Safety Evaluation of Employing Temporal Interference Transcranial Alternating Current Stimulation in Human Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Neurological and Neuropsychology Tests
2.4. EEG Recording
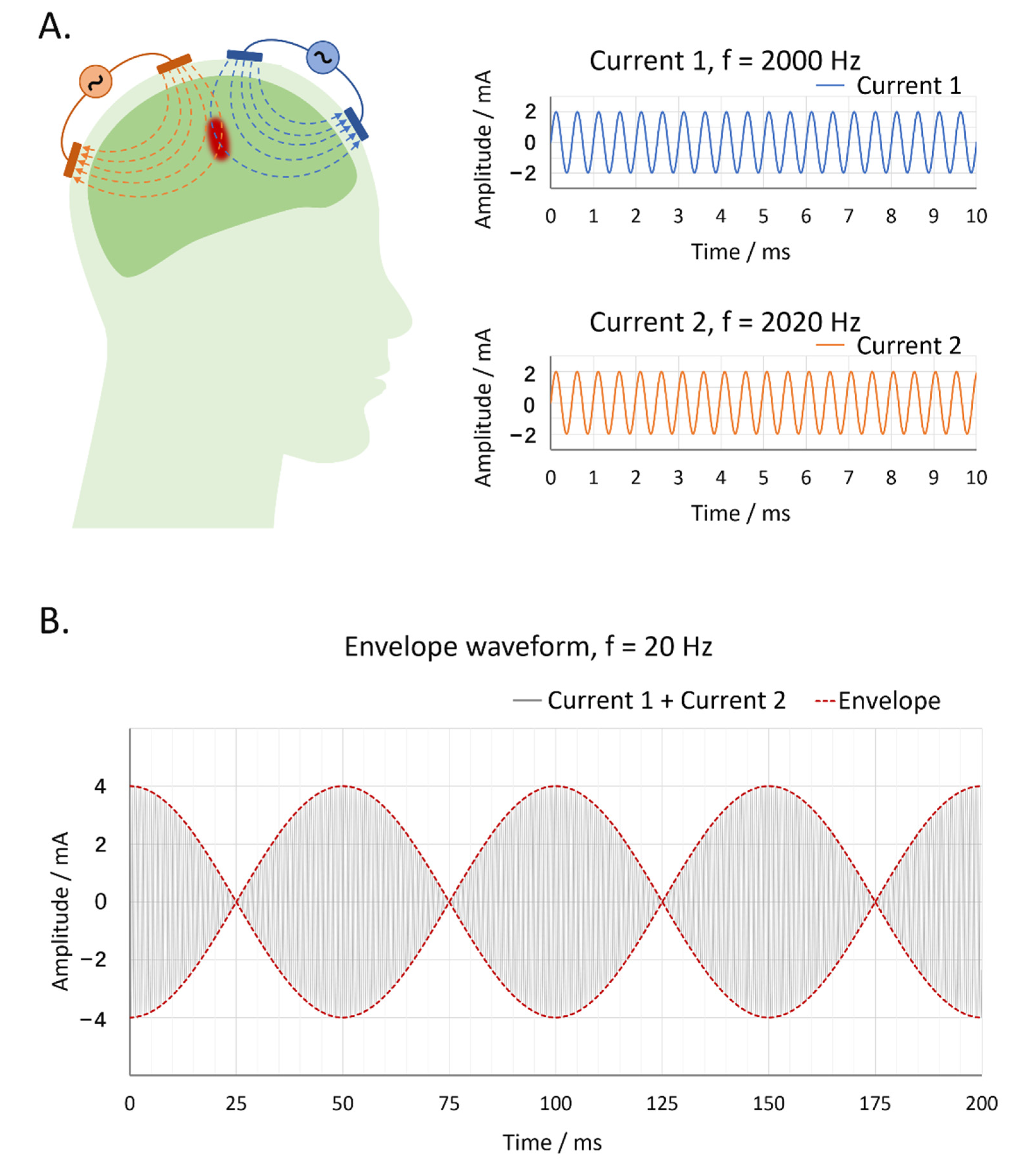
2.5. Temporal Interference Transcranial Alternating Current Stimulation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polania, R.; Nitsche, M.A.; Ruff, C.C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 2018, 21, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041.e16. [Google Scholar] [CrossRef]
- Dmochowski, J.; Bikson, M. Noninvasive Neuromodulation Goes Deep. Cell 2017, 169, 977–978. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N. Modulation without surgical intervention. Science 2018, 361, 461–462. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Z.; Sun, W.; Zhang, J.; Wang, J.; Shi, Y.; Yang, R.; Li, C.; Chen, D.; Wu, J.; et al. Development of a Non-invasive Deep Brain Stimulator With Precise Positioning and Real-Time Monitoring of Bioimpedance. Front. Neuroinform. 2020, 14, 574189. [Google Scholar] [CrossRef]
- Missey, F.; Rusina, E.; Acerbo, E.; Botzanowski, B.; Trebuchon, A.; Bartolomei, F.; Jirsa, V.; Carron, R.; Williamson, A. Orientation of Temporal Interference for Non-invasive Deep Brain Stimulation in Epilepsy. Front. Neurosci. 2021, 15, 633988. [Google Scholar] [CrossRef]
- Song, X.; Zhao, X.; Li, X.; Liu, S.; Ming, D. Multi-channel transcranial temporally interfering stimulation (tTIS): Application to living mice brain. J. Neural. Eng. 2021, 18, 036003. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Attarpour, A.; Amirfattahi, R.; Nezhad, A.Z. Computational analysis of non-invasive deep brain stimulation based on interfering electric fields. Phys. Med. Biol. 2019, 64, 235010. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.; Park, J.; Im, C.H. Individually customized transcranial temporal interference stimulation for focused modulation of deep brain structures: A simulation study with different head models. Sci. Rep. 2020, 10, 11730. [Google Scholar] [CrossRef]
- Wang, H.; Sun, W.; Zhang, J.; Yan, Z.; Wang, C.; Wang, L.; Liu, T.; Li, C.; Chen, D.; Shintaro, F.; et al. Influence of layered skull modeling on the frequency sensitivity and target accuracy in simulations of transcranial current stimulation. Hum. Brain Mapp. 2021, 42, 5345–5356. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.; Choi, D.S.; Lee, C.; Im, C.H. Multipair transcranial temporal interference stimulation for improved focalized stimulation of deep brain regions: A simulation study. Comput. Biol. Med. 2022, 143, 105337. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.; Roig-Solvas, B.; Yarossi, M.; Kulkarni, P.P.; Santarnecchi, E.; Dorval, A.D.; Brooks, D.H. Prospects for transcranial temporal interference stimulation in humans: A computational study. Neuroimage 2019, 202, 116124. [Google Scholar] [CrossRef]
