Astrocyte-Derived Neuronal Transdifferentiation as a Therapy for Ischemic Stroke: Advances and Challenges
Abstract
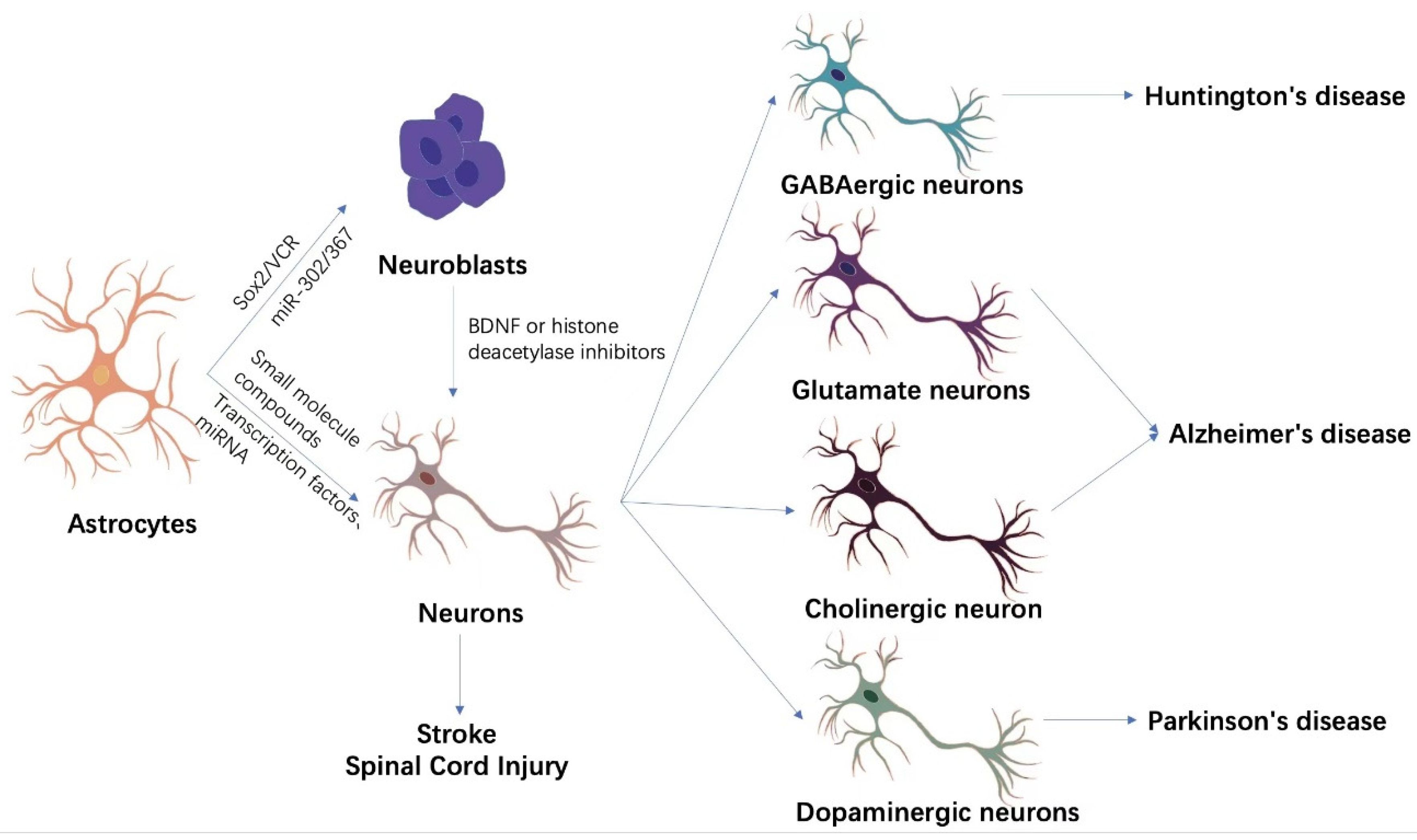
:1. Introduction
2. Transdifferentiation Methods
2.1. Indirect Lineage Conversion
2.1.1. Transcription Factors
2.1.2. miRNA
2.1.3. Small Molecule Compounds
2.2. Direct Transdifferentiation
2.2.1. Transcription Factors
2.2.2. miRNA
2.2.3. Small Molecule Compounds
3. Transdifferentiation Efficiency
4. Transdifferentiation as a Therapy for Ischemic Stroke
5. Applications in Other Diseases
6. Evidence of the Origin of Newborn Neurons
7. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tobin, M.K.; Bonds, J.A.; Minshall, R.D.; Pelligrino, D.A.; Testai, F.D.; Lazarov, O. Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. J. Cereb. Blood Flow Metab. 2014, 34, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Schäbitz, W.R.; Schwab, S.; Spranger, M.; Hacke, W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 1997, 17, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.W.; Platt, S.R.; Lau, V.W.; Grace, H.E.; Holmes, S.P.; Wang, L.; Duberstein, K.J.; Howerth, E.W.; Kinder, H.A.; Stice, S.L.; et al. Induced Pluripotent Stem Cell-Derived Neural Stem Cell Therapy Enhances Recovery in an Ischemic Stroke Pig Model. Sci. Rep. 2017, 7, 10075. [Google Scholar] [CrossRef]
- Prasad, K.; Sharma, A.; Garg, A.; Mohanty, S.; Bhatnagar, S.; Johri, S.; Singh, K.K.; Nair, V.; Sarkar, R.S.; Gorthi, S.P.; et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014, 45, 3618–3624. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Yan, H.; Krishnaney-Davison, R.; Al Sawaf, A.; Zhang, S.C. Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. J. Stroke Cereb. Dis. 2013, 22, 304–308. [Google Scholar] [CrossRef]
- Mollinari, C.; Zhao, J.; Lupacchini, L.; Garaci, E.; Merlo, D.; Pei, G. Transdifferentiation: A new promise for neurodegenerative diseases. Cell Death Dis. 2018, 9, 830. [Google Scholar] [CrossRef]
- Heins, N.; Malatesta, P.; Cecconi, F.; Nakafuku, M.; Tucker, K.L.; Hack, M.A.; Chapouton, P.; Barde, Y.A.; Gotz, M. Glial cells generate neurons: The role of the transcription factor Pax6. Nat. Neurosci. 2002, 5, 308–315. [Google Scholar] [CrossRef]
- Amamoto, R.; Arlotta, P. Development-inspired reprogramming of the mammalian central nervous system. Science 2014, 343, 1239882. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Z.B.; Zhuge, Q.; Zheng, W.; Shao, B.; Wang, B.; Sun, F.; Jin, K. Glial scar formation occurs in the human brain after ischemic stroke. Int. J. Med. Sci. 2014, 11, 344–348. [Google Scholar] [CrossRef]
