Hospital Outcomes among COVID-19 Hospitalizations with Acute Ischemic Stroke: Cross-Sectional Study Results from California State Inpatient Database
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Data Source
2.2. Study Population
2.3. Study Variables and Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns Hopkins Hopkins. Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/ (accessed on 15 November 2020).
- Wang, T.; Du, Z.; Zhu, F.; Cao, Z.; An, Y.; Gao, Y.; Jiang, B. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 2020, 395, E52. [Google Scholar] [CrossRef]
- Merkler, A.E.; Parikh, N.S.; Mir, S.; Gupta, A.; Kamel, H.; Lin, E.; Lantos, J.; Schenck, E.J.; Goyal, P.; Bruce, S.S.; et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs. patients with Influenza. JAMA Neurol. 2020, 77, 1366–1367. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Wang, M.; Zhou, Y.; Chang, J.; Xian, Y.; Wang, D.; Mao, L.; Jin, H.; Hu, B. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc. Neurol. 2020, 5, 279–284. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Yang, S.; Zhang, S.; Chen, M.; Yu, H.; Tian, D.-S.; Wang, W. Clinical characteristics and outcomes of COVID-19 patients with a history of stroke in Wuhan, China. Stroke 2020, 51, 2219–2223. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.-D.; Sacco, C.; Bertuzzi, A.; et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020, 191, 9–14. [Google Scholar] [CrossRef]
- Benussi, A.; Pilotto, A.; Premi, E.; Libri, I.; Giunta, M.; Agosti, C.; Alberici, A.; Baldelli, E.; Benini, M.; Bonacina, S.; et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology 2020, 95, e910–e920. [Google Scholar] [CrossRef]
- Agency for Healthcare Research and Quality. Overview of the State Inpatient Databases (SID). Available online: https://www.hcup-us.ahrq.gov/sidoverview.jsp (accessed on 16 March 2022).
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Strobe Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Baskett, W.I.; Huang, W.; Shyu, D.; Myers, D.; Raju, M.; Lobanova, I.; Suri, M.F.K.; Naqvi, S.H.; French, B.R.; et al. Acute ischemic stroke and COVID-19: An analysis of 27,676 patients. Stroke 2021, 52, 905–912. [Google Scholar] [CrossRef]