- von Conta, J.; Kasten, F.H.; Curcic-Blake, B.; Aleman, A.; Thielscher, A.; Herrmann, C.S. Interindividual variability of electric fields during transcranial temporal interference stimulation (tTIS). Sci. Rep. 2021, 11, 20357. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Xia, X.; Zhang, W.; Lu, Z.; Wu, Q.; Cui, J.; Song, H.; Fan, C.; Chen, X.; Zha, R.; et al. High Gamma and Beta Temporal Interference Stimulation in the Human Motor Cortex Improves Motor Functions. Front. Neurosci. 2021, 15, 800436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xiong, Y.; Chen, Y.; Jiang, Y.; Qian, Z.; Lu, J.; Liu, Y.; Zhuang, J. Temporal Interference (TI) Stimulation Boosts Functional Connectivity in Human Motor Cortex: A Comparison Study with Transcranial Direct Current Stimulation (tDCS). Neural. Plast 2022, 2022, 7605046. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmoller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Floel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef]
- Steinhoff, B.J.; Tumani, H.; Otto, M.; Mursch, K.; Wiltfang, J.; Herrendorf, G.; Bittermann, H.J.; Felgenhauer, K.; Paulus, W.; Markakis, E. Cisternal S100 protein and neuron-specific enolase are elevated and site-specific markers in intractable temporal lobe epilepsy. Epilepsy Res. 1999, 36, 75–82. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Miller, E.N.; Satz, P.; Visscher, B. Computerized and Conventional Neuropsychological Assessment of Hiv-1-Infected Homosexual Men. Neurology 1991, 41, 1608–1616. [Google Scholar] [CrossRef]
- Folstein, M.F.; Luria, R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol. Med. 1973, 3, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kontou, E.; Thomas, S.A.; Lincoln, N.B. Psychometric properties of a revised version of the Visual Analog Mood Scales. Clin. Rehabil. 2012, 26, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Tiffin, J.; Asher, E.J. The Purdue pegboard; norms and studies of reliability and validity. J. Appl. Psychol. 1948, 32, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychoph. 2011, 14, 1133–1145. [Google Scholar] [CrossRef]
- Fertonani, A.; Ferrari, C.; Miniussi, C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin. Neurophysiol. 2015, 126, 2181–2188. [Google Scholar] [CrossRef]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Rommens, N.; Geertsema, E.; Holleboom, L.J.; Cox, F.; Visser, G. Improving staff response to seizures on the epilepsy monitoring unit with online EEG seizure detection algorithms. Epilepsy Behav. 2018, 84, 99–104. [Google Scholar] [CrossRef]
