Sensory Stimulation of the Triceps Surae Muscle Complex Modulates Spinal Reflex Responses—A Comparison between Tapotement Massage and Repetitive Peripheral Magnetic Stimulation (rPMS)
Abstract
1. Introduction
2. Materials and Methods
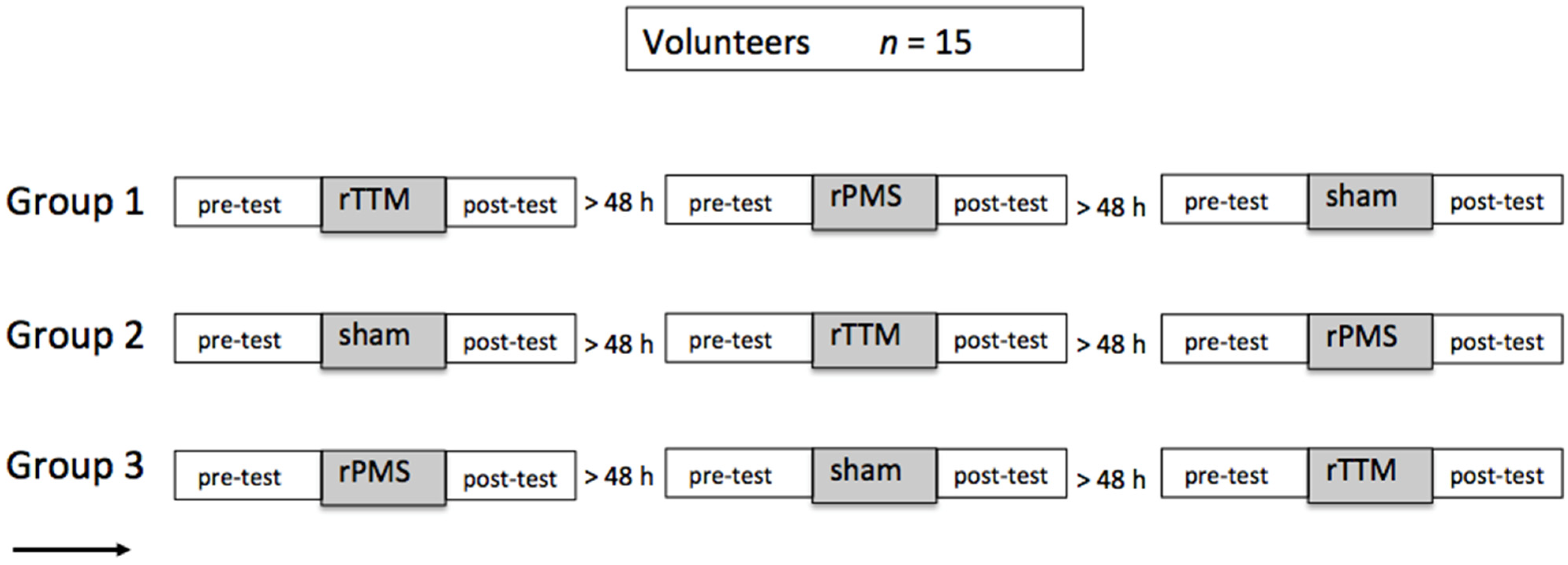
2.1. Participants and Experimental Design
2.2. Data Recording and Processing
2.3. Electromyography
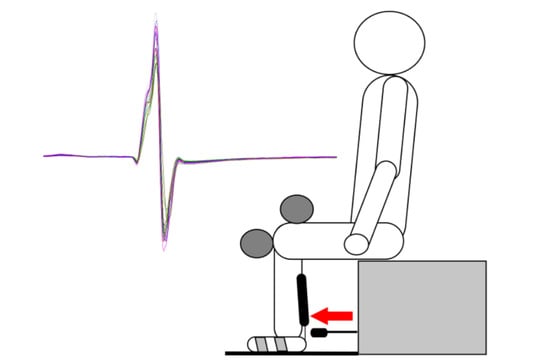
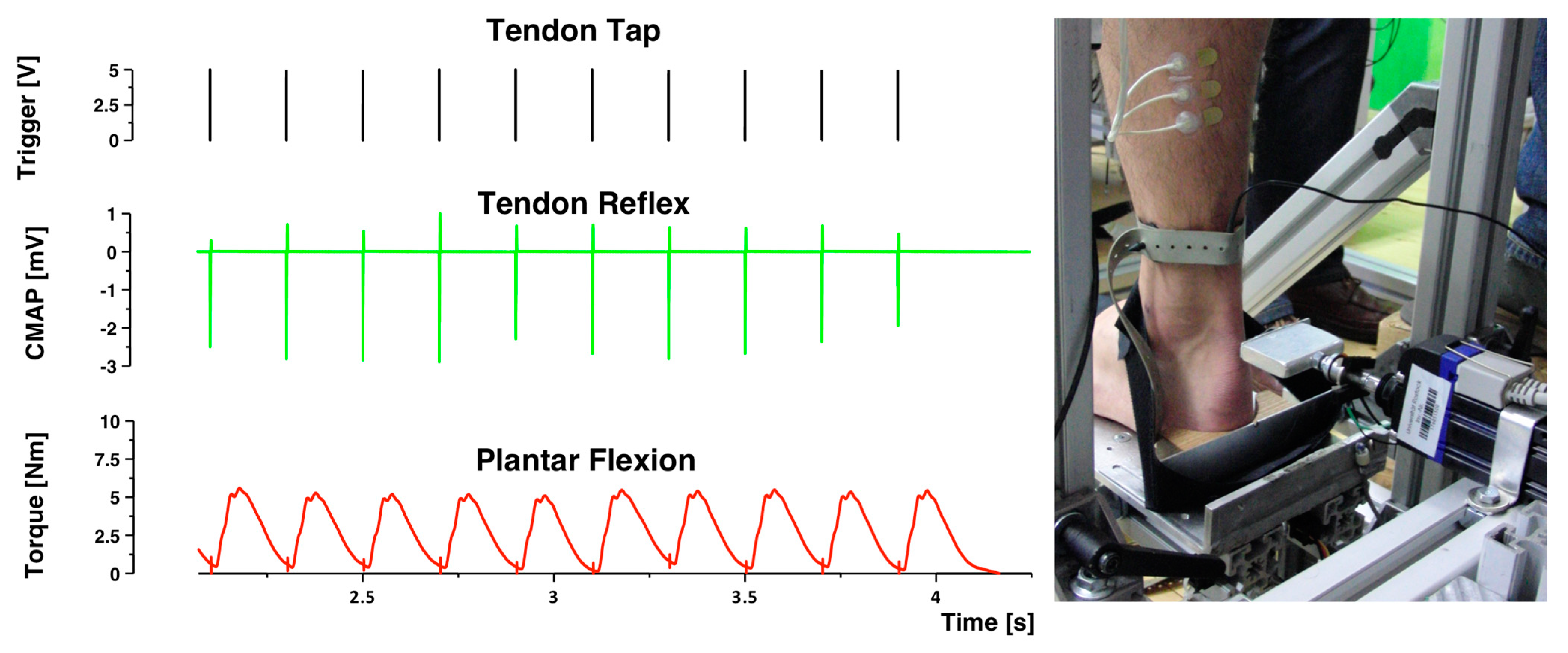
2.4. Tendon Reflex Administration
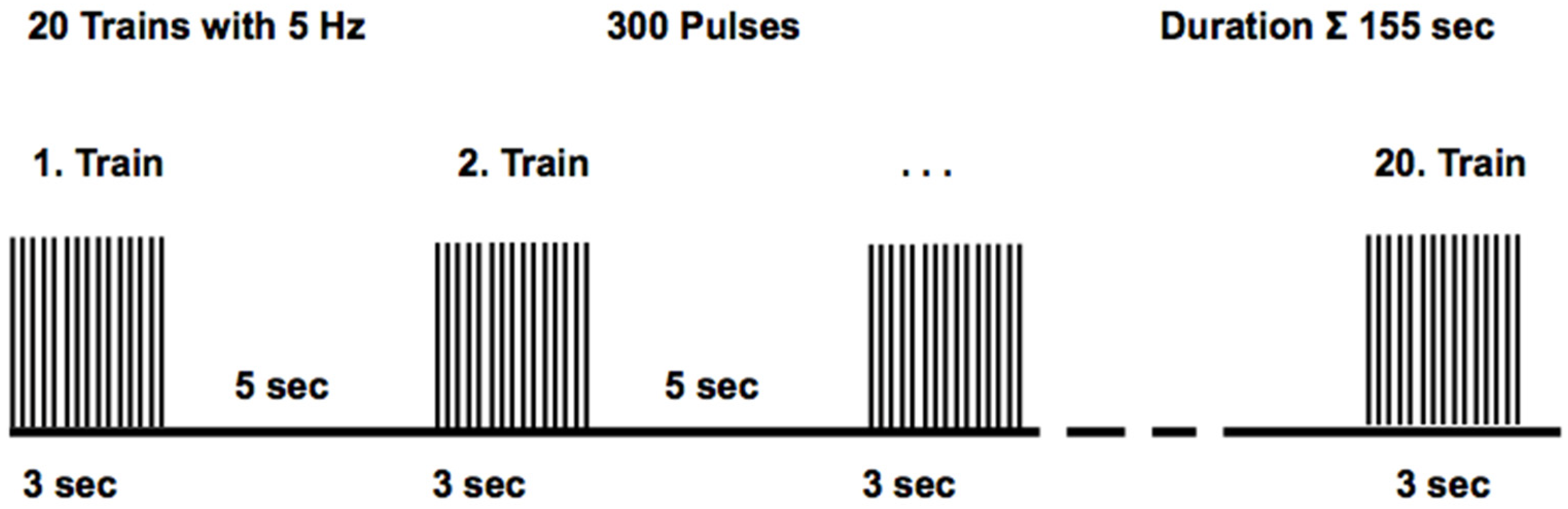
2.5. Repetitive Peripheral Magnetic Stimulation Protocol
2.6. Repetitive Tendon Tapotement Massage Protocol
3. Data Analysis and Statistics
4. Results
5. Discussion
5.1. Magnetic Stimulation
5.2. Mechanical Stimulation
5.3. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayer, N.H. Clinicophysiologic concepts of spasticity and motor dysfunction in adults with an upper motoneuron lesion. Muscle Nerve 1997, 20, 1–14. [Google Scholar] [CrossRef]
- Ivanhoe, C.B.; Reistetter, T.A. Spasticity: The misunderstood part of the upper motor neuron syndrome. Am. J. Phys. Med. Rehabil. 2004, 83, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Sinkjaer, T. Spastic movement disorder: Impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007, 6, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.-M. Physical modalities other than stretch in spastic hypertonia. Phys. Med. Rehabil. Clin. N. Am. 2001, 12, 769–792. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.P. An overview of the clinical management of spasticity. In Upper Motor Neurone Syndrome and Spasticity: Clinical Management and Neurophysiology; Cambridge University Press: Cambridge, UK, 2001; Volume 1. [Google Scholar]
- Katz, R.T. Management of spastic hypertonia after stroke. J. Neurol. Rehabil. 1991, 5, S5–S12. [Google Scholar] [CrossRef]