- Yaghi, S.; Ishida, K.; Torres, J.; Mac Grory, B.; Raz, E.; Humbert, K.; Henninger, N.; Trivedi, T.; Lillemoe, K.; Alam, S.; et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke 2020, 51, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Kirchberger, I.; Berghaus, T.M.; von Scheidt, W.; Linseisen, J.; Meisinger, C. COVID-19 risk perceptions, worries and preventive behaviors in patients with previous pulmonary embolism. Thromb. Res. 2021, 202, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. The unique characteristics of COVID-19 coagulopathy. Crit. Care 2020, 24, 360. [Google Scholar] [CrossRef]
- Sweid, A.; Hammoud, B.; Bekelis, K.; Missios, S.; Tjoumakaris, S.I.; Gooch, M.R.; Herial, N.A.; Zarzour, H.; Romo, V.; DePrince, M.; et al. Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int. J. Stroke 2020, 15, 733–742. [Google Scholar] [CrossRef]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef] [PubMed]
- Morassi, M.; Bagatto, D.; Cobelli, M.; D’Agostini, S.; Gigli, G.L.; Bnà, C.; Vogrig, A. Stroke in patients with SARS-CoV-2 infection: Case series. J. Neurol. 2020, 267, 2185–2192. [Google Scholar] [CrossRef]
- Annie, F.; Bates, M.C.; Nanjundappa, A.; Bhatt, D.L.; Alkhouli, M. Prevalence and outcomes of acute ischemic stroke among patients ≤50 years of age with laboratory confirmed COVID-19 infection. Am. J. Cardiol. 2020, 130, 169–170. [Google Scholar] [CrossRef]
- Harrison, S.L.; Fazio-Eynullayeva, E.; Lane, D.A.; Underhill, P.; Lip, G.Y. Higher mortality of ischaemic stroke patients hospitalized with COVID-19 compared to historical controls. Cerebrovasc. Dis. 2021, 50, 326–331. [Google Scholar] [CrossRef]
- Escalard, S.; Maïer, B.; Redjem, H.; Delvoye, F.; Hébert, S.; Smajda, S.; Ciccio, G.; Desilles, J.-P.; Mazighi, M.; Blanc, R.; et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19: Experience from Paris. Stroke 2020, 51, 2540–2543. [Google Scholar] [CrossRef]