- Yuan, Y.M.; He, C. The glial scar in spinal cord injury and repair. Neurosci. Bull. 2013, 29, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Buffo, A.; Rite, I.; Tripathi, P.; Lepier, A.; Colak, D.; Horn, A.P.; Mori, T.; Gotz, M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. USA 2008, 105, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.L.; Liu, C.W.; Shen, S.W.; Yu, Z.; Mo, J.L.; Chen, X.H.; Sun, F.Y. Striatal astrocytes transdifferentiate into functional mature neurons following ischemic brain injury. Glia 2015, 63, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Shimada, I.S.; LeComte, M.D.; Granger, J.C.; Quinlan, N.J.; Spees, J.L. Self-renewal and differentiation of reactive astrocyte-derived neural stem/progenitor cells isolated from the cortical peri-infarct area after stroke. J. Neurosci. 2012, 32, 7926–7940. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, J.P.; Goritz, C.; Tatarishvili, J.; Dias, D.O.; Smith, E.M.; Lindvall, O.; Kokaia, Z.; Frisen, J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science 2014, 346, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Zang, T.; Zou, Y.; Fang, S.; Smith, D.K.; Bachoo, R.; Zhang, C.L. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 2013, 15, 1164–1175. [Google Scholar] [CrossRef]
- Niu, W.; Zang, T.; Smith, D.K.; Vue, T.Y.; Zou, Y.; Bachoo, R.; Johnson, J.E.; Zhang, C.L. SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem. Cell Rep. 2015, 4, 780–794. [Google Scholar] [CrossRef]
- Su, Z.; Niu, W.; Liu, M.L.; Zou, Y.; Zhang, C.L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014, 5, 3338. [Google Scholar] [CrossRef]
- Weinberg, M.S.; Criswell, H.E.; Powell, S.K.; Bhatt, A.P.; McCown, T.J. Viral Vector Reprogramming of Adult Resident Striatal Oligodendrocytes into Functional Neurons. Mol. Ther. 2017, 25, 928–934. [Google Scholar] [CrossRef]
- Ghasemi-Kasman, M.; Hajikaram, M.; Baharvand, H.; Javan, M. MicroRNA-Mediated In Vitro and In Vivo Direct Conversion of Astrocytes to Neuroblasts. PLoS ONE 2015, 10, e0127878. [Google Scholar] [CrossRef]
- Ghasemi-Kasman, M.; Baharvand, H.; Javan, M. Enhanced neurogenesis in degenerated hippocampi following pretreatment with miR-302/367 expressing lentiviral vector in mice. Biomed. Pharm. 2017, 96, 1222–1229. [Google Scholar] [CrossRef]
- Cheng, L.; Gao, L.; Guan, W.; Mao, J.; Hu, W.; Qiu, B.; Zhao, J.; Yu, Y.; Pei, G. Direct conversion of astrocytes into neuronal cells by drug cocktail. Cell Res. 2015, 25, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Ng, Y.H.; Pang, Z.P.; Sudhof, T.C.; Wernig, M. Induced neuronal cells: How to make and define a neuron. Cell Stem. Cell 2011, 9, 517–525. [Google Scholar] [CrossRef]
- Buffo, A.; Vosko, M.R.; Erturk, D.; Hamann, G.F.; Jucker, M.; Rowitch, D.; Gotz, M. Expression pattern of the transcription factor Olig2 in response to brain injuries: Implications for neuronal repair. Proc. Natl. Acad. Sci. USA 2005, 102, 18183–18188. [Google Scholar] [CrossRef] [PubMed]
