Olive- and Coconut-Oil-Enriched Diets Decreased Secondary Bile Acids and Regulated Metabolic and Transcriptomic Markers of Brain Injury in the Frontal Cortexes of NAFLD Pigs
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Experimental Design
2.2. Pen Activity and Novel Object Recognition Test
2.3. Fatty Acid Composition
2.4. Immunofluorescence Analysis
2.5. Analysis of Metabolites
2.6. Transcriptomic Analysis
2.7. Statistical Methods
3. Results
3.1. COC and OLI Pigs Had Decreased Physical Activity without Changes in Cognitive Function or Neuronal Loss
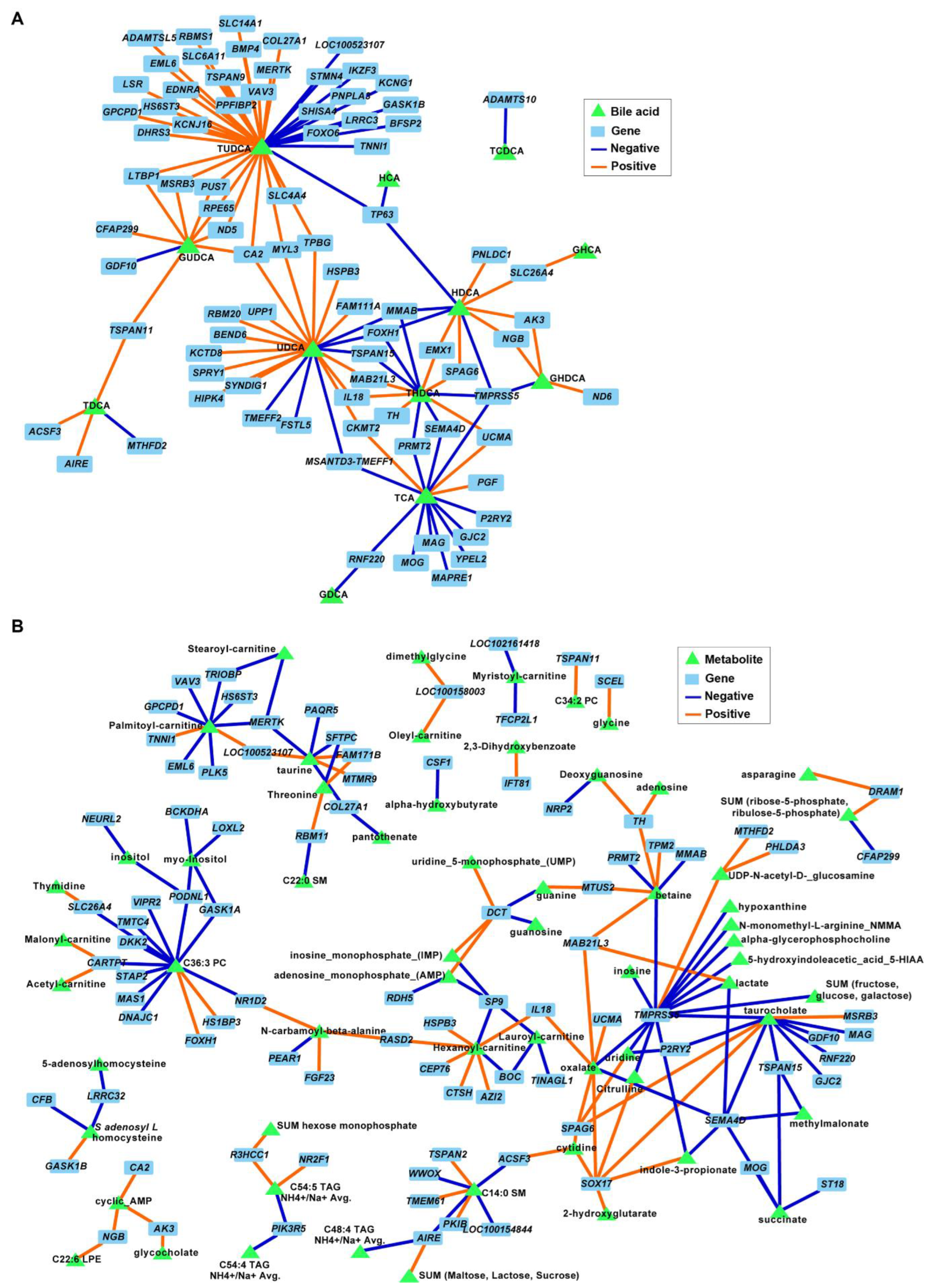
3.2. COC and OLI Diets Decreased Secondary BA Species Compared with LAR
3.3. COC and OLI Decreased One-Carbon Metabolites, Amino Acids, Complex Lipids, and Carboxylic Acids in the Frontal Cortex
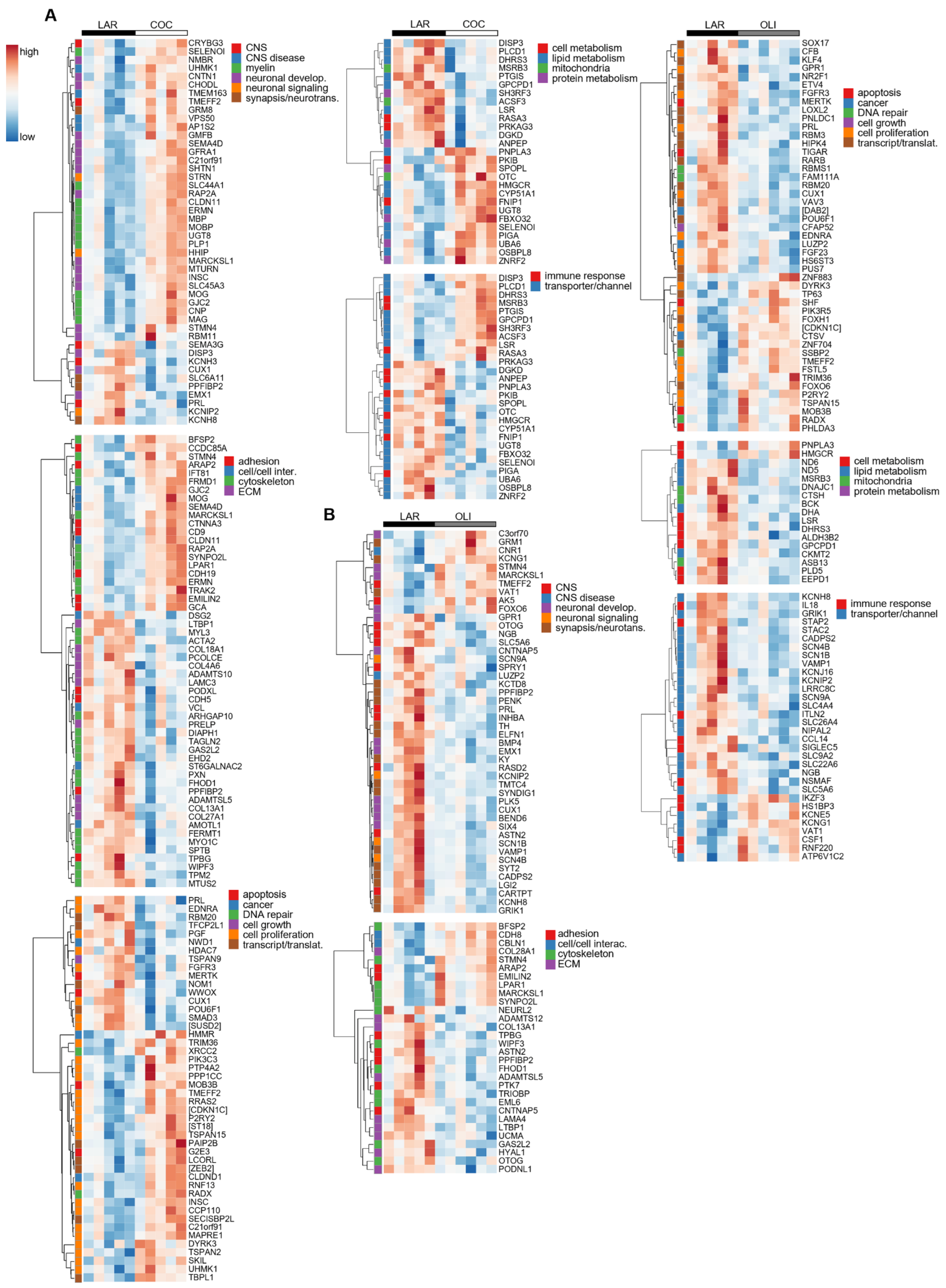
3.4. COC and OLI Diets Regulated Genes Associated with Myelin Formation, Neuronal Development, and Signaling
3.5. Secondary Bile Acids Were Correlated with Expression of Frontal Cortex Genes Involved in Neurogenesis, Neurotransmission, and Extracellular Matrix Organization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alisi, A.; Newton, K.P.; Schwimmer, J.B. Comparison of the phenotype and approach to pediatric vs adult patients with nonalcoholic fatty liver disease. Gastroenterology 2016, 150, 1798–1810. [Google Scholar] [CrossRef] [PubMed]
- Elliott, C.; Frith, J.; Day, C.P.; Jones, D.E.; Newton, J.L. Functional impairment in alcoholic liver disease and non-alcoholic fatty liver disease is significant and persists over 3 years of follow-up. Dig. Dis. Sci. 2013, 58, 2383–2391. [Google Scholar] [CrossRef] [PubMed]
- Filipović, B.; Marković, O.; Durić, V.; Filipović, B. Cognitive Changes and Brain Volume Reduction in Patients with Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2018, 2018, 9638797. [Google Scholar] [CrossRef]
- Seo, S.W.; Gottesman, R.F.; Clark, J.M.; Hernaez, R.; Chang, Y.; Kim, C.; Ha, K.H.; Guallar, E.; Lazo, M. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology 2016, 86, 1136–1142. [Google Scholar] [CrossRef]
- Weinstein, G.; Zelber-Sagi, S.; Preis, S.R.; Beiser, A.S.; DeCarli, C.; Speliotes, E.K.; Satizabal, C.L.; Vasan, R.S.; Seshadri, S. Association of nonalcoholic fatty liver disease with lower brain volume in healthy middle-aged adults in the Framingham Study. JAMA Neurol. 2018, 75, 97–104. [Google Scholar] [CrossRef]
- Bosoi, C.R.; Vandal, M.; Tournissac, M.; Leclerc, M.; Fanet, H.; Mitchell, P.L.; Verreault, M.; Trottier, J.; Virgili, J.; Tremblay, C.; et al. High-fat diet modulates hepatic amyloid β and cerebrosterol metabolism in the triple transgenic mouse model of Alzheimer’s Disease. Hepatol. Commun. 2020, 5, 446–460. [Google Scholar] [CrossRef]
- Kim, D.G.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.A.; Yan, A.; Torres, L.; Bynoe, M.S. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J. Neuroinflammation 2016, 13, 1. [Google Scholar] [CrossRef]
- Zeltser, N.; Meyer, I.; Hernandez, G.V.; Trahan, M.J.; Fanter, R.K.; Abo-Ismail, M.; Glanz, H.; Strand, C.R.; Burrin, D.G.; La Frano, M.R.; et al. Neurodegeneration in juvenile Iberian pigs with diet-induced nonalcoholic fatty liver disease. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E592–E606. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease—An emerging role for gut microbiome. Alzheimer’s Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef]
- Nho, K.; Kueider-Paisley, A.; Ahmad, S.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; et al. Association of altered liver enzymes with Alzheimer disease diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers. JAMA Netw. 2019, 2, e197978. [Google Scholar] [CrossRef]
- Pasquier, F.; Boulogne, A.; Leys, D.; Fontaine, P. Diabetes mellitus and dementia. Diabetes Metab. 2006, 32, 403–414. [Google Scholar] [CrossRef]
- Barnard, N.D.; Bunner, A.E.; Agarwal, U. Saturated and trans fats and dementia: A systematic review. Neurobiol. Aging 2014, 35 (Suppl. S2), S65–S73. [Google Scholar] [CrossRef]
