Patient Safety Comparison of Frameless and Frame-Based Stereotactic Navigation for Brain Biopsy—A Single Center Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Inclusion Criteria
2.2. Indications for Surgery and Surgical Procedure
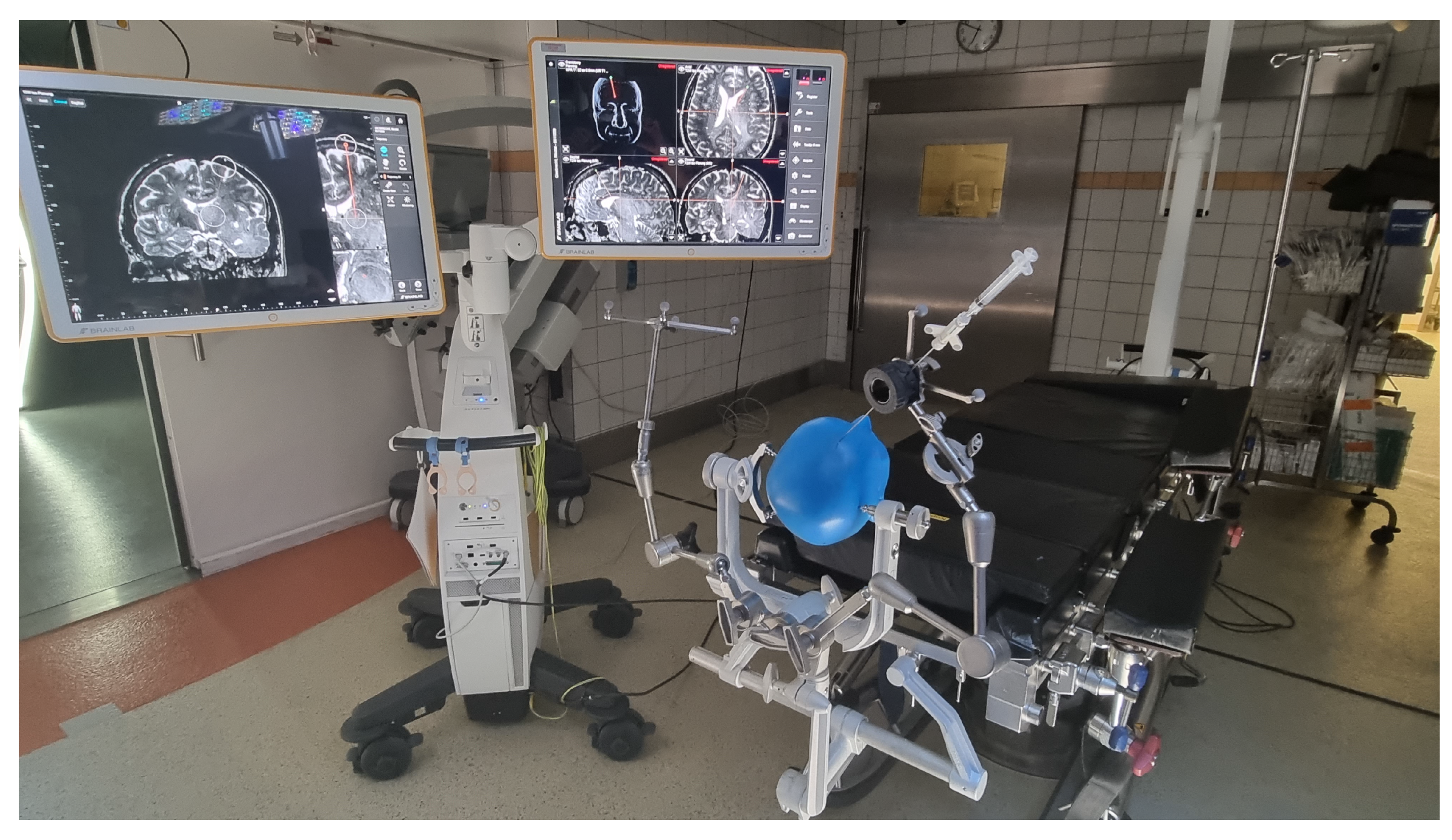
2.3. VagioGuide
2.4. Leksell Stereotactic System
2.5. Postoperative Management
2.6. Statistics
3. Results
3.1. Patient’s Baseline Data
3.2. Patient and Disease-Related Characteristics
3.3. Histological Diagnoses
3.4. Cost Comparison
4. Discussion
4.1. Literature Comparison
4.2. Cost Comparison
4.3. Conclusions
4.4. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.; Murad, G.J.; Mocco, J. Early history of the stereotactic apparatus in neurosurgery. Neurosurg. Focus 2009, 27, E12. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, L.D. Lars Leksell. Notes at the side of a raconteur. Stereotact. Funct. Neurosurg. 1996, 67, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Kogias, E.; Altenmüller, D.M.; Karakolios, K.; Egger, K.; Coenen, V.A.; Schulze-Bonhage, A.; Reinacher, P.C. Electrode placement for SEEG: Combining stereotactic technique with latest generation planning software for intraoperative visualization and postoperative evaluation of accuracy and accuracy predictors. Clin. Neurol. Neurosurg. 2022, 213, 107137. [Google Scholar] [CrossRef]
- Nakagawa, J.M.; Trippel, M.; Doostkam, S.; Mader, I.; Coenen, V.A.; Reinacher, P.C. The stereotactic suboccipitaltranscerebellar approach to lesions of the brainstem and the cerebellum. Clin. Neurol. Neurosurg. 2018, 166, 10–15. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, Y.; Lu, Z. Clinical evaluation of frameless stereotaxy in minimally invasive neurosurgery. Zhonghua Yi Xue Za Zhi 2001, 81, 1042–1045. [Google Scholar] [PubMed]
- Spetzger, U.; Laborde, G.; Gilsbach, J.M. Frameless neuronavigation in modern neurosurgery. Min-Minim. Invasive Neurosurg. 1995, 38, 163–166. [Google Scholar] [CrossRef]
- Dammers, R.; Haitsma, I.K.; Schouten, J.W.; Kros, J.M.; Avezaat, C.J.J.; Vincent, A.J.P.E. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir. 2008, 150, 23–29. [Google Scholar] [CrossRef]
- Eggers, G.; Mühling, J.; Marmulla, R. Image-to-patient registration techniques in head surgery. Int. J. Oral Maxillofac. Surg. 2006, 35, 1081–1095. [Google Scholar] [CrossRef]
- Ringel, F.; Ingerl, D.; Ott, S.; Meyer, B. VarioGuide: A new frameless image-guided stereotactic system–accuracy study and clinical assessment. Oper. Neurosurg. 2009, 64 (Suppl. 5), 365–371. [Google Scholar] [CrossRef]