- Furbass, F.; Kampusch, S.; Kaniusas, E.; Koren, J.; Pirker, S.; Hopfengartner, R.; Stefan, H.; Kluge, T.; Baumgartner, C. Automatic multimodal detection for long-term seizure documentation in epilepsy. Clin. Neurophysiol. 2017, 128, 1466–1472. [Google Scholar] [CrossRef]
- Furbass, F.; Ossenblok, P.; Hartmann, M.; Perko, H.; Skupch, A.M.; Lindinger, G.; Elezi, L.; Pataraia, E.; Colon, A.J.; Baumgartner, C.; et al. Prospective multi-center study of an automatic online seizure detection system for epilepsy monitoring units. Clin. Neurophysio. 2015, 126, 1124–1131. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Meth. 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Rigonatti, S.P.; Ribeiro, R.B.; Myczkowski, M.L.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int. J. Neuropsychopharmacol. 2008, 11, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Valiengo, L.; Baccaro, A.; Zanao, T.A.; de Oliveira, J.F.; Goulart, A.; Boggio, P.S.; Lotufo, P.A.; Bensenor, I.M.; Fregni, F. The sertraline vs. electrical current therapy for treating depression clinical study: Results from a factorial, randomized, controlled trial. JAMA Psychiatry 2013, 70, 383–391. [Google Scholar] [CrossRef]

| Measurements (Range or Unit) | Active Group (Mean ± SD) | Sham Group (Mean ± SD) | Statistical Results | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | F | p | |
| MoCA (0–30) | 27.95 ± 1.47 | 27.68 ± 1.46 | 27.63 ± 1.17 | 27.89 ± 1.49 | 0.973 | 0.331 |
| PPT (times 1) | ||||||
| Right Hand | 16.44 ± 1.56 | 17.21 ± 1.91 | 16.35 ± 1.60 | 17.65 ± 0.97 | 1.985 | 0.167 |
| Left Hand | 15.25 ± 1.86 | 16.10 ± 2.06 | 14.93 ± 1.29 | 15.91 ± 1.42 | 0.298 | 0.588 |
| Both Hands | 12.72 ± 1.68 | 13.35 ± 1.68 | 12.51 ± 1.60 | 13.30 ± 1.62 | 0.307 | 0.583 |
| Assembly | 41.30 ± 5.88 | 45.84 ± 6.68 | 40.23 ± 7.75 | 44.14 ± 6.40 | 0.340 | 0.564 |
| A-CalCAP (ms 2) | ||||||
| SRT | 363.79 ± 82.52 | 357.17 ± 60.90 | 357.64 ± 50.01 | 367.96 ± 54.43 | 0.620 | 0.436 |
| CRT | 428.42 ± 33.54 | 441.08 ± 41.93 | 415.76 ± 35.18 | 422.66 ± 32.58 | 0.349 | 0.558 |
| SPM1 | 501.01 ± 69.28 | 513.59 ± 83.14 | 487.74 ± 61.24 | 481.20 ± 65.78 | 1.589 | 0.216 |
| SPM2 | 593.20 ± 94.59 | 540.83 ± 78.03 | 554.99 ± 67.63 | 535.68 ± 68.15 | 2.886 | 0.098 |
| NSE (ng/mL) | 14.09 ± 3.21 | 16.01 ± 2.94 | 13.40 ± 3.27 | 14.32 ± 3.72 | 0.460 | 0.503 |
| VAMS-R (0–100) | ||||||
| Sad | 1.74 ± 2.16 | 8.63 ± 24.43 | 6.26 ± 9.15 | 9.21 ± 15.51 | 0.418 | 0.522 |
| Confused | 9.00 ± 19.06 | 8.16 ± 22.60 | 15.74 ± 18.47 | 11.00 ± 17.02 | 0.462 | 0.638 |
