clinical trial. Physiol. Behav. 2020, 227, 113135. [Google Scholar] [CrossRef] [PubMed]
- Stuckenschneider, T.; Abeln, V.; Foitschik, T.; Abel, T.; Polidori, M.C.; Strüder, H.K. Disease-inclusive exercise classes improve physical fitness and reduce depressive symptoms in individuals with and without Parkinson’s disease—A feasibility study. Brain Behav. 2021, 11, e2352. [Google Scholar] [CrossRef] [PubMed]
- Sajatovic, M.; Ridgel, A.L.; Walter, E.M.; Tatsuoka, C.M.; Colon-Zimmermann, K.; Ramsey, R.K.; Welter, E.; Gunzler, S.; Whitney, C.M.; Walter, B.L. A randomized trial of individual versus group-format exercise and self-management in individuals with Parkinson’s disease and comorbid depression. Patient Prefer. Adherence 2017, 11, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.S.A.; Heuvel, O.A.V.D.; Rietberg, M.B.; De Groot, V.; Hirsch, M.A.; Van de Berg, W.D.J.; Jaspers, R.T.; Vriend, C.; Vanbellingen, T.; Van Wegen, E.E.H. (HIIT-The Track) High-Intensity Interval Training for People with Parkinson’s Disease: Individual Response Patterns of (Non-)Motor Symptoms and Blood-Based Biomarkers—A Crossover Single-Case Experimental Design. Brain Sci. 2023, 13, 849. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Pardo, J.; Sánchez-Ferro, A.; Monje, M.H.G.; Pavese, N.; Obeso, J. Onset pattern of nigrostriatal denervation in early Parkinson’s disease. Brain 2022, 145, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.C.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef]
- Johansson, M.E.; Cameron, I.G.M.; Van der Kolk, N.M.; de Vries, N.M.; Klimars, E.; Toni, I.; Bloem, B.R.; Helmich, R.C. Aerobic exercise alters brain function and structure in Parkinson’s disease: A randomized controlled trial. Ann. Neurol. 2022, 91, 203–216. [Google Scholar] [CrossRef]
- Sehm, B.; Taubert, M.; Conde, V.; Weise, D.; Classen, J.; Dukart, J.; Draganski, B.; Villringer, A.; Ragert, P. Structural brain plasticity in Parkinson’s disease induced by balance training. Neurobiol. Aging 2013, 35, 232–239. [Google Scholar] [CrossRef]
- Fisher, B.E.; Wu, A.D.; Salem, G.J.; Song, J.E.; Lin, C.H.; Yip, J.; Cen, S.; Gordon, J.; Jacowec, M.; Petzinger, G. The effect of exercise training in improving motor performance and corticomotor excitability in persons with early Parkinson’s disease. Arch. Phys. Med. Rehabil. 2008, 89, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.E.; Li, Q.; Nacca, A.; Salem, G.J.; Song, J.; Yip, J.; Hui, J.S.; Jakowec, M.W.; Petzinger, G.M. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. NeuroReport 2013, 24, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Sacheli, M.A.; Neva, J.L.; Lakhani, B.; Murray, D.K.; Vafai, N.; Shahinfard, E.; English, A.; McCormick, S.; Dinelle, K.; Neilson, N.; et al. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov. Disord. 2019, 34, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Malczynska-Sims, P.; Chalimoniuk, M.; Wronski, Z.; Marusiak, J.; Sulek, A. High-intensity interval training modulates inflammatory response in Parkinson’s disease. Aging Clin. Exp. Res. 2022, 34, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 2011, 77, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Van Wegen, E.E.H.; Hirsch, M.A.; Huiskamp, M.; Kwakkel, G. Harnessing cueing training for neuroplasticity in Parkinson’s disease. Top. Geriatr. Rehabil. 2014, 30, 46–57. [Google Scholar] [CrossRef]