- Ruan, Y.; Tang, J.; Guo, X.; Li, K.; Li, D. Dietary fat intake and risk of Alzheimer’s disease and dementia: A meta-analysis of cohort studies. Curr. Alzheimer Res. 2018, 15, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef]
- Spencer, S.J.; Korosi, A.; Laye, S.; Shukitt-Hale, B.; Barrientos, R.M. Food for thought: How nutrition impacts cognition and emotion. NPJ Sci. Food 2017, 1, 7. [Google Scholar] [CrossRef]
- Meeusen, R. Exercise, Nutrition and the Brain. Sports Med. 2014, 44, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Molina, T.L.; Stoll, B.; Mohammad, M.; Mohila, C.A.; Call, L.; Cui, L.; Guthrie, G.; Kunichoff, D.; Lin, S.; Welch-Jernigan, R.; et al. New generation lipid emulsions increase brain DHA and improve body composition, but not short-term neurodevelopment in parenterally-fed preterm piglets. Brain Behav. Immun. 2020, 85, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; McMillin, M.; Galindo, C.; Frampton, G.; Pae, H.Y.; DeMorrow, S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig. Liver Dis. 2014, 46, 527–534. [Google Scholar] [CrossRef]
- Greenwood, J.; Adu, J.; Davey, A.J.; Abbott, N.J.; Bradbury, M.W.B. The effect of bile salts on the permeability and ultrastructure of the perfused, energy-depleted, rat blood-brain barrier. J. Cereb. Blood Flow Metab. 1991, 11, 644–654. [Google Scholar] [CrossRef] [Green Version]
- Wahler, J.B.; Swain, M.G.; Carson, R.; Bergasa, N.V.; Jones, E.A. Blood-brain barrier permeability is markedly decreased in cholestasis in the rat. Hepatology 1993, 6, 1103–1108. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Quinn, M.; Ashfaq, S.; De Los Santos, M.; Grant, S.; DeMorrow, S. Bile acid signaling is involved in the neurological decline in a murine model of acute liver failure. Am. J. Pathol. 2016, 186, 312–323. [Google Scholar] [CrossRef]
- McMillin, M.; Grant, S.; Frampton, G.; Petrescu, A.D.; Kain, J.; Williams, E.; Haines, R.; Canady, L.; DeMorrow, S. FXR-mediated cortical cholesterol accumulation contributes to the pathogenesis of type A hepatic encephalopathy. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 47–63. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; Frampton, G.; Grant, S.; Khan, S.; Diocares, J.; Petrescu, A.; Wyatt, A.; Kain, J.; Jefferson, B.; DeMorrow, S. Bile acid-mediated sphingosine-1-phosphate receptor 2 signaling promotes neuroinflammation during hepatic encephalopathy in mice. Front. Cell. Neurosci. 2017, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.V.; Smith, V.A.; Melnyk, M.; Burd, M.A.; Sprayberry, K.A.; Edwards, M.S.; Peterson, D.G.; Bennet, D.C.; Fanter, R.K.; Columbus, D.A.; et al. Dysregulated FXR-FGF19 signaling and choline metabolism is associated with gut dysbiosis and hyperplasia in a novel pig model of pediatric NASH. Am. J. Physiol. Liver Physiol. 2020, 318, G582–G609. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signaling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef]
- Puri, P.; Daita, K.; Joyce, A.; Mirshahi, F.; Santhekadur, P.K.; Cazanave, S.; Luketic, V.A.; Siddiqui, M.S.; Boyett, S.; Min, H.K.; et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 2018, 67, 534–548. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Huang, M.; Sun, X.; Liu, B.; Guo, Q.; Chang, Q.; Duan, Z. Taurocholic acid is an active promoting factor, not just a biomarker of progression of liver cirrhosis, evidence from a human metabolomic study and in vitro experiments. BMC Gastroenterol. 2018, 18, 112. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Ridolfi, F.; Hannivoort, R.; Saccomanno, S.; Homan, M.; De Minicis, S.; Jansen, P.L.; Candelaresi, C.; Benedetti, A.; Moshage, H. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology 2005, 128, 1042–1055. [Google Scholar] [CrossRef]
- Xie, G.; Wang, X.; Jiang, R.; Zhao, A.; Yan, J.; Zheng, X.; Huang, F.; Liu, X.; Panee, J.; Rajani, C.; et al. Dysregulated bile acid signaling contributes to the neurological impairment in murine models of acute and chronic liver failure. EBioMedicine 2018, 37, 294–306. [Google Scholar] [CrossRef] [Green Version]
