ER Stress in COVID-19 and Parkinson’s Disease: In Vitro and In Silico Evidences
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Analyses
2.1.1. Gene Set Enrichment Analysis (GSEA) of ER Stress and Oxidative Stress in SARS-CoV-2 Infected Cells
2.1.2. Caspase-4 and SARS-CoV-2 Proteases
2.2. Laboratory-Based Analyses
2.2.1. Chemicals and Antibodies
2.2.2. Cell Culture and Treatments
2.2.3. Western Blot Analysis
2.2.4. Detection and Statistical Analysis of Western Blot Data
2.2.5. Immunocytochemistry
2.2.6. MTT Assay
3. Results
3.1. In Silico Results
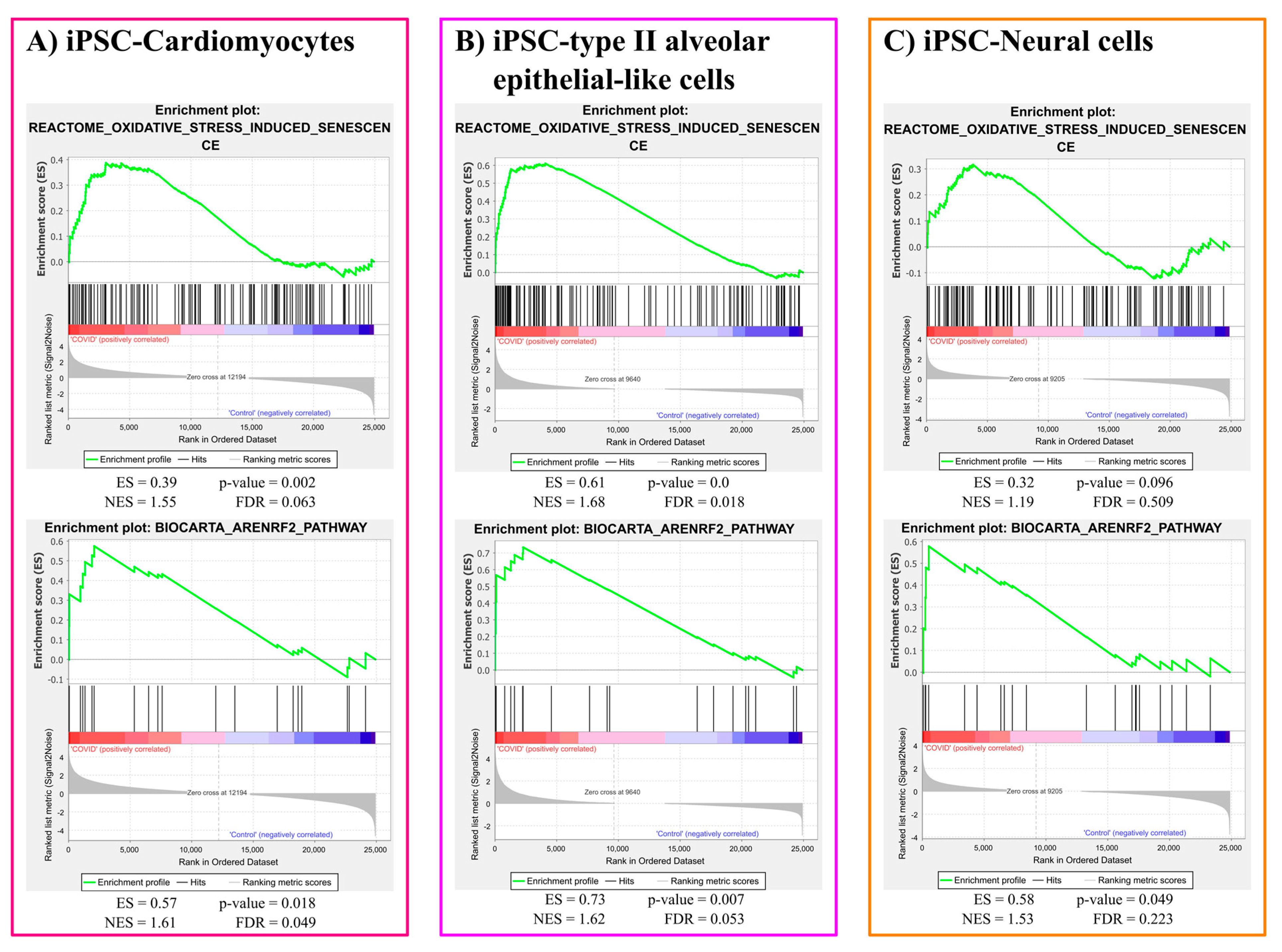
3.1.1. Oxidative Stress Gene Sets Enrichment Analysis Results
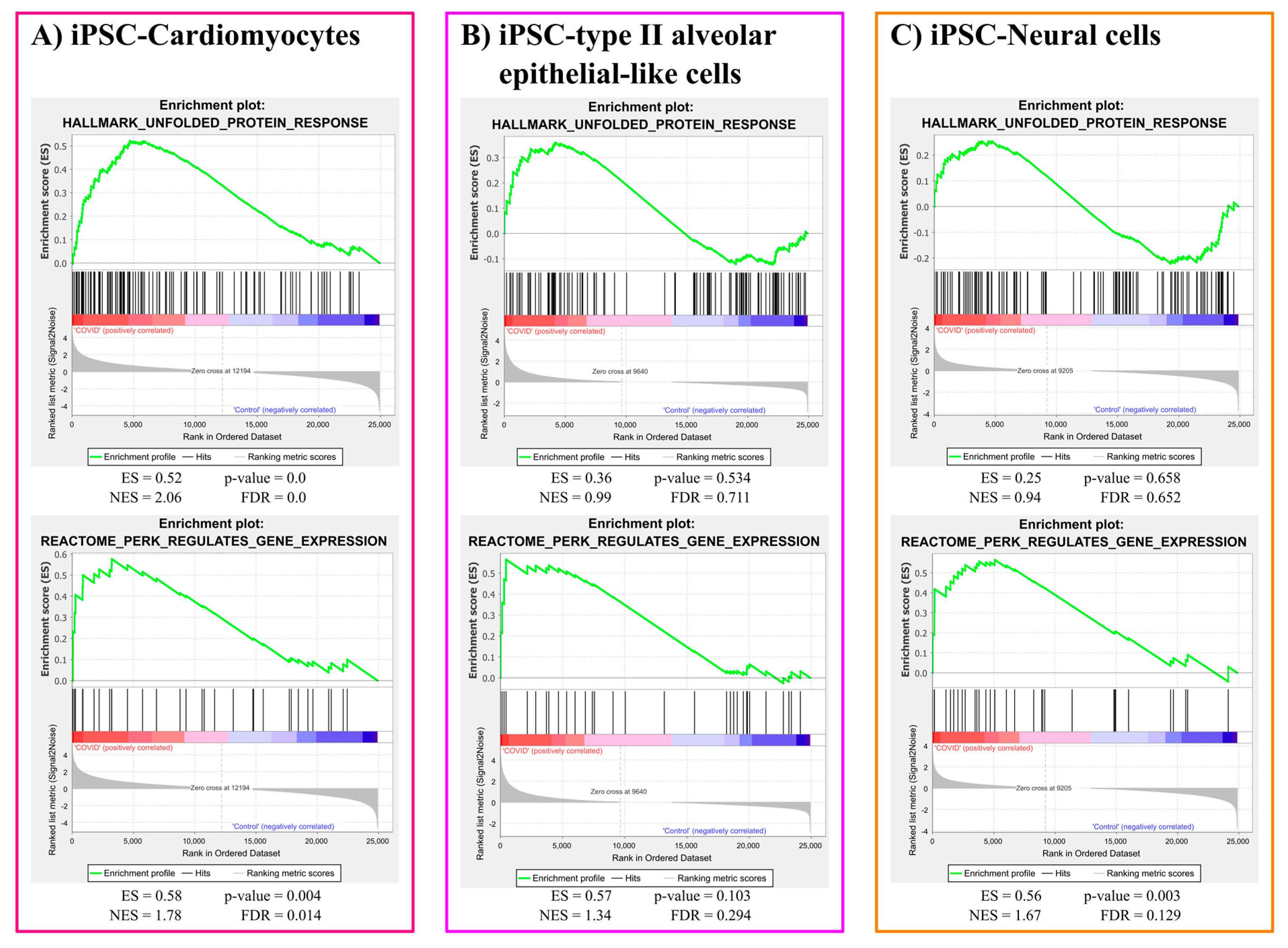
3.1.2. ER Stress Gene Sets Enrichment Analysis
3.1.3. 3CL Protease Is Predicted to Cleave and Activate Caspase-4
3.2. Laboratory-Based Results
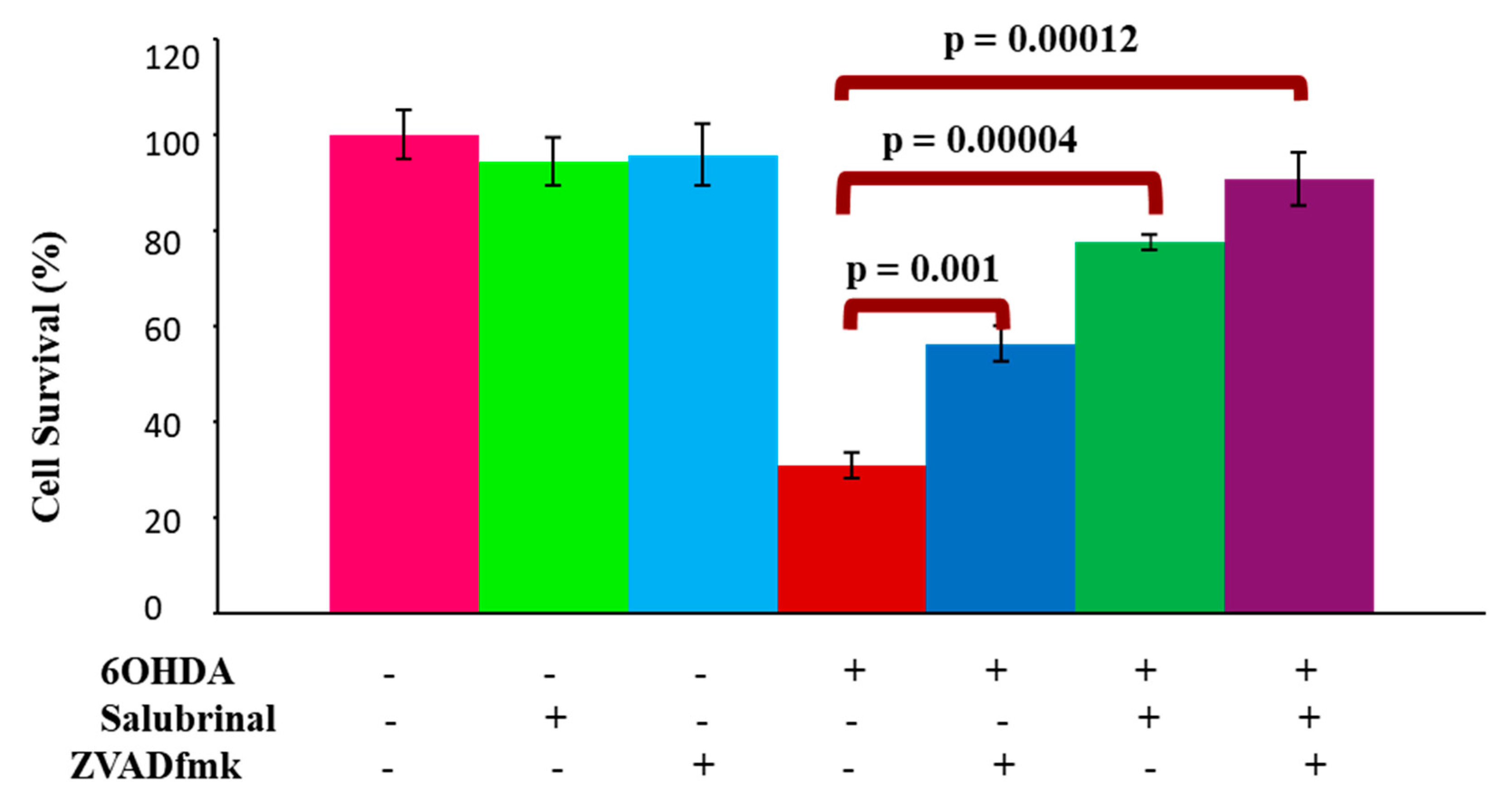
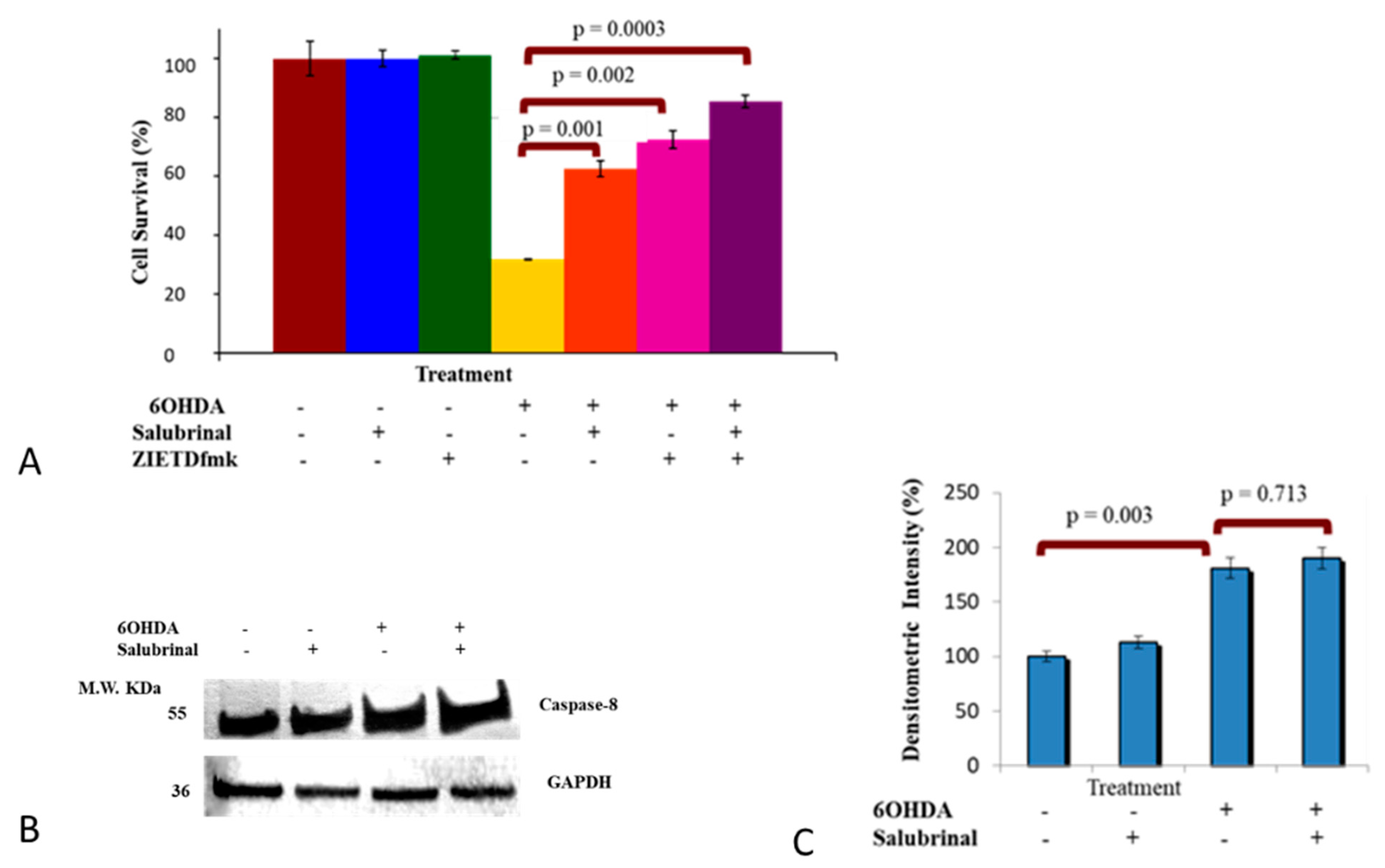
3.2.1. Salubrinal and zVADfmk Promote Survival of Stressed dDCNs
3.2.2. 6OHDA-Induced Oxidative Stress Stimulates Caspase-2 Activation via the ER Stress Pathway in dDCNs
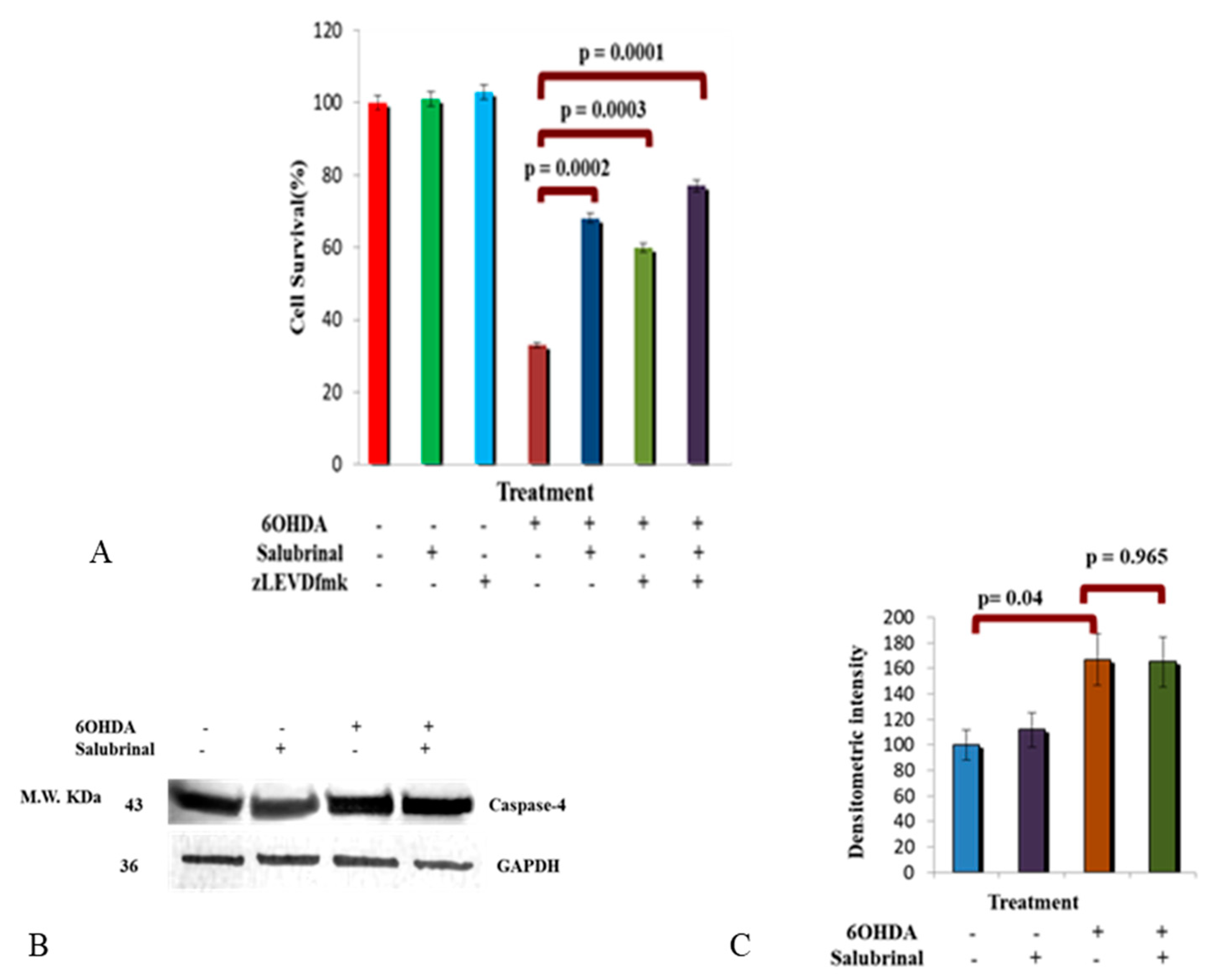
3.2.3. Activation of Caspase-4 in 6OHDA-Treated dDCNs
3.2.4. Caspase-8 Activation by 6OHDA Is Independent on ER Stress Pathway
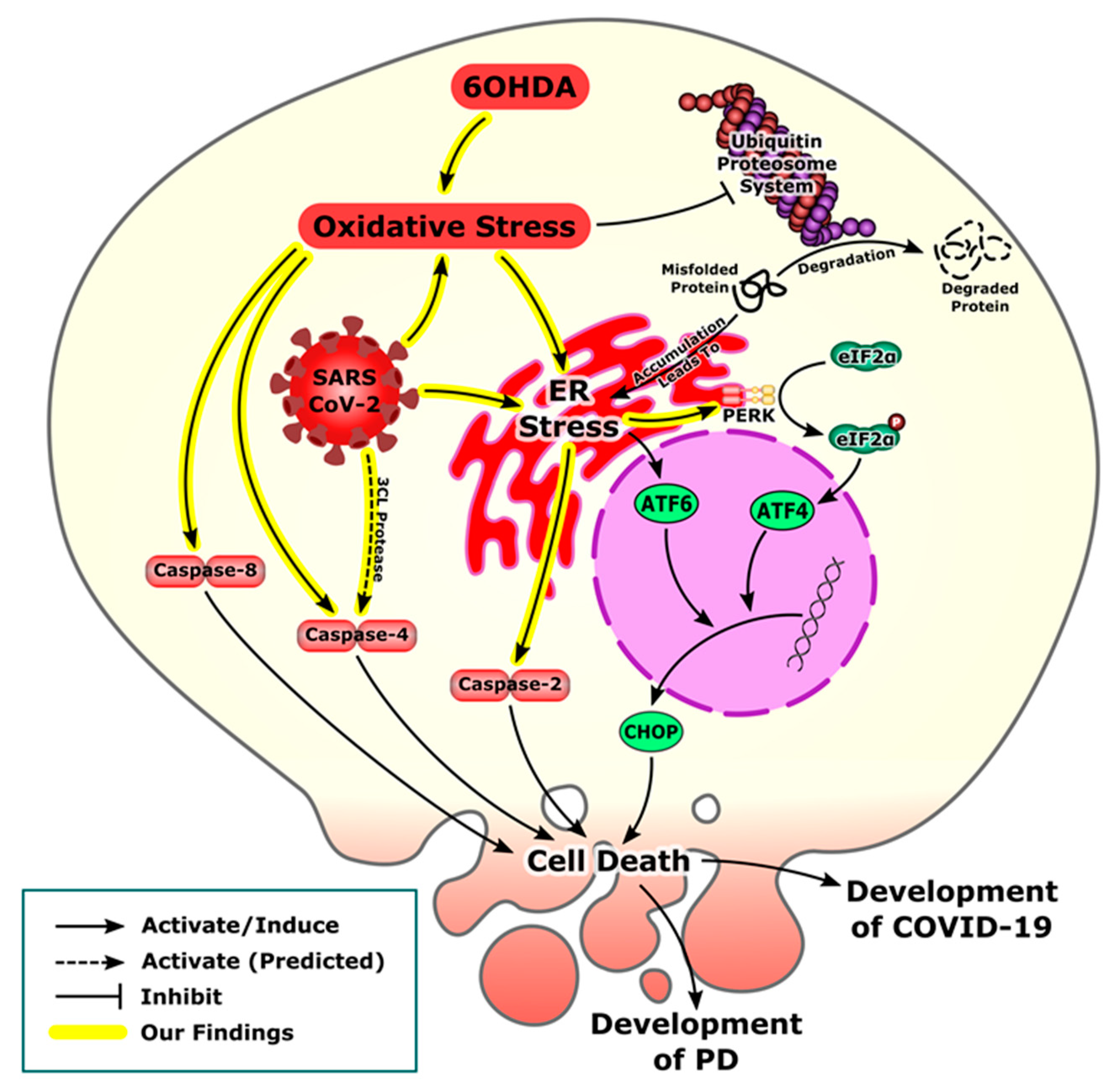
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Köseler, A.; Sabirli, R.; Gören, T.; Türkçüer, I.; Kurt, Ö. Endoplasmic Reticulum Stress Markers in SARS-COV-2 Infection and Pneumonia: Case-Control Study. Vivo 2020, 34, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Santerre, M.; Arjona, S.P.; Allen, C.N.; Shcherbik, N.; Sawaya, B.E. Why Do SARS-CoV-2 NSPs