- Kerleroux, B.; Fabacher, T.; Bricout, N.; Moïse, M.; Testud, B.; Vingadassalom, S.; Ifergan, H.; Janot, K.; Consoli, A.; Hassen, W.B.; et al. Mechanical thrombectomy for acute ischemic stroke amid the COVID-19 outbreak: Decreased activity, and increased care delays. Stroke 2020, 51, 2012–2017. [Google Scholar] [CrossRef]
- Teo, K.-C.; Leung, W.C.-Y.; Wong, Y.-K.; Liu, R.K.C.; Chan, A.H.Y.; Choi, O.M.Y.; Kwok, W.-M.; Leung, K.-K.; Tse, M.-Y.; Cheung, R.T.F.; et al. Delays in stroke onset to hospital arrival time during COVID-19. Stroke 2020, 51, 2228–2231. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | COVID-19 Hospitalizations with AIS n = 1027 (1.1%) | COVID-19 Hospitalizations without AIS n = 90,393 (98.9%) | p Value a | AIS Hospitalizations without COVID-19 n = 58,083 (98.9%) | p Value b |
|---|---|---|---|---|---|
| Age, n (%) | <0.001 | 0.270 | |||
| 18–44 years | 44 (4.3%) | 14,923 (16.5%) | 2346 (4.0%) | ||
| 45–64 years | 262 (25.5%) | 35,121 (38.9%) | 16,132 (27.8%) | ||
| ≥65 years | 721 (70.2%) | 40,349 (44.6%) | 39,605 (68.2%) | ||
| Sex, n (%) | 0.001 | <0.001 | |||
| Male | 616 (60.0%) | 49,745 (55.0%) | 30,966 (53.3%) | ||
| Female | 411 (40.0%) | 40,645 (45.0%) | 27,114 (46.7%) | ||
| Race, n (%) | <0.001 | <0.001 | |||
| White | 360 (35.6%) | 22,847 (25.7%) | 28,909 (50.4%) | ||
| Black | 77 (7.6%) | 5114 (5.7%) | 5456 (9.5%) | ||
| Hispanic | 424 (42.0%) | 48,250 (54.2%) | 13,579 (23.7%) | ||
| Asian, Pacific Islander, Native American | 99 (9.8%) | 8274 (9.3%) | 6855 (11.9%) | ||
| Other | 50 (5.0%) | 4535 (5.1%) | 2587 (4.5%) | ||
| Insurance, n (%) | <0.001 | 0.050 | |||
| Medicare | 685 (66.7%) | 37,966 (42.0%) | 37,047 (63.8%) | ||
| Medicaid | 176 (17.1%) | 25,713 (28.5%) | 9497 (16.4%) | ||
| Private insurance | 132 (12.9%) | 21,833 (24.2%) | 9297 (16.0%) | ||
| Uninsured | 14 (1.4%) | 1668 (1.8%) | 1095 (1.9%) | ||
| Other | 20 (1.9%) | 3169 (3.5%) | 1134 (2.0%) | ||
| Clinical risk profile, n (%) | |||||
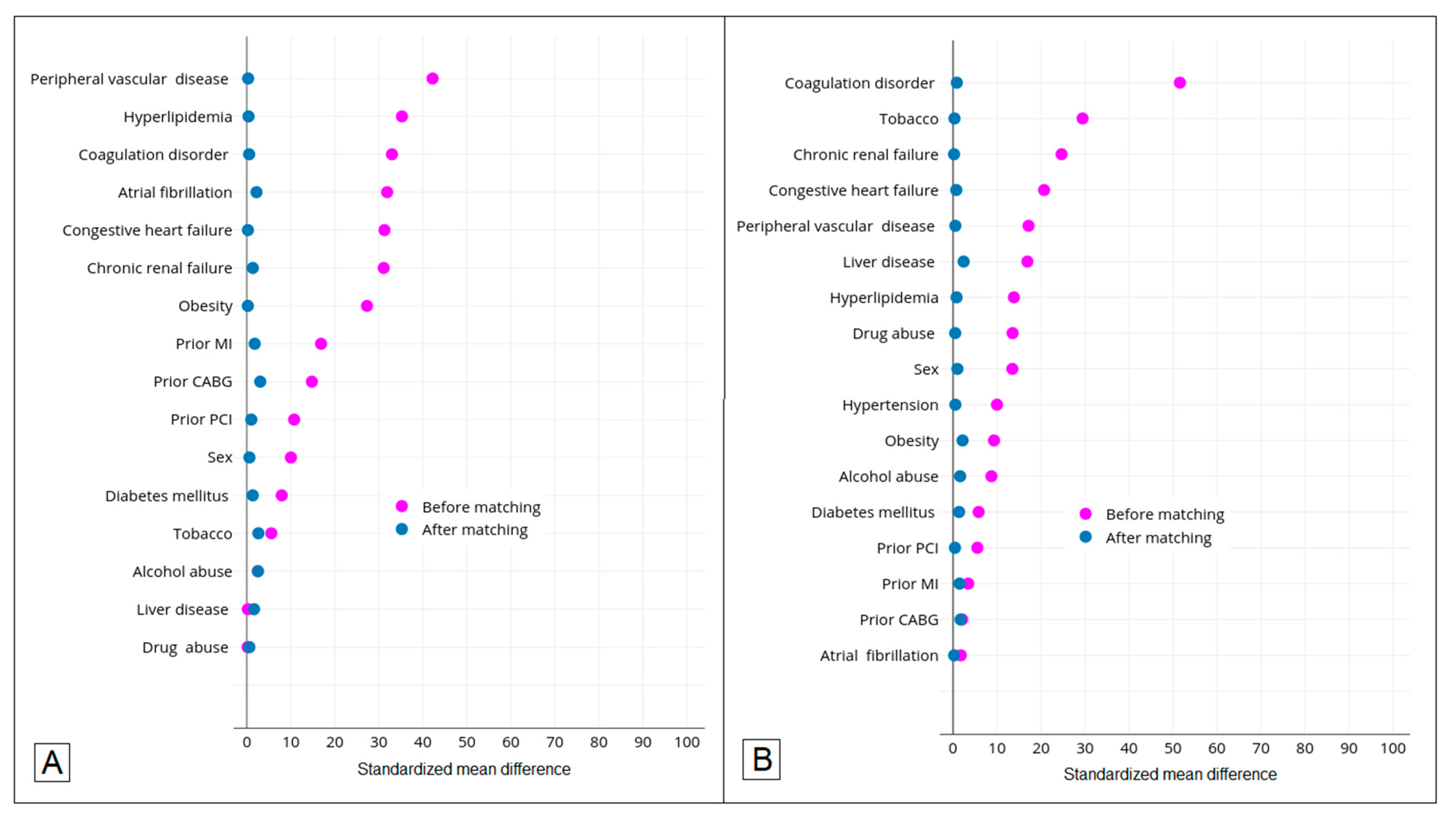
| Hypertension | 843 (82.1%) | 54,659 (60.5%) | <0.001 | 49,803 (85.7%) | <0.001 |
| Diabetes mellitus | 144 (14.0%) | 15,264 (16.9%) | 0.014 | 9350 (16.1%) | 0.072 |