- Petzinger, G.M.; Holschneider, D.P.; Fisher, B.E.; McEwen, S.; Kintz, N.; Halliday, M.; Toy, W.; Walsh, J.W.; Beeler, J.; Jakowec, M.W. The effects of exercise on dopamine neurotransmission in Par-kinson’s disease: Targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast. 2015, 1, 29–39. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2006, 50, 431–438. [Google Scholar] [CrossRef]
- Hanyu, O.; Yamatani, K.; Ikarashi, T.; Soda, S.; Maruyama, S.; Kamimura, T. Brain-derived neurotrophic factor modulates glucagon secretion from pancreatic alpha cells: Its contribution to glucose metabolism. Diabetes Obes. Metab. 2003, 5, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Maldonado, A.; De Álvarez-Buylla, E.R.; Montero, S.; Melnikov, V. Chronic Exercise Increases Plasma Brain-Derived Neurotrophic Factor Levels, Pancreatic Islet Size, and Insulin Tolerance in a TrkB-Dependent Manner. PLoS ONE 2014, 9, e115177. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Suzuki, K.; Higuchi, M.; Balogh, L.; Boldogh, I.; Koltai, E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic. Biol. Med. 2016, 98, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ruchti, E.; Petit, J.-M.; Jourdain, P.; Grenningloh, G.; Allaman, I. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233. [Google Scholar] [CrossRef] [PubMed]
- Marquez, C.M.S.; Vanaudenaerde, B.; Troosters, T.; Wenderoth, N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J. Appl. Physiol. 2015, 119, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Satriotomo, I.; Nichols, N.; Dale, E.; Emery, A.; Dahlberg, J.; Mitchell, G. Repetitive acute intermittent hypoxia increases growth/neurotrophic factor expression in non-respiratory motor neurons. Neuroscience 2016, 322, 479–488. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Brunelli, A.; Dimauro, I.; Sgrò, P.; Emerenziani, G.P.; Magi, F.; Baldari, C.; Guidetti, L.; DI Luigi, L.; Parisi, P.; Caporossi, D. Acute Exercise Modulates BDNF and pro-BDNF Protein Content in Immune Cells. Med. Sci. Sports Exerc. 2012, 44, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Radka, S.F.; Hoist, P.A.; Fritsche, M.; Altar, C.A. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996, 709, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Fernández, P.; Säuberli, K.; Colzani, M.; Moreau, T.; Ghevaert, C.; Barde, Y.-A. Brain-derived Neurotrophic Factor in Megakaryocytes. J. Biol. Chem. 2016, 291, 9872–9881. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, H.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.-I.; Sun, B.; Altar, C.; Tandon, N.N. Brain-derived Neurotrophic Factor Is Stored in Human Platelets and Released by Agonist Stimulation. Arthritis Res. Ther. 2002, 87, 728–734. [Google Scholar] [CrossRef]