- Al-Tehewy, M.M.; Abd Al-Razak, S.E.; Hikal, T.S.; Wahdan, M.M. Association of patient safety indicator 03 and clinical outcome in a surgery hospital. Int. J. Health Care Qual. Assur. 2020, 33, 403–412. [Google Scholar] [CrossRef]
- Stocking, J.C.; Utter, G.H.; Drake, C.; Aldrich, J.M.; Ong, M.K.; Amin, A.; Marmori, R.A.; Godati, L.; Cannessonj, M.; Groppere, M.A.; et al. Postoperative respiratory failure: An update on the validity of the Agency for Healthcare Research and Quality Patient Safety Indicator 11 in an era of clinical documentation improvement programs. Am. J. Surg. 2020, 220, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.R.; Segreto, F.A.; Alas, H.; Bortz, C.; Jackson-Fowl, B.; Brown, A.E.; Pierce, K.E.; Vasquez-Montes, D.; Egers, M.I.; Line, B.G.; et al. Hospital-acquired conditions occur more frequently in elective spine surgery than for other common elective surgical procedures. J. Clin. Neurosci. 2020, 76, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Schneider, G.H.; Vajkoczy, P. Utilization of the Intraoperative Mobile AIRO® CT Scanner in Stereotactic Surgery: Workflow and Effectiveness. Stereotact. Funct. Neurosurg. 2019, 97, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Valles, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J.; et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. J. Am. Soc. Anesthesiol. 2010, 113, 1338–1350. [Google Scholar] [CrossRef]
- Fukui, K.; Fujioka, M.; Yamasaki, K.; Yamakawa, S.; Matsuo, H.; Noguchi, M. Risk Factors for Postoperative Complications among the Elderly after Plastic Surgery Procedures Performed under General Anesthesia. Plast. Surg. Int. 2018, 2018, 7053839. [Google Scholar] [CrossRef]
- Woodworth, G.F.; McGirt, M.J.; Samdani, A.; Garonzik, I.; Olivi, A.; Weingart, J.D. Frameless image-guided stereotactic brain biopsy procedure: Diagnostic yield, surgical morbidity, and comparison with the frame-based technique. J. Neurosurg. 2006, 104, 233–237. [Google Scholar] [CrossRef]
- Furtak, J.; Śledzińska, P.; Bebyn, M.G.; Szylberg, T.; Krajewski, S.; Birski, M.; Harat, M. Infratentorial Stereotactic Biopsy of Brainstem and Cerebellar Lesions. Brain Sci. 2021, 11, 1432. [Google Scholar] [CrossRef]
- Krieger, M.D.; Chandrasoma, P.T.; Zee, C.S.; Apuzzo, M.L. Role of stereotactic biopsy in the diagnosis and management of brain tumors. In Seminars in Surgical Oncology; John Wiley & Sons, Inc.: New York, NY, USA, 1998; Volume 14, pp. 13–25. [Google Scholar]
- Riche, M.; Amelot, A.; Peyre, M.; Capelle, L.; Carpentier, A.; Mathon, B. Complications after frame-based stereotactic brain biopsy: A systematic review. Neurosurg. Rev. 2021, 44, 301–307. [Google Scholar] [CrossRef]