Rush to the ER? J. Neurol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Czinn, S.J.; Reiter, R.J.; Blanchard, T.G. Crosstalk between Endoplasmic Reticulum Stress and Anti-Viral Activities: A Novel Therapeutic Target for COVID-19. Life Sci. 2020, 255, 117842. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Czinn, S.J.; Reiter, R.J.; Blanchard, T.G. Endoplasmic Reticulum as a Potential Therapeutic Target for COVID-19 Infection Management? Eur. J. Pharmacol. 2020, 882, 173288. [Google Scholar]
- de Las Heras, N.; Martín Giménez, V.M.; Ferder, L.; Manucha, W.; Lahera, V. Implications of Oxidative Stress and Potential Role of Mitochondrial Dysfunction in COVID-19: Therapeutic Effects of Vitamin D. Antioxidants 2020, 9, 897. [Google Scholar] [CrossRef]
- Lin, J.H.; Walter, P.; Yen, T.S.B. Endoplasmic Reticulum Stress in Disease Pathogenesis. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 399–425. [Google Scholar] [CrossRef]
- Pereira, C.M.F. Crosstalk between Endoplasmic Reticulum Stress and Protein Misfolding in Neurodegenerative Diseases. ISRN Cell Biol. 2013, 2013, 1–22. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER Stress-Induced Cell Death Mechanisms. Biochim. Biophys. Acta-Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic Reticulum Stress, Obesity and Diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. Signaling the Unfolded Protein Response from the Endoplasmic Reticulum. J. Biol. Chem. 2004, 279, 25935–25938. [Google Scholar] [CrossRef]