- Baloni, P.; Funk, C.C.; Yan, J.; Yurkovich, J.T.; Kueider-Paisley, A.; Nho, K.; Heinken, A.; Jia, W.; Mahmoudiandehkordi, S.; Louie, G.; et al. Metabolic network analysis reveals altered bile acid synthesis and metabolism in Alzheimer’s Disease. Cell Rep. Med. 2020, 1, 100138. [Google Scholar] [CrossRef] [PubMed]
- Marksteiner, J.; Blasko, I.; Kemmler, G.; Koal, T.; Humpel, C. Bile acid quantification of 20 plasma metabolites identifies lithocholic acid as a putative biomarker in Alzheimer’s disease. Metabolomics 2018, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Barbier Saint Hilaire, P.; Colsch, B.; Isnard, F.; Attala, S.; Schaefer, A.; Amador, M.D.; Rudler, M.; Lamari, F.; Sedel, F.; et al. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy. J. Hepatol. 2016, 65, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Bazzari, F.H.; Abdallah, D.M.; El-Abhar, H.S. Chenodeoxycholic acid ameliorates AlCl3-induced Alzheimer’s disease neurotoxicity and cognitive deterioration via enhanced insulin signaling in rats. Molecules 2019, 24, 1992. [Google Scholar] [CrossRef]
- Bell, S.M.; Barnes, K.; Clemmens, H.; Al-Rafiah, A.R.; Al-Ofi, E.A.; Leech, V.; Bandmann, O.; Shaw, P.J.; Blackburn, D.J.; Ferraiuolo, L.; et al. Ursodeoxycholic acid improves mitochondrial function and redistributes drp1 in fibroblasts from patients with either sporadic or familial Alzheimer’s disease. J. Mol. Biol. 2018, 430, 3942–3953. [Google Scholar] [CrossRef]
- Rosa, A.I.; Duarte-Silva, S.; Silva-Fernandes, A.; Nunes, M.J.; Carvalho, A.N.; Rodrigues, E.; Gama, M.J.; Rodrigues, C.M.P.; Maciel, P.; Castro-Caldas, M. Tauroursodeoxycholic acid improves motor symptoms in a mouse model of parkinson’s disease. Mol. Neurobiol. 2018, 55, 9139–9155. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, M.; Spady, D.K.; Dietschy, J.M. Regulation of hepatic cholesterol metabolism in the rat in vivo: Effect of a synthetic fat-free diet on sterol synthesis and low-density lipoprotein transport. Biochim. Biophys. Acta 1995, 1255, 293–300. [Google Scholar] [CrossRef]
- Botham, K.M.; Boyd, G.S. The effect of dietary fat on bile salt synthesis in rat liver. Biochim. Biophys. Acta 1983, 752, 307–314. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Goldsmith, G.A.; Hamilton, J.G.; Miller, O.N. Lowering of serum lipid concentrations: Mechanisms used by unsaturated fats, nicotinic acid, and neomycin: Excretion of sterols and bile acids. Arch. Intern. Med. 1960, 105, 512–517. [Google Scholar] [CrossRef]
- Haust, H.L.; Beveridge, J.M. Effect of varying type and quantity of dietary fat on the fecal excretion of bile acids in humans subsisting on formula diets. Arch. Biochem. Biophys. 1958, 78, 367–375. [Google Scholar] [CrossRef]
- Bisschop, P.H.; Bandsma, R.H.; Stellaard, F.; ter Harmsel, A.; Meijer, A.J.; Sauerwein, H.P.; Kuipers, F.; Romijn, J.A. Low-fat, high-carbohydrate and high-fat, low-carbohydrate diets decrease primary bile acid synthesis in humans. Am. J. Clin. Nutr. 2004, 79, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Manjarin, R.; Dillard, K.; Coffin, M.; Hernandez, G.V.; Smith, V.A.; Noland-Liddell, T.; Gehani, T.R.; Smart, H.J.; Wheeler, K.; Sprayberry, K.A.; et al. Dietary fat composition shapes bile acid metabolism and severity of liver injury in a pig model of pediatric NAFLD. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E187–E206. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Swine, 10th ed.; National Academy Press: Washington, DC, USA, 1998; pp. 110–123. [Google Scholar]
- De Blas, C.; Gasa, J.; Mateos, G.G. Necesidades Nutricionales Para Ganado Porcino; Normas FEDNA (2ª Edición) [Online]; Universidad Politécnica de Madrid: Madrid, Spain; Universidad Autónoma de Barcelona: Barcelona, Spain; Available online: http://www.fundacionfedna.org/sites/default/files/Normas%20PORCINO_2013rev2.pdf (accessed on 8 January 2020).