- Berninger, B.; Costa, M.R.; Koch, U.; Schroeder, T.; Sutor, B.; Grothe, B.; Gotz, M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J. Neurosci. 2007, 27, 8654–8664. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F. Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr. Opin. Cell Biol. 2005, 17, 639–647. [Google Scholar] [CrossRef]
- Heinrich, C.; Blum, R.; Gascon, S.; Masserdotti, G.; Tripathi, P.; Sanchez, R.; Tiedt, S.; Schroeder, T.; Gotz, M.; Berninger, B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010, 8, e1000373. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem. Cell 2014, 14, 188–202. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, Q.; Yuan, J.; Han, S.; Zhang, P.; Li, S.; Rao, Z.; Zhao, W.; Ye, Q.; Geng, J.; et al. Ascl1 Converts Dorsal Midbrain Astrocytes into Functional Neurons In Vivo. J. Neurosci. 2015, 35, 9336–9355. [Google Scholar] [CrossRef]
- Chen, Y.C.; Ma, N.X.; Pei, Z.F.; Wu, Z.; Do-Monte, F.H.; Keefe, S.; Yellin, E.; Chen, M.S.; Yin, J.C.; Lee, G.; et al. A NeuroD1 AAV-Based Gene Therapy for Functional Brain Repair after Ischemic Injury through In Vivo Astrocyte-to-Neuron Conversion. Mol. Ther. 2020, 28, 217–234. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Yu, S.P.; Wei, Z.Z.; Zhong, W.; Cao, W.; Gu, X.; Wu, A.; McCrary, M.R.; Berglund, K.; Wei, L. Conversion of Reactive Astrocytes to Induced Neurons Enhances Neuronal Repair and Functional Recovery After Ischemic Stroke. Front. Aging Neurosci. 2021, 13, 612856. [Google Scholar] [CrossRef]
- Brulet, R.; Matsuda, T.; Zhang, L.; Miranda, C.; Giacca, M.; Kaspar, B.K.; Nakashima, K.; Hsieh, J. NEUROD1 Instructs Neuronal Conversion in Non-Reactive Astrocytes. Stem Cell Rep. 2017, 8, 1506–1515. [Google Scholar] [CrossRef]
- Ge, L.J.; Yang, F.H.; Li, W.; Wang, T.; Lin, Y.; Feng, J.; Chen, N.H.; Jiang, M.; Wang, J.H.; Hu, X.T.; et al. In vivo Neuroregeneration to Treat Ischemic Stroke Through NeuroD1 AAV-Based Gene Therapy in Adult Non-human Primates. Front Cell Dev. Biol. 2020, 8, 590008. [Google Scholar] [CrossRef]
- Torper, O.; Pfisterer, U.; Wolf, D.A.; Pereira, M.; Lau, S.; Jakobsson, J.; Bjorklund, A.; Grealish, S.; Parmar, M. Generation of induced neurons via direct conversion in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 7038–7043. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, W.; Jin, H.; Li, T. Brn2 Alone Is Sufficient to Convert Astrocytes into Neural Progenitors and Neurons. Stem Cells Dev. 2018, 27, 736–744. [Google Scholar] [CrossRef]
- Grande, A.; Sumiyoshi, K.; Lopez-Juarez, A.; Howard, J.; Sakthivel, B.; Aronow, B.; Campbell, K.; Nakafuku, M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat. Commun. 2013, 4, 2373. [Google Scholar] [CrossRef]