- Weerapong, P.; Hume, P.A.; Kolt, G.S. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005, 35, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Sullivan, S.J.; Seaborne, D.E. The effect of two intensities of massage on H-reflex amplitude. Phys. Ther. 1992, 72, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.J.; Seguin, S.; Seaborne, D.; Goldberg, J. Reduction of H-reflex amplitude during the application of effleurage to the triceps surae in neurologically healthy subjects. Physiother. Theory Pract. 1993, 9, 25–31. [Google Scholar] [CrossRef]
- Lapole, T.; Pérot, C. Effects of repeated achilles tendon vibration on triceps surae stiffness and reflex excitability. J. Electromyogr. Kines 2011, 21, 87–94. [Google Scholar] [CrossRef]
- Behm, D.G.; Peach, A.; Maddigan, M.; Aboodarda, S.J.; DiSanto, M.C.; Button, D.C.; Maffiuletti, N.A. Massage and stretching reduce spinal reflex excitability without affecting twitch contractile properties. J. Electromyogr. Kines 2013, 23, 1215–1221. [Google Scholar] [CrossRef]
- Eriksson Crommert, M.; Lacourpaille, L.; Heales, L.; Tucker, K.; Hug, F. Massage induces an immediate, albeit short-term, reduction in muscle stiffness. Scand. J. Med. Sci. Sports 2015, 25, e490–e496. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.-D.; Schneider, C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: A literature review on parameters of application and afferents recruitment. Neurophysiol. Clin. Clin. Neurophysiol. 2015, 45, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Zschorlich, V.R.; Hillebrecht, M.; Tanjour, T.; Qi, F.; Behrendt, F.; Kirschstein, T.; Köhling, R. Repetitive peripheral magnetic nerve stimulation (rPMS) as adjuvant therapy reduces skeletal muscle reflex activity. Front. Neurol. 2019, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.F.; Sinkjær, T. Long-lasting depression of soleus motoneurons excitability following repetitive magnetic stimuli of the spinal cord in multiple sclerosis patients. Mult. Scler. 1997, 3, 18–30. [Google Scholar] [CrossRef]
- Flamand, V.H.; Beaulieu, L.-D.; Nadeau, L.; Schneider, C. Peripheral magnetic stimulation to decrease spasticity in cerebral palsy. Pediatr. Neurol. 2012, 47, 345–348. [Google Scholar] [CrossRef]