- Kincaid, M.M.; Cooper, A.A. ERADicate ER Stress or Die Trying. Antioxid. Redox Signal. 2007, 9, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Brodsky, J.L.; Nakatsukasa, K. Roles of Molecular Chaperones in Endoplasmic Reticulum (ER) Quality Control and ER-Associated Degradation (ERAD). J. Biochem. 2005, 137, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Takahashi, R. Expanding Insights on the Involvement of Endoplasmic Reticulum Stress in Parkinson’s Disease. Antioxid. Redox Signal. 2007, 9, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Matus, S.; Lisbona, F.; Torres, M.; Leon, C.; Thielen, P.; Hetz, C. The Stress Rheostat: An Interplay between the Unfolded Protein Response (UPR) and Autophagy in Neurodegeneration. Curr. Mol. Med. 2008, 8, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, T.; Ando, F.; Murakami, K.; Isobe, K.; Mori, T.; Susa, K.; Nomura, N.; Sohara, E.; Rai, T.; Uchida, S. Tolvaptan Activates the Nrf2/HO-1 Antioxidant Pathway through PERK Phosphorylation. Sci. Rep. 2019, 9, 9245. [Google Scholar] [CrossRef]
- Rochet, J.-C. Novel Therapeutic Strategies for the Treatment of Protein-Misfolding Diseases. Expert Rev. Mol. Med. 2007, 9, 1–34. [Google Scholar] [CrossRef]
- Yoshida, H. ER Stress and Diseases. FEBS J. 2007, 274, 630–658. [Google Scholar] [CrossRef]
- Doyle, K.M.; Kennedy, D.; Gorman, A.M.; Gupta, S.; Healy, S.J.; Samali, A. Unfolded Proteins and Endoplasmic Reticulum Stress in Neurodegenerative Disorders. J. Cell. Mol. Med. 2011, 15, 2025–2039. [Google Scholar] [CrossRef]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular Pathophysiology of Parkinson’s Disease. Annu. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef]
- Skovronsky, D.M.; Lee, V.M.-Y.; Trojanowski, J.Q. Neurodegenerative Diseases: New Concepts of Pathogenesis and Their Therapeutic Implications. Annu. Rev. Pathol. 2006, 1, 151–170. [Google Scholar] [CrossRef]