- El Wazan, L.; Urrutia-Cabrera, D.; Wong, R.C. Using transcription factors for direct reprogramming of neurons in vitro. World J. Stem Cells 2019, 11, 431–444. [Google Scholar] [CrossRef]
- Li, X.; Kozielski, K.; Cheng, Y.H.; Liu, H.; Zamboni, C.G.; Green, J.; Mao, H.Q. Nanoparticle-mediated conversion of primary human astrocytes into neurons and oligodendrocytes. Biomater. Sci. 2016, 4, 1100–1112. [Google Scholar] [CrossRef]
- Rivetti di Val Cervo, P.; Romanov, R.A.; Spigolon, G.; Masini, D.; Martin-Montanez, E.; Toledo, E.M.; La Manno, G.; Feyder, M.; Pifl, C.; Ng, Y.H.; et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat. Biotechnol. 2017, 35, 444–452. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Z.; Xu, J.; Hou, K.; Yu, Y.; Lv, S.; Chen, L.; Li, Y.; Quan, C.; Chi, G. MiR-124 and Small Molecules Synergistically Regulate the Generation of Neuronal Cells from Rat Cortical Reactive Astrocytes. Mol. Neurobiol. 2021, 58, 2447–2464. [Google Scholar] [CrossRef]
- Gao, L.; Guan, W.; Wang, M.; Wang, H.; Yu, J.; Liu, Q.; Qiu, B.; Yu, Y.; Ping, Y.; Bian, X.; et al. Direct Generation of Human Neuronal Cells from Adult Astrocytes by Small Molecules. Stem Cell Rep. 2017, 8, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yin, J.C.; Yeh, H.; Ma, N.X.; Lee, G.; Chen, X.A.; Wang, Y.; Lin, L.; Chen, L.; Jin, P.; et al. Small Molecules Efficiently Reprogram Human Astroglial Cells into Functional Neurons. Cell Stem Cell 2015, 17, 735–747. [Google Scholar] [CrossRef]
- Yin, J.C.; Zhang, L.; Ma, N.X.; Wang, Y.; Lee, G.; Hou, X.Y.; Lei, Z.F.; Zhang, F.Y.; Dong, F.P.; Wu, G.Y.; et al. Chemical Conversion of Human Fetal Astrocytes into Neurons through Modulation of Multiple Signaling Pathways. Stem Cell Rep. 2019, 12, 488–501. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, H.; Du, X.; Wang, L.; Jin, X.; Zhang, Q.; Han, Y.; Sun, S.; Wang, L.; Li, X.; et al. In vivo chemical reprogramming of astrocytes into neurons. Cell Discov. 2021, 7, 12. [Google Scholar] [CrossRef]
- Qian, H.; Kang, X.; Hu, J.; Zhang, D.; Liang, Z.; Meng, F.; Zhang, X.; Xue, Y.; Maimon, R.; Dowdy, S.F.; et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 2020, 582, 550–556. [Google Scholar] [CrossRef]
- Sirko, S.; Behrendt, G.; Johansson, P.A.; Tripathi, P.; Costa, M.; Bek, S.; Heinrich, C.; Tiedt, S.; Colak, D.; Dichgans, M.; et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell 2013, 12, 426–439. [Google Scholar] [CrossRef]
- Livingston, J.; Lee, T.; Daniele, E.; Phillips, C.; Krassikova, A.; Enbar, T.; Kortebi, I.; Bang, K.W.A.; Donville, B.; Ibragimov, O.; et al. Direct Reprogramming of Astrocytes to Neurons Leads to Functional Recovery After Stroke. bioRxiv 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.02.02.929091v1 (accessed on 24 May 2022).