- Hof, P.R.; Morrison, J.H.; Cox, K. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: I. Superior frontal and inferior temporal cortex. J. Comp. Neurol. 1990, 301, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Fleming, S.A.; Dilger, R.N. Young pigs exhibit differential exploratory behavior during novelty preference tasks in response to age, sex, and delay. Behav. Brain Res. 2017, 321, 50–60. [Google Scholar] [CrossRef]
- Gifford, A.K.; Cloutier, S.; Newberry, R.C. Objects as enrichment: Effects of object exposure time and delay interval on object recognition memory of the domestic pig. Appl. Anim. Behav. Sci. 2007, 107, 206–217. [Google Scholar] [CrossRef]
- Pamplona, R.; Portero-Otín, M.; Riba, D.; Ruiz, C.; Prat, J.; Bellmunt, M.J.; Barja, G. Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. J. Lipid Res. 1998, 39, 1989–1994. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Townsend, M.K.; Clish, C.B.; Kraft, P.; Wu, C.; Souza, A.L.; Deik, A.A.; Tworoger, S.S.; Wolpin, B.M. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin. Chem. 2013, 59, 1657–1667. [Google Scholar] [CrossRef] [Green Version]
- Bajad, S.U.; Lu, W.; Kimball, E.H.; Yuan, J.; Peterson, C.; Rabinowitz, J.D. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrome-try. J. Chromatogr. A 2006, 1125, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes predic-tion in humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Piepho, H.P. Data transformation in statistical analysis of field trials with changing treatment variance. Agron. J. 2009, 101, 865–869. [Google Scholar] [CrossRef]
- Manjarin, R.; Maj, M.A.; La Frano, M.R.; Glanz, H. %polynova_2way: A SAS macro for implementation of mixed models for metabolomics data. PLoS ONE 2020, 15, e0244013. [Google Scholar] [CrossRef]
- Troyanskaya, O.; Cantor, M.; Sherlock, G.; Brown, P.; Hastie, T.; Tibshirani, R.; Botstein, D.; Altman, R.B. Missing value estimation methods for DNA microarrays. Bioinformatics 2001, 17, 520–525. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Lê Cao, K.A.; Martin, P.G.; Robert-Granié, C.; Besse, P. Sparse canonical methods for biological data integration: Application to a cross-platform study. BMC Bioinform. 2009, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Cao, K.A.; Davis, M.J.; Déjean, S. Visualizing associations between paired ‘omics’ data sets. BioData Min. 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Pariante, C.M.; Borsini, A. From dried bear bile to molecular investigation: A systematic review of the effect of bile acids on cell apoptosis, oxidative stress and inflammation in the brain, across pre-clinical models of neurological, neurodegenerative and neuropsychiatric disorders. Brain Behav. Immun. 2022, 99, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Karababa, A.; Groos-Sahr, K.; Albrecht, U.; Keitel, V.; Shafigullina, A.; Görg, B.; Häussinger, D. Ammonia Attenuates LPS-Induced Upregulation of Pro-Inflammatory Cytokine mRNA in Co-Cultured Astrocytes and Microglia. Neurochem. Res. 2017, 42, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Wang, X.Q.; Cheng, F.F.; Yan, X.; Luo, J.; Wang, Q.G. Hyodeoxycholic acid protects the neurovascular unit against oxygen-glucose deprivation and reoxygenation-induced injury in vitro. Neural Regen. Res. 2019, 14, 1941–1949. [Google Scholar]
- Mueller, M.; Thorell, A.; Claudel, T.; Jha, P.; Koefeler, H.; Lackner, C.; Hoesel, B.; Fauler, G.; Stojakovic, T.; Einarsson, C.; et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J. Hepatol. 2015, 62, 1398–1404. [Google Scholar] [CrossRef]
- Palmela, I.; Correia, L.; Silva, R.F.; Sasaki, H.; Kim, K.S.; Brites, D.; Brito, M.A. Hydrophilic bile acids protect human blood-brain barrier endothelial cells from disruption by unconjugated bilirubin: An in vitro study. Front. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef]