- McShane, R.; Nagy, Z.; Esiri, M.; King, E.; Joachim, C.; Sullivan, N.; Smith, A. Anosmia in Dementia Is Associated with Lewy Bodies Rather than Alzheimer’s Pathology. J. Neurol. Neurosurg. Psychiatry 2001, 70, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, T.; Kadiane-Oussou, N.; Toko, L.; Royer, P.-Y.; Lepiller, Q.; Gendrin, V.; Zayet, S. Features of Anosmia in COVID-19. Méd. Mal. Infect. 2020, 50, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elshahat, M.E.; Elfiky, A.A. COVID-19 Spike-Host Cell Receptor GRP78 Binding Site Prediction. J. Infect. 2020, 80, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Sabirli, R.; Koseler, A.; Goren, T.; Turkcuer, I.; Kurt, O. High GRP78 Levels in Covid-19 Infection: A Case-Control Study. Life Sci. 2021, 265, 118781. [Google Scholar] [CrossRef]
- Colla, E.; Coune, P.; Liu, Y.; Pletnikova, O.; Troncoso, J.C.; Iwatsubo, T.; Schneider, B.L.; Lee, M.K. Endoplasmic Reticulum Stress Is Important for the Manifestations of -Synucleinopathy In Vivo. J. Neurosci. 2012, 32, 3306–3320. [Google Scholar] [CrossRef]
- Chaudhry, Z.L.; Klenja, D.; Janjua, N.; Cami-Kobeci, G.; Ahmed, B.Y. COVID-19 and Parkinson’s Disease: Shared Inflammatory Pathways Under Oxidative Stress. Brain Sci. 2020, 10, 807. [Google Scholar] [CrossRef]
- Ferro, M.M.; Bellissimo, M.I.; Anselmo-Franci, J.A.; Angellucci, M.E.M.; Canteras, N.S.; Da Cunha, C. Comparison of Bilaterally 6-OHDA- and MPTP-Lesioned Rats as Models of the Early Phase of Parkinson’s Disease: Histological, Neurochemical, Motor and Memory Alterations. J. Neurosci. Methods 2005, 148, 78–87. [Google Scholar] [CrossRef]