- Prigent-Tessier, A.; Quirié, A.; Maguin-Gaté, K.; Szostak, J.; Mossiat, C.; Nappey, M.; Devaux, S.; Marie, C.; Demougeot, C. Physical training and hypertension have opposite effects on endothelial brain-derived neurotrophic factor expression. Cardiovasc. Res. 2013, 100, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Kim, W.J.; Lok, J.; Lee, S.-R.; Besancon, E.; Luo, B.-H.; Stins, M.F.; Wang, X.; Dedhar, S.; Lo, E.H. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 7582–7587. [Google Scholar] [CrossRef] [PubMed]
- Monnier, A.; Prigent-Tessier, A.; Quirié, A.; Bertrand, N.; Savary, S.; Gondcaille, C.; Garnier, P.; Demougeot, C.; Marie, C. Brain-derived neurotrophic factor of the cerebral microvasculature: A forgotten and nitric oxide-dependent contributor of brain-derived neurotrophic factor in the brain. Acta Physiol. 2016, 219, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Junior, R.S.; Cevada, T.; Oliveira, B.R.; Lattari, E.; Portugal, E.M.; Carvalho, A.; Deslandes, A.C. We need to move more: Neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med. Hypotheses 2015, 85, 537–541. [Google Scholar] [CrossRef]
- Janssen Daalen, J.M.; Schootemeijer, S.; Richard, E.; Darweesh, S.K.L.; Bloem, B.R. Lifestyle interventions for the prevention of Parkinson disease: A recipe for action. Neurology 2022, 99 (Suppl. S1), 42–51. [Google Scholar] [CrossRef]
- Mougeot, J.L.; Hirsch, M.A.; Stevens, C.B.; Mougeot, F. Oral biomarkers in exercise-induced neuroplasticity in Parkinson’s disease. Oral Dis. 2016, 22, 745–753. [Google Scholar] [CrossRef]
- Thomas, M.; Knoblich, N.; Wallisch, A.; Glowacz, K.; Becker-Sadzio, J.; Gundel, F.; Brückmann, C.; Nieratschker, V. Increased BDNF methylation in saliva, but not blood, of patients with borderline personality disorder. Clin. Epigenetics 2018, 10, 109. [Google Scholar] [CrossRef]
- Harpham, C.; Gunn, H.; Marsden, J.; Connolly, L. The feasibility, safety, physiological and clinical effects of high-intensity interval training for people with Parkinson’s: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2023, 35, 497–523. [Google Scholar] [CrossRef]
- Goldman, J.G.; Volpe, D.; Ellis, T.D.; Hirsch, M.; Johnson, J.; Wood, J.; Aragon, A.; Biundo, R.; Di Rocco, A.; Kasman, G.S.; et al. Delivering Multidisciplinary Rehabilitation Care in Parkinson’s Disease: An International Consensus Statement. J. Parkinson’s Dis. 2024, 14, 135–166. [Google Scholar] [CrossRef]
- Patterson, C.G.; Joslin, E.; Gil, A.B.; Spigle, W.; Nemet, T.; Chahine, L.; Christiansen, C.L.; Melanson, E.; Kohrt, W.M.; Mancini, M.; et al. Study in Parkinson’s disease of exercise phase 3 (SPARX3): Study protocol for a randomized controlled trial. Trials 2022, 23, 1–26. [Google Scholar] [CrossRef]
- Cammisuli, D.M.; Bonuccelli, U.; Daniele, S.; Martini, C.; Fusi, J.; Franzoni, F. Aerobic Exercise and Healthy Nutrition as Neuroprotective Agents for Brain Health in Patients with Parkinson’s Disease: A Critical Review of the Literature. Antioxidants 2020, 9, 380. [Google Scholar] [CrossRef]