- Pan, X.; Elliott, C.T.; McGuinness, B.; Passmore, P.; Kehoe, P.G.; Hölscher, C.; McClean, P.L.; Graham, S.F.; Green, B.D. Metabolomic Profiling of Bile Acids in Clinical and Experimental Samples of Alzheimer’s Disease. Metabolites 2017, 7, 28. [Google Scholar] [CrossRef]
- Parry, G.J.; Rodrigues, C.M.; Aranha, M.M.; Hilbert, S.J.; Davey, C.; Kelkar, P.; Low, W.C.; Steer, C.J. Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic Acid in patients with amyotrophic lateral sclerosis. Clin. Neuropharmacol. 2010, 33, 17–21. [Google Scholar] [CrossRef]
- Pai, R.; French, D.; Ma, N.; Hotzel, K.; Plise, E.; Salphati, L.; Setchell, K.D.; Ware, J.; Lauriault, V.; Schutt, L.; et al. Antibody-mediated inhibition of fibroblast growth factor 19 results in increased bile acids synthesis and ileal malabsorption of bile acids in cynomolgus monkeys. Toxicol. Sci. 2012, 126, 446–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.; Hagenbuch, B.; Kullak-Ublick, G.A.; Benke, D.; Aguzzi, A.; Meier, P.J. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J. Pharmacol. Exp. Ther. 2000, 294, 73–79. [Google Scholar] [PubMed]
- Maeda, K.; Kambara, M.; Tian, Y.; Hofmann, A.F.; Sugiyama, Y. Uptake of ursodeoxycholate and its conjugates by human hepatocytes: Role of Na(+)-taurocholate cotransporting polypeptide (NTCP), organic anion transporting polypeptide (OATP) 1B1 (OATP-C), and oatp1B3 (OATP8). Mol. Pharm. 2006, 3, 70–77. [Google Scholar] [CrossRef]
- Dayon, L.; Guiraud, S.P.; Corthésy, J.; Da Silva, L.; Migliavacca, E.; Tautvydaitė, D.; Oikonomidi, A.; Moullet, B.; Henry, H.; Métairon, S.; et al. One-carbon metabolism, cognitive impairment and CSF measures of Alzheimer pathology: Homocysteine and beyond. Alzheimer’s Res. Ther. 2017, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.W.; Bradshaw, P.C. Amino Acid Catabolism in Alzheimer’s Disease Brain: Friend or Foe? Oxid. Med. Cell. Longev. 2017, 2017, 5472792. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, X.; Dang, R.; Zhang, W.; Zhang, J.; Yao, Z. Lactate deficit in an Alzheimer Disease mouse model: The relationship with neuronal damage. J. Neuropathol. Exp. Neurol. 2018, 77, 1163–1176. [Google Scholar] [CrossRef]
- Conway, M.E. Alzheimer’s disease: Targeting the glutamatergic system. Biogerontology 2020, 21, 257–274. [Google Scholar] [CrossRef]
- Balez, R.; Ooi, L. Getting to NO Alzheimer’s Disease: Neuroprotection versus neurotoxicity mediated by nitric oxide. Oxid. Med. Cell. Longev. 2016, 2016, 3806157. [Google Scholar] [CrossRef]
- Kuiper, M.A.; Teerlink, T.; Visser, J.J.; Bergmans, P.L.; Scheltens, P.; Wolters, E.C. L-glutamate, L-arginine and L-citrulline levels in cerebrospinal fluid of Parkinson’s disease, multiple system atrophy, and Alzheimer’s disease patients. J. Neural Transm. 2000, 107, 183–189. [Google Scholar] [CrossRef]
- Jadavji, N.M.; Deng, L.; Leclerc, D.; Malysheva, O.; Bedell, B.J.; Caudill, M.A.; Rozen, R. Severe methylenetetrahydrofolate reductase deficiency in mice results in behavioral anomalies with morphological and biochemical changes in hippocampus. Mol. Genet. Metab. 2012, 106, 149–159. [Google Scholar] [CrossRef]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Getty, C.M.; Sutton, B.P.; Dilger, R.N. Perinatal choline deficiency delays brain development and alters metabolite concentrations in the young pig. Nutr. Neurosci. 2016, 19, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006, 20, 43–49. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: An important nutrient in brain development, liver function and carcinogenesis. J. Am. Coll. Nutr. 1992, 5, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Buckman, L.B.; Hasty, A.H.; Flaherty, D.K.; Buckman, C.T.; Thompson, M.M.; Matlock, B.K.; Weller, K.; Ellacott, K.L. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav. Immun. 2014, 35, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef]