| Hyperlipidemia | 454 (44.2%) | 55,623 (61.5%) | <0.001 | 36,346 (62.6%) | <0.001 |
| Obesity | 176 (17.1%) | 25,751 (28.5%) | <0.001 | 8000 (13.8%) | 0.002 |
| Atrial fibrillation | 214 (20.8%) | 8622 (9.5%) | <0.001 | 11,690 (20.1%) | 0.573 |
| Coagulation disorder | 240 (23.4%) | 10,018 (11.1%) | <0.001 | 3343 (5.8%) | <0.001 |
| Peripheral vascular disease | 207 (20.2%) | 5595 (6.2%) | <0.001 | 7980 (13.7%) | <0.001 |
| Liver disease | 62 (6.0%) | 5409 (6.0%) | 0.943 | 1517 (2.6%) | <0.001 |
| Chronic renal failure | 326 (31.7%) | 16,655 (18.4%) | <0.001 | 12,174 (21.0%) | <0.001 |
| Tobacco use | 60 (5.8%) | 4162 (4.6%) | 0.060 | 8528 (14.7%) | <0.001 |
| Alcohol abuse | 27 (2.6%) | 2030 (2.2%) | 0.410 | 2447 (4.2%) | 0.012 |
| Drug abuse | 30 (2.9%) | 2615 (2.9%) | 0.957 | 3285 (5.7%) | <0.001 |
| Congestive heart failure | 256 (24.9%) | 11,601 (12.8%) | <0.001 | 9629 (16.6%) | <0.001 |
| Prior MI | 71 (6.9%) | 2917 (3.2%) | <0.001 | 4537 (7.8%) | 0.287 |
| Prior PCI | 50 (4.9%) | 2536 (2.8%) | <0.001 | 3559 (6.1%) | 0.094 |
| Prior CABG | 54 (5.3%) | 2192 (2.4%) | <0.001 | 3328 (5.7%) | 0.518 |
| Elixhauser comorbidity index, n (%) | --- | --- | |||
| 0 | --- | 6712 (7.4%) | 1668 (2.9%) | ||
| 1 or 2 | 140 (13.6%) | 31,371 (34.7%) | 21,444 (36.9%) | ||
| ≥3 | 882 (85.9%) | 52,310 (57.9%) | 34,971 (60.2%) | ||
| Treatment | --- | --- | |||
| Intravenous thrombolysis only | 0 (0%) | --- | 0 (0%) | ||
| Mechanical thrombectomy only | --- | --- | 4289 (7.4%) | ||
| Both | 32 (3.1%) | --- | 6681 (11.5%) | ||
| None | 987 (96.1%) | --- | 47,113 (81.1%) |
| Characteristic | Odds Ratio | p Value |
|---|---|---|
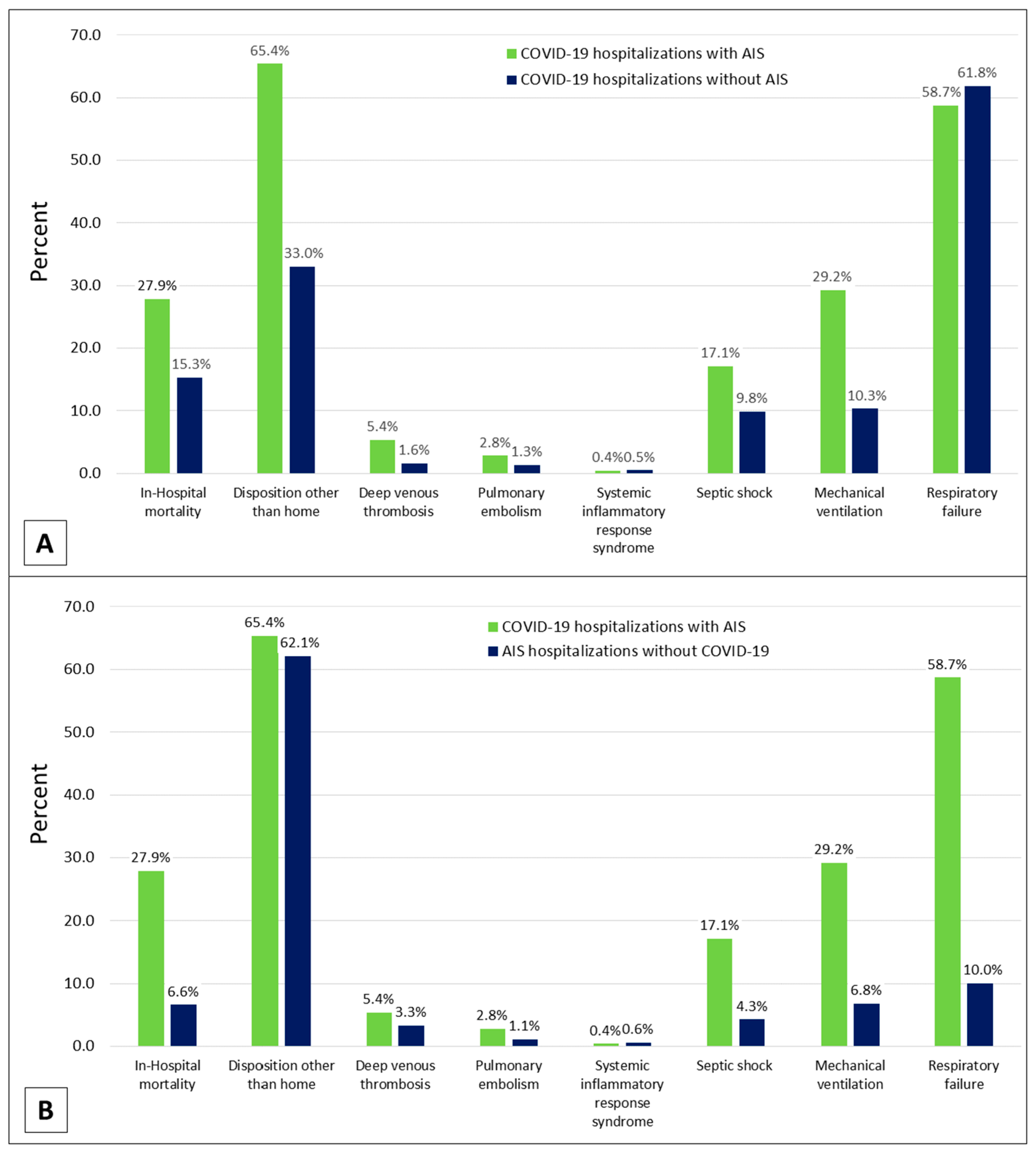
| In-Hospital mortality | 2.16 (1.72–2.73) | <0.001 |
| Disposition other than home | 2.61 (2.10–3.25) | <0.001 |
| Deep venous thrombosis | 3.43 (2.03–5.80) | <0.001 |
| Pulmonary embolism | 2.30 (1.27–4.16) | 0.006 |
| Systemic inflammatory response syndrome | 0.93 (0.38–2.30) | 0.877 |
| Septic shock | 2.07 (1.57–2.74) | <0.001 |
| Mechanical ventilation | 4.69 (3.56–6.16) | <0.001 |