- Wu, Z.; Parry, M.; Hou, X.Y.; Liu, M.H.; Wang, H.; Cain, R.; Pei, Z.F.; Chen, Y.C.; Guo, Z.Y.; Abhijeet, S.; et al. Gene therapy conversion of striatal astrocytes into GABAergic neurons in mouse models of Huntington’s disease. Nat. Commun. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Tai, W.; Wu, W.; Wang, L.L.; Ni, H.; Chen, C.; Yang, J.; Zang, T.; Zou, Y.; Xu, X.M.; Zhang, C.L. In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell 2021, 28, 923–937.e4. [Google Scholar] [CrossRef]
- Wang, L.L.; Serrano, C.; Zhong, X.; Ma, S.; Zou, Y.; Zhang, C.L. Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell 2021, 184, 5465–5481.e16. [Google Scholar] [CrossRef]
| In Vivo/In Vitro | Original Cell | Target Cell | Reprogramming Factor | Efficiency | Ref |
|---|---|---|---|---|---|
| in vitro | Mouse Ast | Neurons | VCR | NeuN+ > 20% | [21] |
| in vitro | Mouse Ast | Neurons | Ngn2, Mash1 | Ngn2 > 70% Mash1 > 30% | [24] |
| in vivo | Mouse Ast | GABAergic neurons Glutamate neurons | Neurog2/Dlx2 | Ngn2 > 70% Dlx2 > 35.9% | [26] |
| in vivo | Mouse Ast | Glutamate neurons | NeuroD1 | 7-day conversion efficiency: >90% | [27] |
| in vitro | Mouse Ast | GABAergic neurons | Ascl1 | 13.2 ± 4.2% glutamatergic neurons 6.5 ± 2.2% GABAergic neurons | [28] |
| in vivo | Mouse Ast | GABAergic neurons Glutamate neurons | NeuroD1 | 17-day conversion efficiency: >70% | [29] |
| in vitro | Mouse Ast | Neurons | NeuroD1 | 66% | [30] |
| in vivo | Mouse Ast | Neurons | NeuroD1 | 2.42% | [31] |
| in vivo | Rhesus astrocytes | Neurons | NeuroD1 | 42-day conversion efficiency: >90% | [32] |
| in vitro | Human Ast/Mouse Ast | Dopaminergic neurons | NeuroD1 + Ascl1 + Lmx1a + miR128 | 16% | [38] |
| in vitro | Adult Ast | Cholinergic neuron Glutamate neurons | Small molecule compounds | 8% | [40] |
| in vitro | Human Ast | Neurons | Small molecule compounds | 68.7 ± 4.2% | [41] |
| in vitro | Human fetal astrocytes | Glutamate neurons GABAergic neurons | Small molecule compounds | 40% | [42] |
| in vivo | Mouse Ast | Glutamate neurons GABAergic neurons | Small molecule compounds | Striatum: 11.3% Cerebral cortex: 10.7% | [43] |
| in vivo | Mouse Ast | Dopaminergic neurons | AAV2 system downgrades PTBP1 | 80% NeuN+ neurons, 30%~35% TH+ neurons | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, S.; Shao, H.; Cai, X.; Zhu, J. Astrocyte-Derived Neuronal Transdifferentiation as a Therapy for Ischemic Stroke: Advances and Challenges. Brain Sci. 2022, 12, 1175. https://doi.org/10.3390/brainsci12091175
Gong S, Shao H, Cai X, Zhu J. Astrocyte-Derived Neuronal Transdifferentiation as a Therapy for Ischemic Stroke: Advances and Challenges. Brain Sciences. 2022; 12(9):1175. https://doi.org/10.3390/brainsci12091175
Chicago/Turabian StyleGong, Siqi, Han Shao, Xiuying Cai, and Juehua Zhu. 2022. "Astrocyte-Derived Neuronal Transdifferentiation as a Therapy for Ischemic Stroke: Advances and Challenges" Brain Sciences 12, no. 9: 1175. https://doi.org/10.3390/brainsci12091175
APA StyleGong, S., Shao, H., Cai, X., & Zhu, J. (2022). Astrocyte-Derived Neuronal Transdifferentiation as a Therapy for Ischemic Stroke: Advances and Challenges. Brain Sciences, 12(9), 1175. https://doi.org/10.3390/brainsci12091175