- Petrov, D.; Pedrós, I.; Artiach, G.; Sureda, F.X.; Barroso, E.; Pallàs, M.; Casadesús, G.; Beas-Zarate, C.; Carro, E.; Ferrer, I.; et al. High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiencies contribute to Alzheimer disease pathology in rodents. Biochim. Biophys. Acta 2015, 1852, 1687–1699. [Google Scholar] [CrossRef]
- Francis, H.M.; Stevenson, R.J. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav. Neurosci. 2011, 125, 943–955. [Google Scholar] [CrossRef]
- Holloway, C.J.; Cochlin, L.E.; Emmanuel, Y.; Murray, A.; Codreanu, I.; Edwards, L.M.; Szmigielski, C.; Tyler, D.J.; Knight, N.S.; Saxby, B.K.; et al. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am. J. Clin. Nutr. 2011, 93, 748–755. [Google Scholar] [CrossRef]
- Mazza, E.; Fava, A.; Ferro, Y.; Rotundo, S.; Romeo, S.; Bosco, D.; Pujia, A.; Montalcini, T. Effect of the replacement of dietary vegetable oils with a low dose of extravirgin olive oil in the Mediterranean Diet on cognitive functions in the elderly. J. Transl. Med. 2018, 16, 10. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solfrizzi, V.; Colacicco, A.M.; D’Introno, A.; Capurso, C.; Torres, F.; Rizzo, C.; Capurso, A.; Panza, F. Dietary intake of unsaturated fatty acids and age-related cognitive decline: A 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol. Aging 2006, 27, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Amtul, Z.; Westaway, D.; Cechetto, D.F.; Rozmahel, R.F. Oleic acid ameliorates amyloidosis in cellular and mouse models of Alzheimer’s disease. Brain Pathol. 2011, 21, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Iuliano, L.; Praticò, D. Extra-virgin olive oil ameliorates cognition and neuropathology of the 3xTg mice: Role of autophagy. Ann. Clin. Transl. Neurol. 2017, 4, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Nenov, M.; Dincer, O.; Iuliano, L.; Praticò, D. Extra virgin olive oil improves synaptic activity, short-term plasticity, memory, and neuropathology in a tauopathy model. Aging Cell. 2020, 19, e13076. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Coleman, P.D.; Cribbs, D.H.; Rogers, J.; Gillen, D.L.; Cotman, C.W. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1653–1661. [Google Scholar] [CrossRef]
- Kang, H.J.; Voleti, B.; Hajszan, T.; Rajkowska, G.; Stockmeier, C.A.; Licznerski, P.; Lepack, A.; Majik, M.S.; Jeong, L.S.; Banasr, M.; et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 2012, 18, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.L.; Barr, S.; Jeanes, Y.M. Habitual fat intake predicts memory function in younger women. Front. Hum. Neurosci. 2013, 7, 838. [Google Scholar] [CrossRef]
- Cherbuin, N.; Anstey, K.J. The Mediterranean diet is not related to cognitive change in a large prospective investigation: The PATH through Life study. Am. J. Geriatr. Psychiatry 2012, 20, 635–639. [Google Scholar] [CrossRef]
- Howe, A.M.; Burke, S.; O’Reilly, M.E.; McGillicuddy, F.C.; Costello, D.A. Palmitic Acid and Oleic Acid Differently Modulate TLR2-Mediated Inflammatory Responses in Microglia and Macrophages. Mol. Neurobiol. 2022, 59, 2348–2362. [Google Scholar] [CrossRef]
- Fan, R.; Hua, Y.; Shen, J.; Xiao, R.; Ma, W. Dietary fatty acids affect learning and memory ability via regulating inflammatory factors in obese mice. J. Nutr. Biochem. 2022, 103, 108959. [Google Scholar] [CrossRef]
- Kim, H.R.; Young, C.N. Inhibition of forebrain microglia ameliorates non-alcoholic fatty liver disease during obesity. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Hadjihambi, A.; Konstantinou, C.; Klohs, J.; Monsorno, K.; Le Guennec, A.; Donnelly, C.; Cox, J.; Kusumbe, A.; Hosford, P.S.; Soffientini, U.; et al. Partial MCT1 invalidation protects against diet-induced non-alcoholic fatty liver disease and the associated brain dysfunction. J. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mitchell, E.S. Cognition and Synaptic-Plasticity Related Changes in Aged Rats Supplemented with 8- and 10-Carbon Medium Chain Triglycerides. PLoS ONE 2016, 11, e0160159. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Larson, B.; Araujo, J.A.; Lau, W.; de Rivera, C.; Santana, R.; Gore, A.; Milgram, N.W. Dietary supplementation with medium-chain TAG has long-lasting cognition-enhancing effects in aged dogs. Br. J. Nutr. 2010, 103, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- De la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Julián Rochina, M.; Aguilar, M.A.; Hu Yang, I. Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimer’s Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef]