- Murray, T.K.; Whalley, K.; Robinson, C.S.; Ward, M.A.; Hicks, C.A.; Lodge, D.; Vandergriff, J.L.; Baumbarger, P.; Siuda, E.; Gates, M.; et al. LY503430, a Novel α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptor Potentiator with Functional, Neuroprotective and Neurotrophic Effects in Rodent Models of Parkinson’s Disease. J. Pharmacol. Exp. Ther. 2003, 306, 752–762. [Google Scholar] [CrossRef]
- Pienaar, I.S.; van de Berg, W. A Non-Cholinergic Neuronal Loss in the Pedunculopontine Nucleus of Toxin-Evoked Parkinsonian Rats. Exp. Neurol. 2013, 248, 213–223. [Google Scholar] [CrossRef]
- Arduino, D.M.; Esteves, A.R.; Domingues, A.F.; Pereira, C.M.; Cardoso, S.M.; Oliveira, C.R. ER-Mediated Stress Induces Mitochondrial-Dependent Caspases Activation in NT2 Neuron-like Cells. BMB Rep. 2009, 42, 719–724. [Google Scholar] [CrossRef]
- Ahmed, B.Y.; Husnain, O.; Stafford, R.; Howard, M.; Gujar, A.S.; Moradiya, V.; Patel, K.K.; Sihotra, S. Hyperphosphorylation of CREB in Human Dopaminergic Neurons. Neuroreport 2013, 24, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Madetko, N.; Koziorowski, D.; Friedman, A. Microglial Activation and Inflammation as a Factor in the Pathogenesis of Progressive Supranuclear Palsy (PSP). Front. Neurosci. 2020, 14, 893. [Google Scholar] [CrossRef] [PubMed]
- Koziorowski, D.; Figura, M.; Milanowski, Ł.M.; Szlufik, S.; Alster, P.; Madetko, N.; Friedman, A. Mechanisms of Neurodegeneration in Various Forms of Parkinsonism—Similarities and Differences. Cells 2021, 10, 656. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The Harmonizome: A Collection of Processed Datasets Gathered to Serve and Mine Knowledge about Genes and Proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—Update. Nucleic Acids Res. 2013, 41, D991-5. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hume, A.J.; Abo, K.M.; Werder, R.B.; Villacorta-Martin, C.; Alysandratos, K.-D.; Beermann, M.L.; Simone-Roach, C.; Lindstrom-Vautrin, J.; Olejnik, J.; et al. SARS-CoV-2 Infection of Pluripotent Stem Cell-Derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response. Cell Stem Cell 2020, 27, 962–973.e7. [Google Scholar] [CrossRef]
- Jacob, F.; Pather, S.R.; Huang, W.-K.; Zhang, F.; Wong, S.Z.H.; Zhou, H.; Cubitt, B.; Fan, W.; Chen, C.Z.; Xu, M.; et al. Human Pluripotent Stem Cell-Derived Neural Cells and Brain Organoids Reveal SARS-CoV-2 Neurotropism Predominates in Choroid Plexus Epithelium. Cell Stem Cell 2020, 27, 937–950.e9. [Google Scholar] [CrossRef]
- Jacob, F.; Pather, S.R.; Huang, W.-K.; Zhang, F.; Wong, S.Z.H.; Zhou, H.; Cubitt, B.; Fan, W.; Chen, C.Z.; Xu, M.; et al. Disease Modeling and Disease Gene Discovery in Cardiomyopathies: A Molecular Study of Induced Pluripotent Stem Cell Generated Cardiomyocytes. Int. J. Mol. Sci. 2021, 22. [Google Scholar]
- Sharma, A.; Garcia Jr, G.; Wang, Y.; Plummer, J.T.; Morizono, K.; Arumugaswami, V.; Svendsen, C.N. Human IPSC-Derived Cardiomyocytes Are Susceptible to SARS-CoV-2 Infection. Cell Rep. Med. 2020, 1, 100052. [Google Scholar] [CrossRef]
- RStudio Team RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2020.
- R Core Team R: A Language and Environment for Statistical Computing 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/nutrients-in-freshwater/r-core-team-2013 (accessed on 10 April 2022).