| Q | η (n) | Q | η (n) | Q | η (n) | Q | η (n) | Q | η (n) |
| 1 | 0.990 | 11 | 1.307 | 21 | 1.327 | 31 | 1.334 | 41 | 1.338 |
| 2 | 1.144 | 12 | 1.311 | 22 | 1.328 | 32 | 1.334 | 42 | 1.338 |
| 3 | 1.206 | 13 | 1.313 | 23 | 1.329 | 33 | 1.335 | 43 | 1.338 |
| 4 | 1.239 | 14 | 1.316 | 24 | 1.330 | 34 | 1.335 | 44 | 1.338 |
| 5 | 1.260 | 15 | 1.318 | 25 | 1.330 | 35 | 1.336 | 45 | 1.339 |
| 6 | 1.274 | 16 | 1.320 | 26 | 1.331 | 36 | 1.336 | 46 | 1.339 |
| 7 | 1.284 | 17 | 1.322 | 27 | 1.332 | 37 | 1.336 | 47 | 1.339 |
| 8 | 1.292 | 18 | 1.323 | 28 | 1.332 | 38 | 1.337 | 48 | 1.339 |
| 9 | 1.298 | 19 | 1.324 | 29 | 1.333 | 39 | 1.337 | 49 | 1.339 |
| 10 | 1.303 | 20 | 1.326 | 30 | 1.333 | 40 | 1.337 | 50 | 1.340 |
| Quality Criteria | DiCagno | Frazzitta | Freidle | O’Callaghan | Szymura |
|---|---|---|---|---|---|
| #1 | + | + | + | + | + |
| #2 | + | + | + | + | CD |
| #3 | + | + | + | + | NR |
| #4 | - | - | - | - | - |
| #5 | + | + | + | + | - |
| #6 | + | + | + | + | + |
| #7* | + | + | - | + | + |
| #8* | + | + | - | + | + |
| #9 | NR | NR | - | + | NR |
| #10 | + | + | + | + | + |
| #11 | + | + | + | + | + |
| #12 | + | + | + | - | - |
| #13 | + | + | + | + | + |
| #14* | + | + | + | + | + |
| Total score | 12/14 | 12/14 | 10/14 | 12/14 | 8/14 |
| Rating | good | good | Poor * | good | fair |
| Effect size (SMD [range]) | 0.71 [−0.31–1.73] 0.80 [−0.23–1.83] | 1.73 [0.76–2.70] | 0.69 [1.28–1.11] | 0.68 [−0.10–1.46] 0.56 [−0.42–1.54] | 4.00 [2.67–5.33] |
| PPI | yes | no | no | no | no |
| Dosage | 480 | 3600 | 1200 | 2160 | 2160 |
| Author (Year) | Intervention | Control | Protocol/Exercise Components | Results | Proposed Mechanism |
|---|---|---|---|---|---|
| DiCagno et al. (2023) [51] | n = 8 (HFG) Age 72.37 ± 7.40 Male 87.5% H&Y stage 1.87 ± 0.35 n = 8 (LFG) Age 73.13 ± 2.85 Male 62.5% H&Y stage 1.44 ± 0.62 | n = 8 Age 70.87 ± 7.77 Male 75% H&Y stage 2.19 ± 0.65 | RCT, 12 wks HFG: strength training + WB-EMS 2x/wk LFG: AT + WB-EMS 2x/wk CG: UC strength training + WB-EMS: strength training (20 min) consisting of half squat, full squat, bent over, core rotation, and crunch, combined with WB-EMS (rectangular stimulation at 85 Hz, 350 µs, 4 s stimulation/4 s rest) AT + WB-EMS: aerobic training on rowing machine (20 min), combined with WB-EMS (rectangular stimulation 7 Hz 350 µs, with a continuous pulse duration) HRR 60–80% | HFG BDNF* 2131.5 pg/mL ± 628.0 à 1999.4 pg/mL ± 1074.9 (p > 0.05) LFG BDNF* 1426.5 pg/mL ± 1426.5 à 2042.2 pg/mL ± 567.5 (p > 0.05) CG BDNF* 1657.9 pg/mL ± 1035.9 à 862 pg/mL ± 760 (p < 0.05) Statistically significant change in BDNF between LFG and CG (p < 0.05) HFG 6MWT 280.5 m (49.3) à 267.5 m (41.25) (p > 0.05) LFG 6MWT 347 m (89.13) à 469 m (94.25) (p < 0.05) CG 6MWT 302 m (46.5) à 287.5 m (30.25) (p > 0.05) Statistically significant differences in 6MWT found in LFG compared to HFG and CG and HFG, and CG compared to LFG (p < 0.05) HFG Tinetti’s test 21.5 (4.5) à 21 (4) (p > 0.05) LFG Tinetti’s test 