- Henderson, S.T.; Poirier, J. Pharmacogenetic analysis of the effects of polymorphisms in APOE, IDE and IL1B on a ketone body based therapeutic on cognition in mild to moderate Alzheimer’s disease; a randomized, double-blind, placebo-controlled study. BMC Med. Genet. 2011, 12, 137. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2016, 44, 951–953. [Google Scholar] [CrossRef]
- Orosco, M.; Rouch, C.; Daugé, V. Behavioral responses to ingestion of different sources of fat. Involvement of serotonin? Behav. Brain Res. 2002, 132, 103–109. [Google Scholar] [CrossRef]
- Butt, A.M.; Rivera, A.D.; Fulton, D.; Azim, K. Targeting the Subventricular Zone to Promote Myelin Repair in the Aging Brain. Cells 2022, 11, 1809. [Google Scholar] [CrossRef]
- El Khoury, D. Study of Myelin Gene Expression in the Central Nervous System Using Real-Time PCR. Methods Mol. Biol. 2019, 2011, 659–670. [Google Scholar] [PubMed]
- El Waly, B.; Macchi, M.; Cayre, M.; Durbec, P. Oligodendrogenesis in the normal and pathological central nervous system. Front. Neurosci. 2014, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Cescon, M.; Chen, P.; Castagnaro, S.; Gregorio, I.; Bonaldo, P. Lack of collagen VI promotes neurodegeneration by impairing autophagy and inducing apoptosis during aging. Aging 2016, 8, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- White, C.J.; Lee, J.; Choi, J.; Chu, T.; Scafidi, S.; Wolfgang, M.J. Determining the Bioenergetic Capacity for Fatty Acid Oxidation in the Mammalian Nervous System. Mol. Cell Biol. 2020, 40, e00037-20. [Google Scholar] [CrossRef] [PubMed]

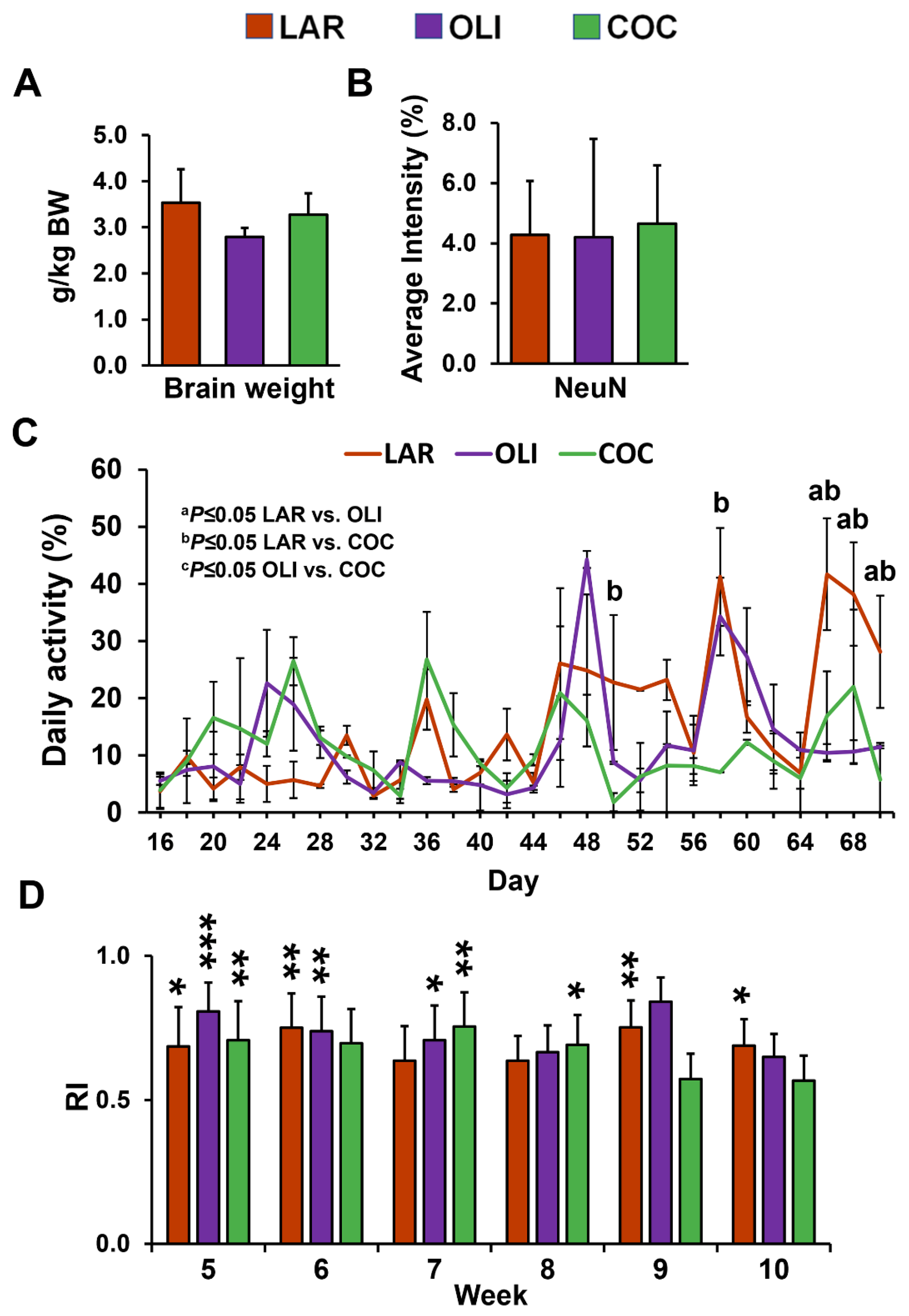

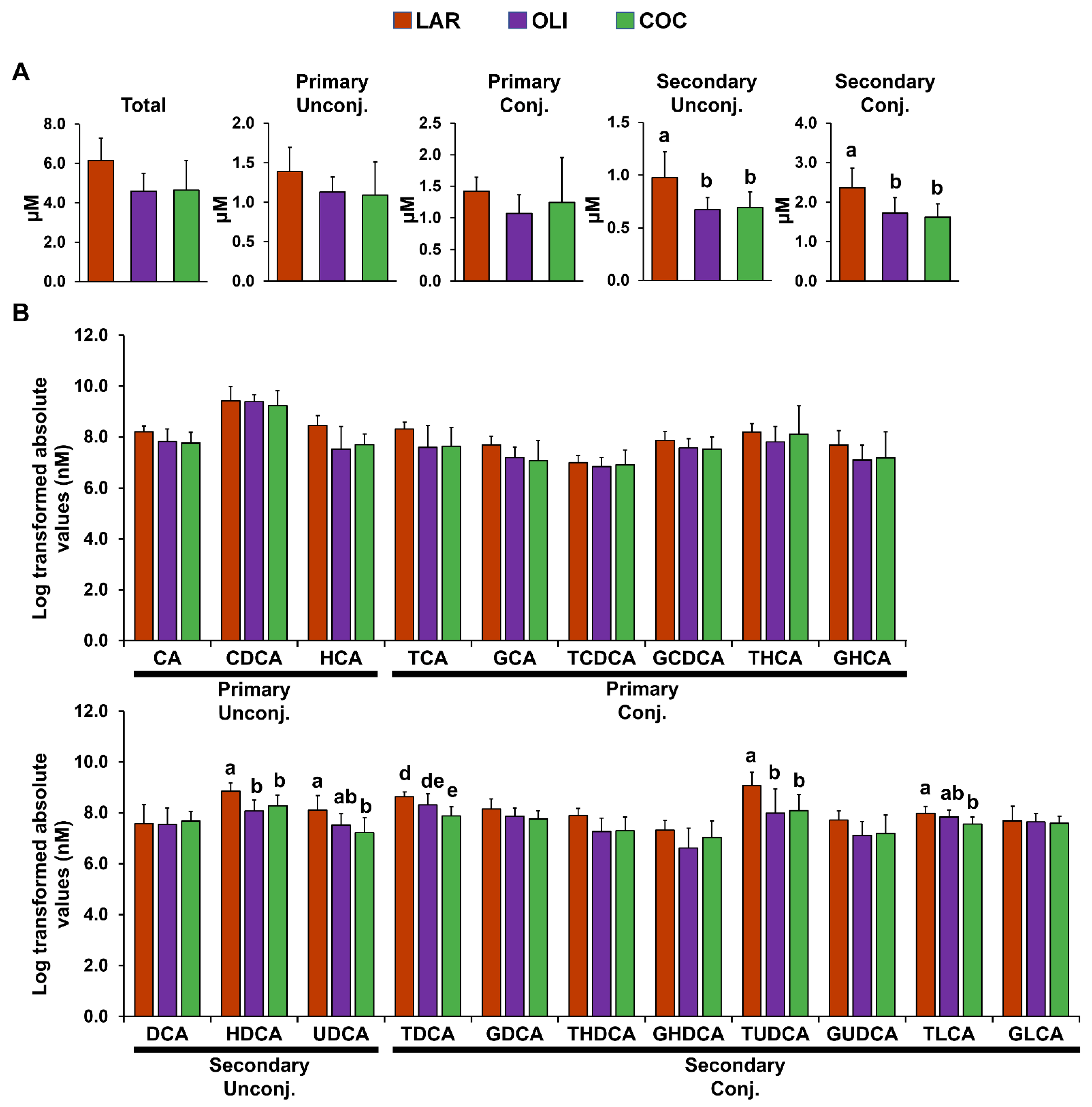
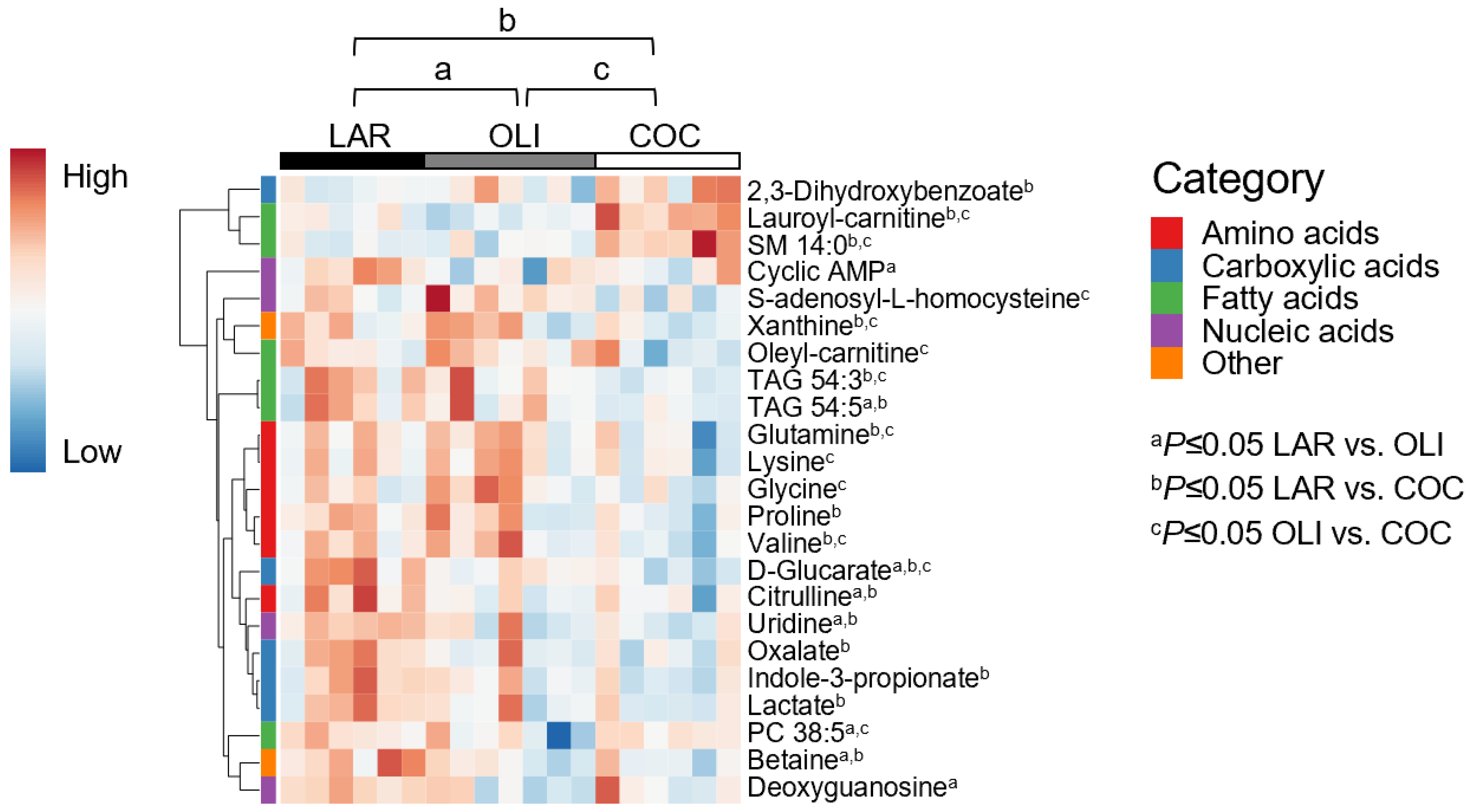
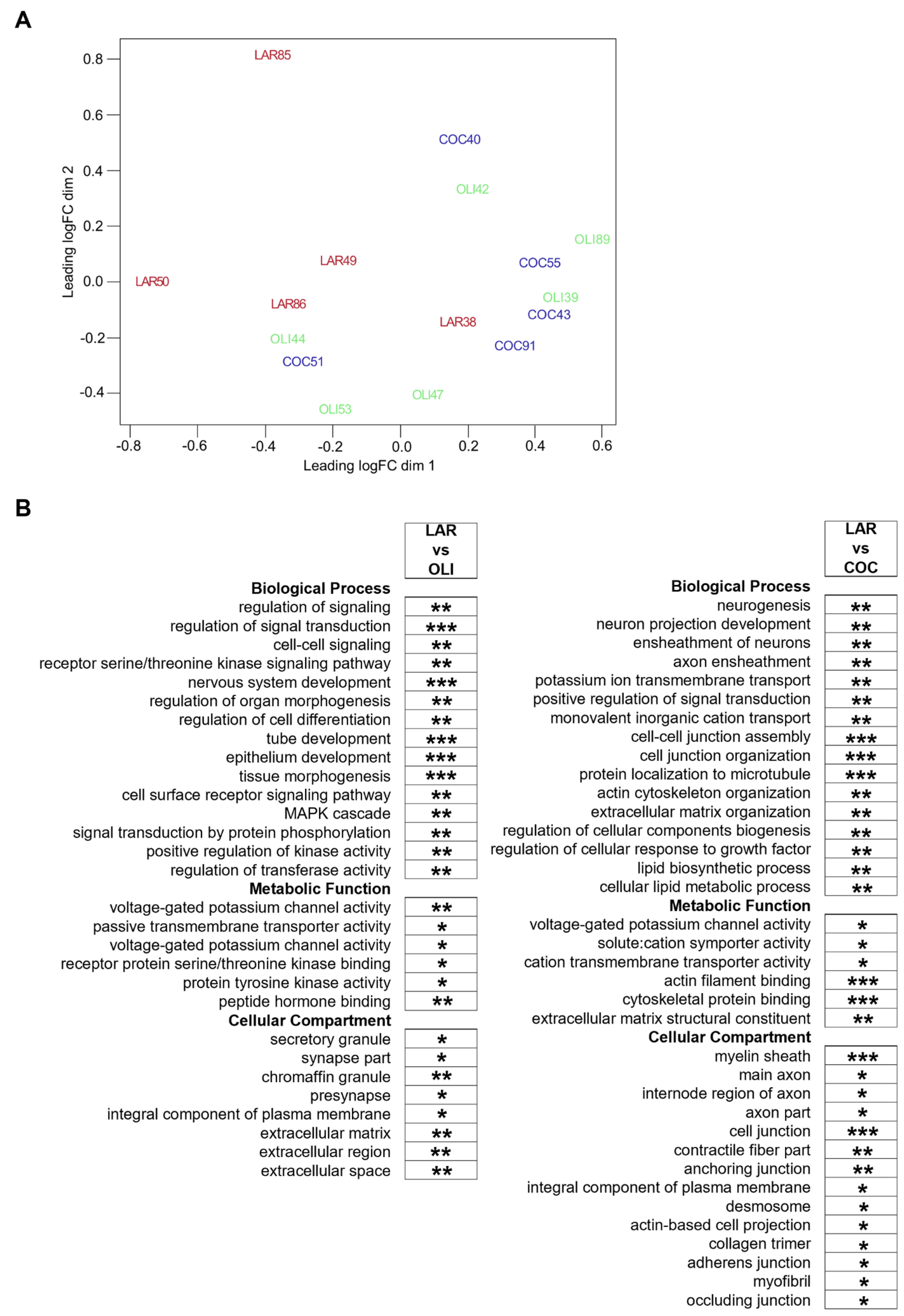
| Behavior | Description |
|---|---|
| Pig activity | |
| Standing | Both pigs in the pen are standing on four legs |
| Resting | Both pigs in the pen are lying down on the floor |
| Standing/resting | One pig is standing and one pig is lying down |
| Novel object recognition test | |
| Learning phase | |
| Sample Object I | Pig exploring sample object on left side |
| Sample Object II | Pig exploring sample object on right side |
| Memory phase | |
| Sample Object | Pig exploring sample object on left side |
| Novel Object | Pig exploring novel object on right side |
| Item 1 | LAR | OLI | COC | ||||
|---|---|---|---|---|---|---|---|
| Nº pigs (pen) | 10 (5) | 10 (5) | 10 (5) | ||||
| Sex (M/F) | 6/4 | 7/3 | 6/4 | ||||
| Liver histology 2 | |||||||
| Steatosis | 2.90 a ± 0.32 | 3.40 ab ± 0.52 | 3.50 b ± 0.53 | ||||
| Ballooning | 0 ± 0 | 0.40 ± 0.52 | 0.30 ± 0.48 | ||||
| Mallory–Denk Bodies | 0.10 ± 0.32 | 0.20 ± 0.42 | 0.30 ± 0.48 | ||||
| Inflammation | 1.20 ± 0.42 | 1.20 ± 0.42 | 1.33 ± 0.42 | ||||
| Necrosis | 0 d ± 0 | 1.00 e ± 0.47 | 0.70 e ± 0.58 | ||||
| Ki67+ cells 3 | 8.23 a ± 3.45 | 9.56 ab ± 3.23 | 14.7 b ± 6.85 | ||||
| Composite lesion score | 4.20 d ± 0.42 | 6.20 e ± 1.23 | 6.00 e ± 0.82 | ||||
| Serum biochemistry | |||||||
| Alanine aminotransferase, U·L−1 | 34.3 a ± 3.5 | 70.6 b ± 18.0 | 62.2 b ± 40.4 | ||||
| Aspartate aminotransferase, U·L−1 | 65.7 ± 48.5 | 180.5 ± 75.6 | 199.6 ± 149.2 | ||||
| Alkaline phosphatase, U·L−1 | 292.6 ± 80.7 | 387.7 ± 120.8 | 391.6 ± 149.2 | ||||
| γ-glutamyl transferase, U·L−1 | 33.3 a ± 9.9 | 37.3 ab ± 4.8 | 56.8 b ± 29.4 | ||||