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Kiemer, L.; Lund, O.; Brunak, S.; Blom, N. Coronavirus 3CLpro Proteinase Cleavage Sites: Possible Relevance to SARS Virus Pathology. BMC Bioinform. 2004, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A.; Papa, F.R. The Role of Endoplasmic Reticulum Stress in Human Pathology. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 173–194. [Google Scholar] [CrossRef]
- Lebeau, J.; Saunders, J.M.; Moraes, V.W.; Madhavan, A.; Madrazo, N.; Anthony, M.C.; Wiseman, R.L. The PERK Arm of the Unfolded Protein Response Regulates Mitochondrial Morphology during Acute Endoplasmic Reticulum Stress. Cell Rep. 2018, 22, 2827–2836. [Google Scholar] [CrossRef]
- Sharif-Askari, N.S.; Sharif-Askari, F.S.; Mdkhana, B.; Alsayed, H.A.H.; Alsafar, H.; Alrais, Z.F.; Hamid, Q.; Halwani, R. Upregulation of Oxidative Stress Gene Markers during SARS-COV-2 Viral Infection. Free Radic. Biol. Med. 2021, 172, 688–698. [Google Scholar] [CrossRef]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy–Bien, J.-P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue Damage from Neutrophil-Induced Oxidative Stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Andrzejewski, K.; Jampolska, M.; Zaremba, M.; Joniec-Maciejak, I.; Boguszewski, P.M.; Kaczyńska, K. Respiratory Pattern and Phrenic and Hypoglossal Nerve Activity during Normoxia and Hypoxia in 6-OHDA-Induced Bilateral Model of Parkinson’s Disease. J. Physiol. Sci. 2020, 70, 16. [Google Scholar] [CrossRef]
- Deumens, R.; Blokland, A.; Prickaerts, J. Modeling Parkinson’s Disease in Rats: An Evaluation of 6-OHDA Lesions of the Nigrostriatal Pathway. Exp. Neurol. 2002, 175, 303–317. [Google Scholar] [CrossRef]
- Prieto-Lloret, J.; Donnelly, D.F.; Rico, A.J.; Moratalla, R.; Gonzalez, C.; Rigual, R.J. Hypoxia Transduction by Carotid Body Chemoreceptors in Mice Lacking Dopamine D 2 Receptors. J. Appl. Physiol. 2007, 103, 1269–1275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tirmenstein, M.A.; Hu, C.X.; Scicchitano, M.S.; Narayanan, P.K.; McFarland, D.C.; Thomas, H.C.; Schwartz, L.W. Effects of 6-Hydroxydopamine on Mitochondrial Function and Glutathione Status in SH-SY5Y Human Neuroblastoma Cells. Toxicol. Vitr. 2005, 19, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.-F.; Benabid, A.-L.; Sadoul, R.; Verna, J.-M. Molecular Pathways Involved in the Neurotoxicity of 6-OHDA, Dopamine and MPTP: Contribution to the Apoptotic Theory in Parkinson’s Disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Glinka, Y.Y.; Youdim, M.B.H. Inhibition of Mitochondrial Complexes I and IV by 6-Hydroxydopamine. Eur. J. Pharmacol. 1995, 292, 329–332. [Google Scholar] [CrossRef]
- Wek, R.C.; Jiang, H.-Y.; Anthony, T.G. Coping with Stress: EIF2 Kinases and Translational Control. Biochem. Soc. Trans. 2006, 34, 7. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Siddiqi, A.; Mella, J.; Lupo, A.; Li, E.; Hollien, J.; Johnson, J.; Lai, K. Salubrinal Enhances EIF2α Phosphorylation and Improves Fertility in a Mouse Model of Classic Galactosemia. Biochim. Biophys. Acta. Mol. basis Dis. 2019, 1865, 165516. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New Insights into the Roles of CHOP-Induced Apoptosis in ER Stress. Acta Biochim. Biophys. Sin. 2014, 46, 629–640. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef]
- Gupta, M.K.; Vemula, S.; Donde, R.; Gouda, G.; Behera, L.; Vadde, R. In-Silico Approaches to Detect Inhibitors of the Human Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel. J. Biomol. Struct. Dyn. 2020, 39, 2617–2627. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, Y.; Sanyal, S. Membrane Heist: Coronavirus Host Membrane Remodeling during Replication. Biochimie 2020, 179, 229–236. [Google Scholar] [CrossRef]