21.5 (3) à 28 (2) (p < 0.05) CG Tinetti’s test 22 (3.25) à 22.5 (2.5) (p > 0.05) Statistically significant differences in Tinetti’s test for balance found in LFG compared to HFG and CG HFG PFS-16 3.13 (0.45) à 2.8 (0.1) (p > 0.05) LFG PFS-16 3.23 (0.3) à 1.8 (0.25) (p < 0.05) CG PFS-16 3 (0.48) à 3.8 (0.47) (p > 0.05) Statistically significant differences in PFS-16 found in LFG compared to HFG and CG, and significant worsening compared with pre-test in CG | Exercise (specifically aerobic exercise) increased BDNF levels (no specific mechanism cited) |
| Frazzitta et al. (2014) [52] | n = 14 Age 67 ± 5 PD duration 8 ± 5 H&Y stage 1–1.5 UPDRS-III 16.4 ± 3.5 | n = 10 Age 65 ± 4 PD duration 8 ± 2 H&Y stage 1–1.5 UPDRS-III 15.6 ± 1.5 | RCT, 4 wks IG: IRT 5x/wk CG: UC IRT: stretching, warm-up, and ROM (60 min), balance, gait (30 min), treadmill plus (30 min), occupational therapy (60 min) ≤60% HRR Total: 180 min | IG BDNF 21,640 pg/mL ± 3400 à 24,770 pg/mL ± 6400 (p = 0.017) IG UPDRS-III 16.4 ± 3.5 à 8.8 ± 3.2 (p < 0.0001) IG UPDRS 25.43 ± 5.6 à 14.79 ± 6.0 (p < 0.0001) IG 6MWT 383 m ± 94 à 477m ± 79 (p = 0.0001) IG BBS 48.64 ± 6.1 à 54.00 ± 2.4 (p = 0.0016) CG BDNF 22870 pg/mL ± 4000 à 22580 pg/mL ± 4300 (p > 0.5) CG UPDRS-III 15.5 ± 1.4 à 15.8 ± 1.1 (p = 0.1934)No significant correlation between BDNF level change and changes in UPDRS-III (r = −0.13; p = 0.65), UPDRS (r = −0.18; p = 0.52), 6MWT (r = 0.05; p = 0.88), and BBS (r = −0.11; p = 0.69) ANOVA found significant inter-group differences in BDNF: F(3,66) = 5.63 (p = 0.0017) and UPDRS-III: F(3,66) = 66.5 (p < 0.001) | No mechanisms discussed |
| Freidle et al. (2022) [55] | n = 48 Age 71 ± 5.9 Male 62.5% PD duration 5.5 (7) H&Y stage 2.18 [2–3] UPDRS-III 31.2 ± 11.9 | n = 47 Age 71.1 ± 6.3 Male 63.8% PD duration 3 (4) H&Y stage 2.28 [2–3] UPDRS-III 31.8 ± 10.3 | RCT, 10 wks IG: HiBalance 2x/wk CG: HiCommunication 2x/wk HiBalance: group training focused on balance and cognitive and motor dual tasks (60 min) HiCommunication: training focused on improving speech and communication (60 min) | IG BDNF 38,010.8 pg/mL ± 7956.7 à 37169.4 pg/mL ± 5928.3 IG UPDRS 51.0 ± 18.8 à 48.2 ± 17.8 IG UPDRS-III 31.2 ± 11.9 à 29.1 ± 11.9 CG BDNF 37,805.3 pg/mL ± 8044.6 à 35,945.8 pg/mL ± 6208.5 CG UPDRS 50.4 ± 15.5 à 45.8 ± 16.8 CG UPDRS-III 31.8 ± 10.3 à 28.8 ± 10.7 No significant group X time interaction for BDNF outcome (p = 0.94) No significant group X time interaction for UPDRS outcome (p = 0.93) No significant group X time interaction for UPDRS-III outcome (p = 0.87) | No mechanisms discussed |
| O’Callaghan et al. (2020a) [53] | n = 13 Age 70.4 ± 7.217 Male 69.2% H&Y stage 2.07 [2–3] | n = 14 Age 64.6 ± 8.581 Male 57.1% H&Y stage 1.86 [1–2] | RCT, 12 wks IG: MICT 3x/wk CG: UC MICT: warm-up (10 min), aerobic exercise on treadmill (24 min), resistance training (12 min) 60–80% HRmax Total 45–60 min | IG BDNF* 1st session 1,433,133 pg/mL ± 605,390 à 1,626,033 pg/mL ± 861,609 IG BDNF* 12th session 1,457,900 pg/mL ± 606,219 à 1,481,967 pg/mL ± 1,441,211 Δ1st–12th (p = 0.650) CG BDNF* 1,386,000 pg/mL ± 1,192,620 à 989,233 pg/mL ± 1,024,793 (p = 0.140) | HIIT (as compared to aerobic exercise) induces hypoxia which triggers release of BDNF from cells |