- Bernstein, M.; Parrent, A.G. Complications of CT-guided stereotactic biopsy of intra-axial brain lesions. J. Neurosurg. 1994, 81, 165–168. [Google Scholar] [CrossRef]
- Nishihara, M.; Sasayama, T.; Kudo, H.; Kohmura, E. Morbidity of stereotactic biopsy for intracranial lesions. Kobe J. Med. Sci. 2011, 56, E148–E153. [Google Scholar]
- Grossman, R.; Sadetzki, S.; Spiegelmann, R.; Ram, Z. Haemorrhagic complications and the incidence of asymptomatic bleeding associated with stereotactic brain biopsies. Acta Neurochir. 2005, 147, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Hsu, P.W.; Wu, T.W.E.; Lee, S.T.; Chang, C.N.; Wei, K.C.; Chuang, C.C.; Wu, C.T.; Liu, T.N.; Hsu, Y.H.; et al. Stereotactic brain biopsy: Single center retrospective analysis of complications. Clin. Neurol. Neurosurg. 2009, 111, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Krishnan, R.; Zimmermann, M.; Seifert, V. Neuronavigation oder Stereotaxie? Eine Standortbestimmung zur Wahl des Verfahrens [Frame-less and frame-based stereotaxy? How to choose the appropriate procedure]. Zentralblatt Neurochir. 2003, 64, 1–5. [Google Scholar]
- Bradac, O.; Steklacova, A.; Nebrenska, K.; Vrana, J.; de Lacy, P.; Benes, V. Accuracy of VarioGuide Frameless Stereotactic System Against Frame-Based Stereotaxy: Prospective, Randomized, Single-Center Study. World Neurosurg. 2017, 104, 831–840. [Google Scholar] [CrossRef]
- Sciortino, T.; Fernandes, B.; Nibali, M.C.; Gay, L.G.; Rossi, M.; Lopci, E.; Colombo, A.E.; Elefante, M.G.; Pessina, F.; Bello, L.; et al. Frameless stereotactic biopsy for precision neurosurgery: Diagnostic value, safety, and accuracy. Acta Neurochir. 2019, 161, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Vera, A. Die “Industrialisierung” des Krankenhauswesens durch DRG-Fallpauschalen–eine interdisziplinäre Analyse [The “Industrialisation” of the hospital sector by DRG-based prospective payment systems–an interdisciplinary analysis]. Gesundheitswesen 2009, 71, e10–e17. [Google Scholar] [CrossRef] [PubMed]

| VarioGuide | LSS | p Values | |
|---|---|---|---|
| Age, years, (q1–q3) | 74 (62–80) | 67 (57–76) | 0.030 |
| Gender, F:M | 17:23 | 34:35 | 0.467 |
| Primary tumor site | |||
| Frontal | 10 | 24 | n.s. |
| Parietal | 7 | 18 | n.s. |
| Temporal | 8 | 8 | n.s. |
| Occipital | 6 | 0 | n.s. |
| Basal ganglia | 7 | 15 | n.s. |
| Brain stem | 0 | 2 | n.s. |
| Cerebellum | 0 | 2 | n.s. |
| Intraventicular | 2 | 0 | n.s. |
| Site of the disease | |||
| Left | 20 | 32 | n.s. |