- Krewer, C.; Hartl, S.; Müller, F.; Koenig, E. Effects of repetitive peripheral magnetic stimulation on upper-limb spasticity and impairment in patients with spastic hemiparesis: A randomized, double-blind, sham-controlled study. Arch. Phys. Med. Rehabil. 2014, 95, 1039–1047. [Google Scholar] [CrossRef]
- Ridding, M.; Brouwer, B.; Miles, T.; Pitcher, J.; Thompson, P. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp. Brain Res. 2000, 131, 135–143. [Google Scholar] [CrossRef]
- Kaelin-Lang, A.; Luft, A.R.; Sawaki, L.; Burstein, A.H.; Sohn, Y.H.; Cohen, L.G. Modulation of human corticomotor excitability by somatosensory input. J. Physiol. 2002, 540, 623–633. [Google Scholar] [CrossRef]
- Harriss, D.; Atkinson, G. Ethical standards in sport and exercise science research: 2014 update. Int. J. Sports Med. 2013, 34, 1025–1028. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef]
- Fattorini, L.; Rodio, A.; Pettorossi, V.E.; Filippi, G.M. Is the focal muscle vibration an effective motor conditioning intervention? A systematic review. J. Funct. Morphol. Kinesiol. 2021, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Filippi, G.M.; Rodio, A.; Fattorini, L.; Faralli, M.; Ricci, G.; Pettorossi, V.E. Plastic changes induced by muscle focal vibration: A possible mechanism for long-term motor improvements. Front. Neurosci. 2023, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Nito, M.; Katagiri, N.; Yoshida, K.; Koseki, T.; Kudo, D.; Nanba, S.; Tanabe, S.; Yamaguchi, T. Repetitive peripheral magnetic stimulation of wrist extensors enhances cortical excitability and motor performance in healthy individuals. Front. Neurosci. 2021, 15, 632716. [Google Scholar] [CrossRef] [PubMed]
- Zschorlich, V.R. Digital filtering of EMG-signals. Electromyogr. Clin. Neurophysiol. 1989, 29, 81–86. [Google Scholar] [PubMed]
- Zschorlich, V.R.; Qi, F.; Wolff, N. Comparing different filter-parameter for pre-processing of brain-stimulation evoked motor potentials. Brain Sci. 2021, 11, 1118. [Google Scholar] [CrossRef] [PubMed]