| Lactate dehydrogenase, U·L−1 | 1819.8 a ± 1078.3 | 3986.5 b ± 829.1 | 3114.2 b ± 1492.7 | ||||
| Total bilirubin, mg·dL−1 | 0.04 ± 0.04 | 0.02 ± 0.02 | 0.05 ± 0.03 | ||||
| LAR | OLI | COC | ||
|---|---|---|---|---|
| FAs | ||||
| Caprylic | C8:0 | 515.8 ± 77.2 | 514.1 ± 69.3 | 497.5 ± 56.3 |
| Myristic | C14:0 | 620.1 ± 63.0 | 621.6 ± 76.8 | 664.0 ± 35.3 |
| Pentanedecanoic | C15:1 (n-5) | 1122.3 ± 206.4 | 1134.8 ± 125.1 | 1267.1 ± 259.2 |
| Palmitic | C16:0 | 7705.1 ± 721.1 | 7741.0 ± 520.6 | 7935.6 ± 774.3 |
| Palmitoleic | C16:1 (n-7) | 587.2 ± 49.0 | 585.8 ± 49.1 | 591.7 ± 38.8 |
| Heptadecanoic | C17:0 | 371.8 ± 32.9 | 392.8 ± 84.2 | 357.8 ± 27.7 |
| Heptadecenoic | C17:1 (n-7) | 302.1 ± 22.8 | 309.5 ± 45.9 | 295.5 ± 29.5 |
| Stearic | C18:0 | 7470.6 ± 930.5 | 7277.3 ± 678.8 | 7682.3 ± 1207.3 |
| Oleic | C18:1 (n-9) | 6305.8 ± 493.6 | 6572.8 ± 769.5 | 6559.4 ± 1388.3 |
| Linoleic | C18:2 (n-6) | 473.1 ± 22.8 | 472.5 ± 78.2 | 449.2 ± 41.9 |
| Arachidic | C20:0 | 617.2 ± 87.8 | 576.8 ± 67.6 | 559.1 ± 35.4 |
| Eicosenoic | C20:1 (n-9) | 478.3 ± 37.6 | 469.9 ± 43.3 | 449.4 ± 49.2 |
| Eicosadienoic | C20:2 (n-6) | 524.7 ± 74.2 | 573.9 ± 58.4 | 578.6 ± 78.3 |
| Heneicosanoic | C21:0 | 323.5 ± 27.9 | 305.5 ± 20.1 | 323.8 ± 60.0 |
| Eicosatrienoic | C20:3 (n-6) | 396.6 ± 35.0 | 422.0 ± 70.8 | 421.3 ± 93.6 |
| Arachidonic | C20:4 (n-6) | 4119.0 ± 376.6 | 4228.3 ± 234.0 | 4224.5 ± 591.4 |
| Behenic | C22:0 | 600.9 ± 109.3 | 534.0 ± 112.8 | 493.6 ± 68.5 |
| Erucic | C22:1 (n-9) | 182.5 ± 11.8 | 167.9 ± 21.4 | 238.6 ± 109.4 |
| Nervonic | C24:1 (n-9) | 2583.2 ± 362.1 | 2581.6 ± 129.0 | 2578.5 ± 416.4 |
| Length of FAs | ||||
| MCFAs | C6-12 | 515.8 ± 77.2 | 514.1 ± 69.3 | 497.5 ± 56.3 |
| LCFAs | C13-21 | 31,102.3 ± 2368.6 | 31,041.9 ± 2624.9 | 30,955.5 ± 4997.8 |
| VLCFAs | C22-24 | 3205.6 ± 391.5 | 2982.6 ± 373.4 | 2926.3 ± 379.2 |
| Saturation of FAs | ||||
| Saturated | 17,860.2 ± 1619.5 | 17,180.1 ± 1555.1 | 17,807.1 ± 2266.9 | |
| Unsaturated | 16,963.5 ± 1182.3 | 17,358.5 ± 1229.7 | 16,572.3 ± 3217.1 | |
| MUFAs | 11,450.2 ± 895.8 | 11,661.8 ± 1004.3 | 10,898.6 ± 2934.4 | |
| PUFAs | 5513.3 ± 357.0 | 5696.7 ± 292.5 | 5673.7 ± 575.2 | |
| Ratio Sat/Unsat | 1.1 ± 0.1 | 1.0 ± 0.0 | 1.1 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maj, M.A.; Gehani, T.R.; Immoos, C.; Medrano, M.S.; Fanter, R.K.; Strand, C.R.; Glanz, H.; Piccolo, B.D.; Abo-Ismail, M.K.; La Frano, M.R.; et al. Olive- and Coconut-Oil-Enriched Diets Decreased Secondary Bile Acids and Regulated Metabolic and Transcriptomic Markers of Brain Injury in the Frontal Cortexes of NAFLD Pigs. Brain Sci. 2022, 12, 1193. https://doi.org/10.3390/brainsci12091193
Maj MA, Gehani TR, Immoos C, Medrano MS, Fanter RK, Strand CR, Glanz H, Piccolo BD, Abo-Ismail MK, La Frano MR, et al. Olive- and Coconut-Oil-Enriched Diets Decreased Secondary Bile Acids and Regulated Metabolic and Transcriptomic Markers of Brain Injury in the Frontal Cortexes of NAFLD Pigs. Brain Sciences. 2022; 12(9):1193. https://doi.org/10.3390/brainsci12091193
Chicago/Turabian StyleMaj, Magdalena A., Tanvi R. Gehani, Chad Immoos, Mikaelah S. Medrano, Rob K. Fanter, Christine R. Strand, Hunter Glanz, Brian D. Piccolo, Mohammed K. Abo-Ismail, Michael R. La Frano, and et al. 2022. "Olive- and Coconut-Oil-Enriched Diets Decreased Secondary Bile Acids and Regulated Metabolic and Transcriptomic Markers of Brain Injury in the Frontal Cortexes of NAFLD Pigs" Brain Sciences 12, no. 9: 1193. https://doi.org/10.3390/brainsci12091193
APA StyleMaj, M. A., Gehani, T. R., Immoos, C., Medrano, M. S., Fanter, R. K., Strand, C. R., Glanz, H., Piccolo, B. D., Abo-Ismail, M. K., La Frano, M. R., & Manjarín, R. (2022). Olive- and Coconut-Oil-Enriched Diets Decreased Secondary Bile Acids and Regulated Metabolic and Transcriptomic Markers of Brain Injury in the Frontal Cortexes of NAFLD Pigs. Brain Sciences, 12(9), 1193. https://doi.org/10.3390/brainsci12091193