| O’Callaghan et al. (2020b) [53] | n = 9 Age 68.8 ± 7.902 Male 55.6% H&Y stage 2.33 [2–3] | n = 8 Age 69.0 ± 6.633 Male 50% H&Y stage 2.25 [1–3] | RCT, 12 wks IG: HIIT 3x/wk CG: UC HIIT: warm-up (10 min), 4–6 × 4 min on Speedflex machine, 5 min cooldown ≥85% HRmax Total: 45–60 min | IG BDNF* 1st session 671,000 pg/mL ± 75,350 à 683,900 pg/mL ± 123,339 IG BDNF* 12th session 765,800 pg/mL ± 135,052 à 723,933 pg/mL ± 111,363 Δ1st–12th (p = 0.010) CG BDNF* 655,767 pg/mL ± 241,783 à 645,100 pg/mL ± 227,448 (p = 0.401) | HIIT (as compared to aerobic exercise) induces hypoxia which triggers release of BDNF from cells |
| Szymura et al. (2020) [54] | n = 16 (PDBT) Age 66.00 ± 2.59 Male 68,8% H&Y stage 2.44 [2–3] n = 16 (HBT) Age 67.25 ± 2.52 Male 62.5% | n = 13 (PDNT) Age 65.23 ± 7.40 Male 61.5% H&Y stage 2.31 [2–3] n = 16 (HNT) Age 65.69 ± 3.70 Male 62.5% | RCT, 12 wks PDBT: balance training 3x/wk PDNT: balance training 3x/wk HBT: UC HNT: UC Balance training: warm-up (5 min), balance training (50 min), cooldown (5 min) 60–70% HRmax Total 60 min | PDBT BDNF 21,190 pg/mL ± 8360 à 30370 pg/mL ± 6330 (p = 0.011) PDNT BDNF 30,080 pg/mL ± 8040 à 25780 pg/mL ± 11,720 (p > 0.05) HBT BDNF 20,210 pg/mL ± 13,330 à 34980 pg/mL ± 20,620 (p < 0.001) HNT BDNF 28,100 pg/mL ± 12,330 à 33,130 pg/mL ± 17,740 (p > 0.05) | Injured muscle (HIIT) recruit chemotactic factors (e.g., fractalkine) which induce release of BDNF from cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaagman, D.G.M.; van Wegen, E.E.H.; Cignetti, N.; Rothermel, E.; Vanbellingen, T.; Hirsch, M.A. Effects and Mechanisms of Exercise on Brain-Derived Neurotrophic Factor (BDNF) Levels and Clinical Outcomes in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Brain Sci. 2024, 14, 194. https://doi.org/10.3390/brainsci14030194
Kaagman DGM, van Wegen EEH, Cignetti N, Rothermel E, Vanbellingen T, Hirsch MA. Effects and Mechanisms of Exercise on Brain-Derived Neurotrophic Factor (BDNF) Levels and Clinical Outcomes in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Brain Sciences. 2024; 14(3):194. https://doi.org/10.3390/brainsci14030194
Chicago/Turabian StyleKaagman, Daan G. M., Erwin E. H. van Wegen, Natalie Cignetti, Emily Rothermel, Tim Vanbellingen, and Mark A. Hirsch. 2024. "Effects and Mechanisms of Exercise on Brain-Derived Neurotrophic Factor (BDNF) Levels and Clinical Outcomes in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis" Brain Sciences 14, no. 3: 194. https://doi.org/10.3390/brainsci14030194
APA StyleKaagman, D. G. M., van Wegen, E. E. H., Cignetti, N., Rothermel, E., Vanbellingen, T., & Hirsch, M. A. (2024). Effects and Mechanisms of Exercise on Brain-Derived Neurotrophic Factor (BDNF) Levels and Clinical Outcomes in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Brain Sciences, 14(3), 194. https://doi.org/10.3390/brainsci14030194