- Stam, J.; Van Crevel, H. Measurement of tendon reflexes by surface electromyography in normal subjects. J. Neurol. 1989, 236, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Avela, J.; Kyröläinen, H.; Komi, P.V. Altered reflex sensitivity after repeated and prolonged passive muscle stretching. J. Appl. Physiol. 1999, 86, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Perot, C.; Goubel, F.; Mora, I. Quantification of T-and H-responses before and after a period of endurance training. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 63, 368–375. [Google Scholar] [CrossRef]
- Stam, J.; Tan, K. Tendon reflex variability and method of stimulation. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 463–467. [Google Scholar] [CrossRef]
- Gallasch, E.; Christova, M.; Kunz, A.; Rafolt, D.; Golaszewski, S. Modulation of sensorimotor cortex by repetitive peripheral magnetic stimulation. Front. Hum. Neurosci. 2015, 9, 407. [Google Scholar] [CrossRef]
- Nilsson, J.; Panizza, M.; Roth, B.J.; Basser, P.J.; Cohen, L.G.; Caruso, G.; Hallett, M. Determining the site of stimulation during magnetic stimulation of a peripheral nerve. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1992, 85, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Zangrandi, A.; Sollmann, N.; Bonfert, M.V.; Beaulieu, L.-D.; rPMS Consensus Group. Checklist on the quality of the repetitive peripheral magnetic stimulation (rPMS) methods in research: An international Delphi study. Front. Neurol. 2022, 13, 852848. [Google Scholar] [CrossRef] [PubMed]
- Hagbarth, K.; Eklund, G. The muscle vibrator—A useful tool in neurological therapeutic work. J. Rehabil. Med. 1969, 1, 26–34. [Google Scholar] [CrossRef]
- Casale, R. Focal, local or segmental vibration? Eur. J. Phys. Rehabil. Med. 2015, 51, 507–508. [Google Scholar] [PubMed]
- Vickers, A.J.; Altman, D.G. Analysing controlled trials with baseline and follow up measurements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Richardson, J.T. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Nielsen, J.F.; Klemar, B.; Hansen, H.J.; Sinkjær, T. A new treatment of spasticity with repetitive magnetic stimulation in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1995, 58, 254. [Google Scholar] [CrossRef][Green Version]