| Right | 14 | 24 | n.s. |
| Size of the lesion, cm3 (mean ± SD) | 18.31 (±26.35) | 12.63 (±14.62) | 0.15 |
| KPS (q1–q3) | 80 (70–90) | 90 (80–90) | 0.08 * |
| ASA score ≥ 3 | 21 | 25 | 0.097 |
| Preoperative neurological deficit | 17 | 4 | <0.001 * |
| VarioGuide | LSS | p Values | |
|---|---|---|---|
| False negative biopsy rate | 3 | 1 | 0.13 |
| Surgery duration, min (q1–q3) | 28 (20–38) | 30 (IQR 25–39) | 0.1352 |
| Anesthesia duration, min (q1–q3) | 163 (138–194) | 193 (167–215) | <0.001 * |
| Postoperative complications | 3 | 4 | n.s. |
| Author (Year) | Number of Patients | VarioGuide Evaluation |
|---|---|---|
| Vychopen et al. (2022) | 109 | Safe alternative to LSS |
| Ringel et al. (2009) [9] | 27 | 100% Target localisation |
| Bradac et al. (2017) [25] | 53 | Comparable reliability to LSS |
| Sciortino et al. (2019) [26] | 140 | Comparable reliability to LSS |
| VarioGuide | Leksell Stereotactic System | |
|---|---|---|
| Pros | Time-sparing procedure No need of pre-op CTA | More accurate, exclusive use in brainstem biopsies |
| Cons | Less accurate in eloquent lesions (cerebellum) | Time consuming CT-A is necessary for trajectory verification |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vychopen, M.; Wach, J.; Borger, V.; Schneider, M.; Eichhorn, L.; Maciaczyk, J.; Bara, G.; Vatter, H.; Banat, M.; Hamed, M. Patient Safety Comparison of Frameless and Frame-Based Stereotactic Navigation for Brain Biopsy—A Single Center Cohort Study. Brain Sci. 2022, 12, 1178. https://doi.org/10.3390/brainsci12091178
Vychopen M, Wach J, Borger V, Schneider M, Eichhorn L, Maciaczyk J, Bara G, Vatter H, Banat M, Hamed M. Patient Safety Comparison of Frameless and Frame-Based Stereotactic Navigation for Brain Biopsy—A Single Center Cohort Study. Brain Sciences. 2022; 12(9):1178. https://doi.org/10.3390/brainsci12091178
Chicago/Turabian StyleVychopen, Martin, Johannes Wach, Valeri Borger, Matthias Schneider, Lars Eichhorn, Jaroslaw Maciaczyk, Gregor Bara, Hartmut Vatter, Mohammed Banat, and Motaz Hamed. 2022. "Patient Safety Comparison of Frameless and Frame-Based Stereotactic Navigation for Brain Biopsy—A Single Center Cohort Study" Brain Sciences 12, no. 9: 1178. https://doi.org/10.3390/brainsci12091178
APA StyleVychopen, M., Wach, J., Borger, V., Schneider, M., Eichhorn, L., Maciaczyk, J., Bara, G., Vatter, H., Banat, M., & Hamed, M. (2022). Patient Safety Comparison of Frameless and Frame-Based Stereotactic Navigation for Brain Biopsy—A Single Center Cohort Study. Brain Sciences, 12(9), 1178. https://doi.org/10.3390/brainsci12091178