| Respiratory failure | 0.86 (0.72–1.03) | 0.102 |
| Characteristic | Odds Ratio | p Value |
|---|---|---|
| In-Hospital mortality | 5.65 (4.20–7.61) | <0.001 |
| Disposition other than home | 1.19 (0.96–1.48) | 0.110 |
| Deep venous thrombosis | 1.82 (1.14–2.91) | 0.012 |
| Pulmonary embolism | 2.61 (1.24–5.49) | 0.011 |
| Systemic inflammatory response syndrome | 0.73 (0.30–1.79) | 0.490 |
| Septic shock | 4.74 (3.29–6.84) | <0.001 |
| Mechanical ventilation | 6.17 (4.55–8.38) | <0.001 |
| Respiratory failure | 15.37 (11.86–19.92) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubens, M.; Saxena, A.; Ramamoorthy, V.; Ahmed, M.A.; Zhang, Z.; McGranaghan, P.; Veledar, E.; McDermott, M.; De Los Rios La Rosa, F. Hospital Outcomes among COVID-19 Hospitalizations with Acute Ischemic Stroke: Cross-Sectional Study Results from California State Inpatient Database. Brain Sci. 2022, 12, 1177. https://doi.org/10.3390/brainsci12091177
Rubens M, Saxena A, Ramamoorthy V, Ahmed MA, Zhang Z, McGranaghan P, Veledar E, McDermott M, De Los Rios La Rosa F. Hospital Outcomes among COVID-19 Hospitalizations with Acute Ischemic Stroke: Cross-Sectional Study Results from California State Inpatient Database. Brain Sciences. 2022; 12(9):1177. https://doi.org/10.3390/brainsci12091177
Chicago/Turabian StyleRubens, Muni, Anshul Saxena, Venkataraghavan Ramamoorthy, Md Ashfaq Ahmed, Zhenwei Zhang, Peter McGranaghan, Emir Veledar, Michael McDermott, and Felipe De Los Rios La Rosa. 2022. "Hospital Outcomes among COVID-19 Hospitalizations with Acute Ischemic Stroke: Cross-Sectional Study Results from California State Inpatient Database" Brain Sciences 12, no. 9: 1177. https://doi.org/10.3390/brainsci12091177
APA StyleRubens, M., Saxena, A., Ramamoorthy, V., Ahmed, M. A., Zhang, Z., McGranaghan, P., Veledar, E., McDermott, M., & De Los Rios La Rosa, F. (2022). Hospital Outcomes among COVID-19 Hospitalizations with Acute Ischemic Stroke: Cross-Sectional Study Results from California State Inpatient Database. Brain Sciences, 12(9), 1177. https://doi.org/10.3390/brainsci12091177