| Afraid | 7.05 ± 22.69 | 6.32 ± 21.76 | 4.53 ± 9.06 | 5.68 ± 11.33 | 0.060 | 0.808 |
| Happy | 47.00 ± 34.39 | 43.89 ± 34.41 | 49.26 ± 31.58 | 49.37 ± 27.95 | 0.098 | 0.756 |
| Tired | 31.21 ± 32.54 | 35.16 ± 30.03 | 35.47 ± 34.30 | 31.47 ± 29.62 | 0.928 | 0.342 |
| Angry | 7.05 ± 22.41 | 9.68 ± 25.74 | 2.53 ± 4.61 | 5.42 ± 10.60 | 0.006 | 0.941 |
| Tense | 16.68 ± 30.35 | 8.05 ± 22.31 | 5.74 ± 8.85 | 8.89 ± 20.32 | 2.361 | 0.133 |
| Energetic | 49.21 ± 28.49 | 47.84 ± 28.75 | 60.00 ± 30.01 | 49.16 ± 30.87 | 1.692 | 0.202 |
| SAS (1–5) | ||||||
| Concentration | 3.74 ± 0.73 | 3.26 ± 0.81 | 3.32 ± 0.48 | 3.21 ± 0.63 | 3.196 | 0.082 |
| Calmness | 4.11 ± 0.74 | 3.79 ± 0.92 | 3.68 ± 0.75 | 3.58 ± 0.84 | 0.475 | 0.495 |
| Fatigue | 2.58 ± 0.84 | 3.05 ± 1.03 | 2.37 ± 0.96 | 3.16 ± 0.96 | 1.317 | 0.259 |
| Visual perception | 3.68 ± 0.67 | 3.37 ± 0.60 | 3.37 ± 0.90 | 3.32 ± 1.00 | 1.573 | 0.218 |
| Items | Active Group | Sham Group | χ2 | p |
|---|---|---|---|---|
| Itching | mild: 2 | none | 2.111 | 0.146 |
| Headache | none | mild: 3 | 3.257 | 0.071 |
| Burning | none | none | - 1 | - |
| Warmth | mild: 1 | mild: 1 | 0.000 | 1.000 |
| Tingling | mild: 1 | none | 1.027 | 0.311 |
| Metallic taste | none | none | - | - |
| Fatigue | mild: 2; considerable: 1 | mild: 2; moderate: 2 | 0.175 | 0.676 2 |
| Vertigo | moderate: 1 3 | mild: 4 | 2.073 | 0.150 |
| Nausea | none | none | - | - |
| Phosphene | none | none | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, Y.; Ma, R.; Weng, Y.; Fan, C.; Xia, X.; Zhang, W.; Zeng, G.Q.; Wang, Y.; Lu, Z.; Cui, J.; et al. Safety Evaluation of Employing Temporal Interference Transcranial Alternating Current Stimulation in Human Studies. Brain Sci. 2022, 12, 1194. https://doi.org/10.3390/brainsci12091194
Piao Y, Ma R, Weng Y, Fan C, Xia X, Zhang W, Zeng GQ, Wang Y, Lu Z, Cui J, et al. Safety Evaluation of Employing Temporal Interference Transcranial Alternating Current Stimulation in Human Studies. Brain Sciences. 2022; 12(9):1194. https://doi.org/10.3390/brainsci12091194
Chicago/Turabian StylePiao, Yi, Ru Ma, Yaohao Weng, Chuan Fan, Xinzhao Xia, Wei Zhang, Ginger Qinghong Zeng, Yan Wang, Zhuo Lu, Jiangtian Cui, and et al. 2022. "Safety Evaluation of Employing Temporal Interference Transcranial Alternating Current Stimulation in Human Studies" Brain Sciences 12, no. 9: 1194. https://doi.org/10.3390/brainsci12091194
APA StylePiao, Y., Ma, R., Weng, Y., Fan, C., Xia, X., Zhang, W., Zeng, G. Q., Wang, Y., Lu, Z., Cui, J., Wang, X., Gao, L., Qiu, B., & Zhang, X. (2022). Safety Evaluation of Employing Temporal Interference Transcranial Alternating Current Stimulation in Human Studies. Brain Sciences, 12(9), 1194. https://doi.org/10.3390/brainsci12091194