- Nielsen, J.F.; Sinkjaer, T.; Jakobsen, J. Treatment of spasticity with repetitive magnetic stimulation; a double-blind placebo-controlled study. Mult. Scler. 1996, 2, 227–232. [Google Scholar] [CrossRef]
- Lance, J.W. The control of muscle tone, reflexes, and movement: Robert wartenbeg lecture. Neurology 1980, 30, 1303. [Google Scholar] [CrossRef]
- Aymard, C.; Katz, R.; Lafitte, C.; Lo, E.; Penicaud, A.; Pradat-Diehl, P.; Raoul, S. Presynaptic inhibition and homosynaptic depression: A comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain 2000, 123, 1688–1702. [Google Scholar] [CrossRef]
- Nielsen, J.; Crone, C.; Hultborn, H. The spinal pathophysiology of spasticity–from a basic science point of view. Acta Physiol. 2007, 189, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Grey, M.J.; Klinge, K.; Crone, C.; Lorentzen, J.; Biering-Sørensen, F.; Ravnborg, M.; Nielsen, J.B. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp. Brain Res. 2008, 185, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Petersen, N.; Crone, C. Changes in transmission across synapses of Ia afferents in spastic patients. Brain 1995, 118, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Crone, C.; Christenhuis, D.; Petersen, N.; Nielsen, J. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain 2001, 124, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, A.; Ashby, P. Postsynaptic potentials in individual soleus motoneurons in man produced by achilles tendon taps and electrical stimulation of tibial nerve. Electroencephalogr. Clin. Neurophysiol. 1982, 54, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.; Gandevia, S.C.; McKeon, B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J. Neurophysiol. 