- Jiang, P.; Gan, M.; Ebrahim, A.S.; Lin, W.-L.; Melrose, H.L.; Yen, S.-H.C. ER Stress Response Plays an Important Role in Aggregation of α-Synuclein. Mol. Neurodegener. 2010, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-Y.; Xie, K.-X.; Wang, S.-L.; Yuan, L.-W. Inflammatory Caspase-Related Pyroptosis: Mechanism, Regulation and Therapeutic Potential for Inflammatory Bowel Disease. Gastroenterol. Rep. 2018, 6, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Casson, C.N.; Yu, J.; Reyes, V.M.; Taschuk, F.O.; Yadav, A.; Copenhaver, A.M.; Nguyen, H.T.; Collman, R.G.; Shin, S. Human Caspase-4 Mediates Noncanonical Inflammasome Activation against Gram-Negative Bacterial Pathogens. Proc. Natl. Acad. Sci. USA 2015, 112, 6688–6693. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Strittmatter, G.E.; Kistowska, M.; French, L.E.; Beer, H.-D. Caspase-4 Is Required for Activation of Inflammasomes. J. Immunol. 2012, 188, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, Q.; Zhong, X.; Zeng, M.; Zeng, H.; Shi, X.; Li, Z.; Wang, Y.; Zhao, Q.; Shao, F.; et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell 2020, 180, 941–955.e20. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, K.-S.; Lee, H.J.; Kim, D.H.; Noh, Y.H.; Yu, K.; Jung, H.-Y.; Lee, S.H.; Lee, J.Y.; Youn, Y.C.; et al. Activation of PERK Signaling Attenuates Aβ-Mediated ER Stress. PLoS ONE 2010, 5, e10489. [Google Scholar] [CrossRef]
- Higuchi, M.; Tomioka, M.; Takano, J.; Shirotani, K.; Iwata, N.; Masumoto, H.; Maki, M.; Itohara, S.; Saido, T.C. Distinct Mechanistic Roles of Calpain and Caspase Activation in Neurodegeneration as Revealed in Mice Overexpressing Their Specific Inhibitors. J. Biol. Chem. 2005, 280, 15229–15237. [Google Scholar] [CrossRef]
- Smith, W.W.; Jiang, H.; Pei, Z.; Tanaka, Y.; Morita, H.; Sawa, A.; Dawson, V.L.; Dawson, T.M.; Ross, C.A. Endoplasmic Reticulum Stress and Mitochondrial Cell Death Pathways Mediate A53T Mutant Alpha-Synuclein-Induced Toxicity. Hum. Mol. Genet. 2005, 14, 3801–3811. [Google Scholar] [CrossRef]
- Unudurthi, S.D.; Luthra, P.; Bose, R.J.; McCarthy, J.R.; Kontaridis, M.I. Cardiac Inflammation in COVID-19: Lessons from Heart Failure. Life Sci. 2020, 260, 118482. [Google Scholar] [CrossRef]
- Bhalerao, A.; Raut, S.; Noorani, B.; Mancuso, S.; Cucullo, L. Molecular Mechanisms of Multi-Organ Failure in COVID-19 and Potential of Stem Cell Therapy. Cells 2021, 10, 2878. [Google Scholar] [CrossRef]

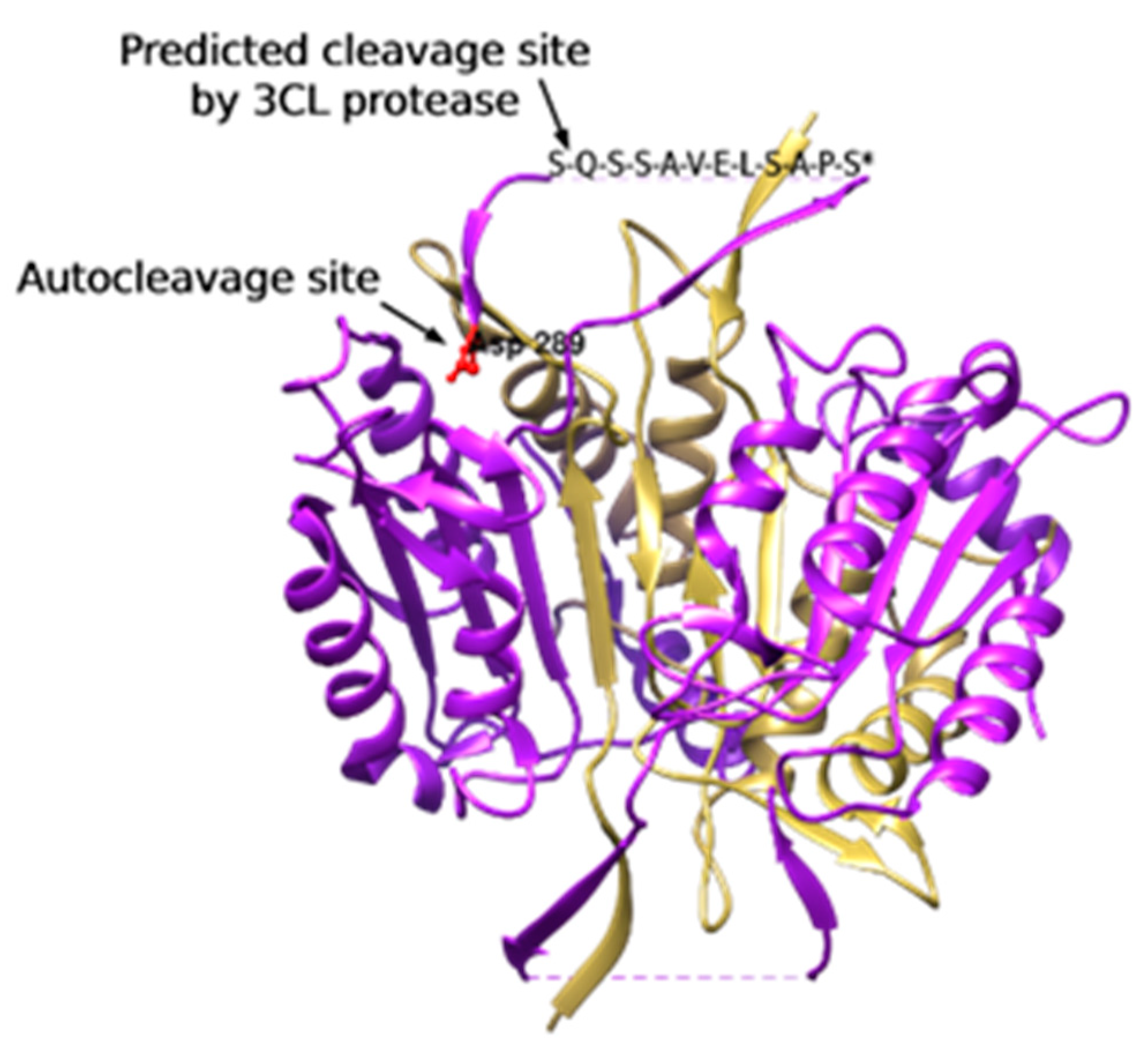
| Residue Position | Sequence | Cleavage Score |
|---|---|---|
| 32 | NLVEQ^NVLNW | 0.088 |
| 62 | ADSMQ^EKQRM | 0.107 |
| 65 | MQEKQ^RMAGQ | 0.062 |
| 70 | RMAGQ^MLLQT | 0.074 |
| 74 | QMLLQ^TFFNI | 0.091 |
| 81 | FNIDQ^ISPNK | 0.071 |
| 236 | DTIFQ^IFNNR | 0.139 |
| 256 | VIIVQ^ACRGA | 0.112 |
| 281 | VASSQ^SSENL | 0.630 * |
| 325 | IFITQ^LITCF | 0.061 |
| 331 | ITCFQ^KYSWC | 0.082 |
| 347 | FRKVQ^QSFET | 0.076 |
| 348 | RKVQQ^SFETP | 0.379 |
| 358 | RAKAQ^MPTIE | 0.095 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhry, Z.L.; Gamal, M.; Ferhati, I.; Warda, M.; Ahmed, B.Y. ER Stress in COVID-19 and Parkinson’s Disease: In Vitro and In Silico Evidences. Brain Sci. 2022, 12, 507. https://doi.org/10.3390/brainsci12040507
Chaudhry ZL, Gamal M, Ferhati I, Warda M, Ahmed BY. ER Stress in COVID-19 and Parkinson’s Disease: In Vitro and In Silico Evidences. Brain Sciences. 2022; 12(4):507. https://doi.org/10.3390/brainsci12040507
Chicago/Turabian StyleChaudhry, Zahara L., Mahmoud Gamal, Ingrid Ferhati, Mohamad Warda, and Bushra Y. Ahmed. 2022. "ER Stress in COVID-19 and Parkinson’s Disease: In Vitro and In Silico Evidences" Brain Sciences 12, no. 4: 507. https://doi.org/10.3390/brainsci12040507
APA StyleChaudhry, Z. L., Gamal, M., Ferhati, I., Warda, M., & Ahmed, B. Y. (2022). ER Stress in COVID-19 and Parkinson’s Disease: In Vitro and In Silico Evidences. Brain Sciences, 12(4), 507. https://doi.org/10.3390/brainsci12040507