1984, 52, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Machetanz, J.; Bischoff, C.; Pichlmeier, R.; Riescher, H.; Meyer, B.U.; Sader, A.; Conrad, B. Magnetically induced muscle contraction is caused by motor nerve stimulation and not by direct muscle activation. Muscle Nerve 1994, 17, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Schrader, M.; Wernicke, S.; Bryl, B.; Hesse, S. Repetitive peripheral magnetic stimulation (rPMS) in combination with muscle stretch decreased the wrist and finger flexor muscle spasticity in chronic patients after cns lesion. Int. J. Phys. Med. Rehabil. 2016, 4, 10–4172. [Google Scholar]
- Pujol, J.; Pascual-Leone, A.; Dolz, C.; Delgado, E.; Dolz, J.L.; Aldoma, J. The effect of repetitive magnetic stimulation on localized musculoskeletal pain. Neuroreport 1998, 9, 1745–1748. [Google Scholar] [CrossRef]
- Heldmann, B.; Kerkhoff, G.; Struppler, A.; Havel, P.; Jahn, T. Repetitive peripheral magnetic stimulation alleviates tactile extinction. Neuroreport 2000, 11, 3193–3198. [Google Scholar] [CrossRef]
- Werner, C.; Zschorlich, V.; Hesse, S. Repetitive peripheral magnetic stimulation as a muscle stiffness therapy after CNS-lesion (Repetitive periphere Magnetstimulation als Therapie der Muskelstiffness nach ZNS-Läsion). In 22. Jahrestagung der Deutschen Gesellschaft für Neurorehabilitation (DGNR); German Neuroscience Society (GNS): Fürth, Germany, 2012. (In German) [Google Scholar]
- McKenney, K.; Elder, A.S.; Elder, C.; Hutchins, A. Myofascial release as a treatment for orthopaedic conditions: A systematic review. J. Athl. Train. 2013, 48, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Ajimsha, M.; Al-Mudahka, N.R.; Al-Madzhar, J. Effectiveness of myofascial release: Systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2015, 19, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Mau-Möller, A.; Zschorlich, V.; Bruhn, S. Repetitive peripheral magnetic stimulation (15 hz rPMS) of the human soleus muscle did not affect spinal excitability. J. Sports Sci. Med. 2011, 10, 39. [Google Scholar] [PubMed]
- El Nahas, N.; Kenawy, F.F.; Abd Eldayem, E.H.; Roushdy, T.M.; Helmy, S.M.; Akl, A.Z.; Ashour, A.A.; Emara, T.H.; Moawad, M.M.; Amin, R.M. Peripheral magnetic theta burst stimulation to muscles can effectively reduce spasticity: A randomized controlled trial. J. Neuroeng. Rehabil. 2022, 19, 1–7. [Google Scholar] [CrossRef]
- Steyvers, M.; Levin, O.; Verschueren, S.; Swinnen, S. Frequency-dependent effects of muscle tendon vibration on corticospinal excitability: A tms study. Exp. Brain Res. 2003, 151, 9–14. [Google Scholar] [CrossRef]
- Steyvers, M.; Levin, O.; Van Baelen, M.; Swinnen, S.P. Corticospinal excitability changes following prolonged muscle tendon vibration. Neuroreport 2003, 14, 2001–2004. [Google Scholar] [CrossRef]
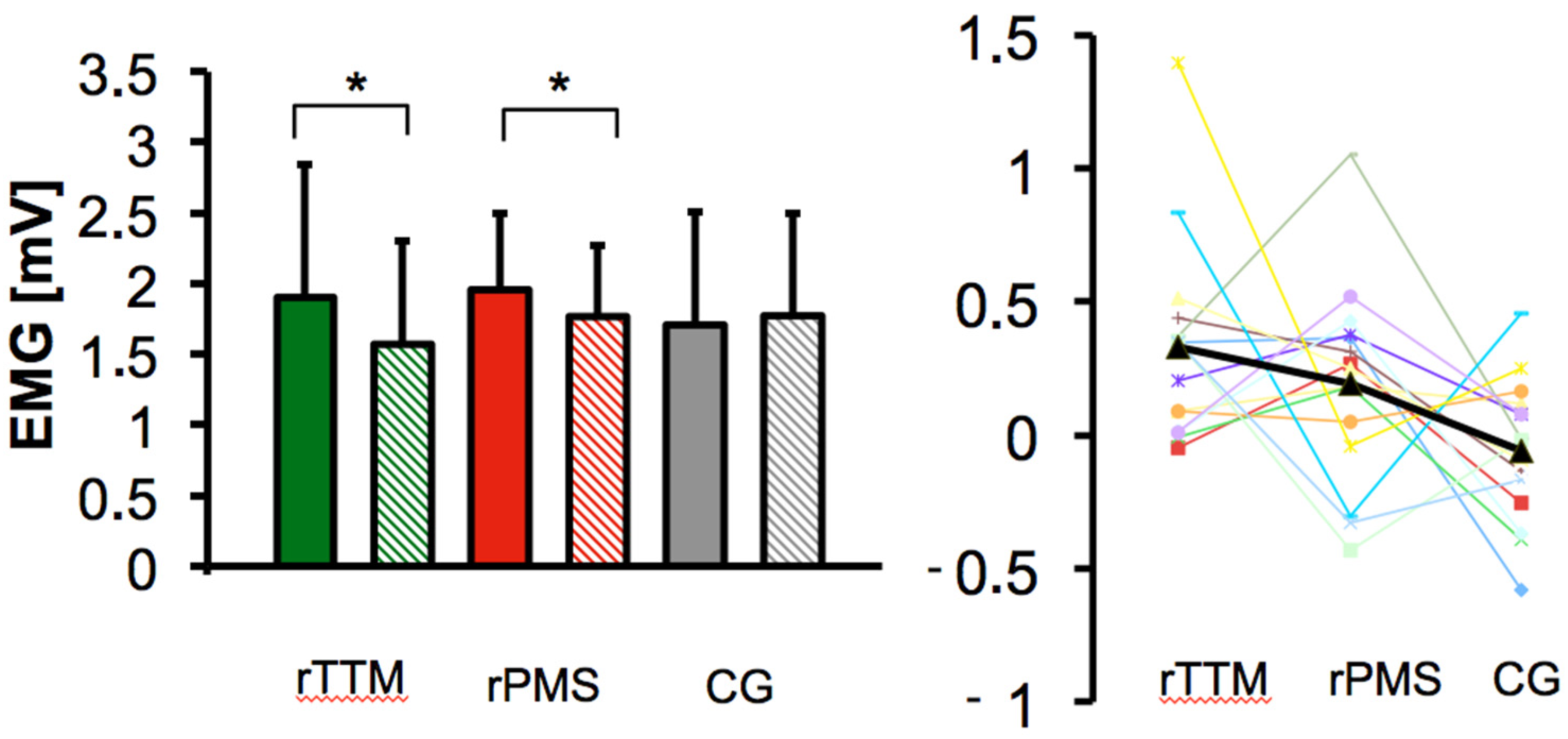
| Group | CMAPpre | CMAPpost | Group Effect | |
|---|---|---|---|---|
| F | p-Value ηp2 Partial | |||
| rTTM | 1.90 ± 0.94 | 1.57 ± 0.73 | 4.86 | * 0.013 0.188 |
| rPMS | 1.96 ± 0.54 | 1.77 ± 0.51 | ||
| sham | 1.71 ± 0.79 | 1.77 ± 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zschorlich, V.R.; Qi, F.; Schorer, J.; Büsch, D. Sensory Stimulation of the Triceps Surae Muscle Complex Modulates Spinal Reflex Responses—A Comparison between Tapotement Massage and Repetitive Peripheral Magnetic Stimulation (rPMS). Brain Sci. 2024, 14, 119. https://doi.org/10.3390/brainsci14020119
Zschorlich VR, Qi F, Schorer J, Büsch D. Sensory Stimulation of the Triceps Surae Muscle Complex Modulates Spinal Reflex Responses—A Comparison between Tapotement Massage and Repetitive Peripheral Magnetic Stimulation (rPMS). Brain Sciences. 2024; 14(2):119. https://doi.org/10.3390/brainsci14020119
Chicago/Turabian StyleZschorlich, Volker R., Fengxue Qi, Jörg Schorer, and Dirk Büsch. 2024. "Sensory Stimulation of the Triceps Surae Muscle Complex Modulates Spinal Reflex Responses—A Comparison between Tapotement Massage and Repetitive Peripheral Magnetic Stimulation (rPMS)" Brain Sciences 14, no. 2: 119. https://doi.org/10.3390/brainsci14020119
APA StyleZschorlich, V. R., Qi, F., Schorer, J., & Büsch, D. (2024). Sensory Stimulation of the Triceps Surae Muscle Complex Modulates Spinal Reflex Responses—A Comparison between Tapotement Massage and Repetitive Peripheral Magnetic Stimulation (rPMS). Brain Sciences, 14(2), 119. https://doi.org/10.3390/brainsci14020119