Brain Correlates of Chinese Handwriting and Their Relation to Reading Development in Children: An fMRI Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Handwriting Tests
2.3. Reading Skill Tests
2.4. fMRI Analysis
2.4.1. Stimuli and Procedures
2.4.2. Imaging Data Acquisition
2.4.3. Preprocessing
2.5. Statistical Analysis
2.5.1. Brain Activation Analysis
2.5.2. Lateralization Analysis
2.5.3. Correlation between Brain Activation and Functional Lateralization during Handwriting and Reading
2.5.4. Post Hoc Power Analysis
3. Results
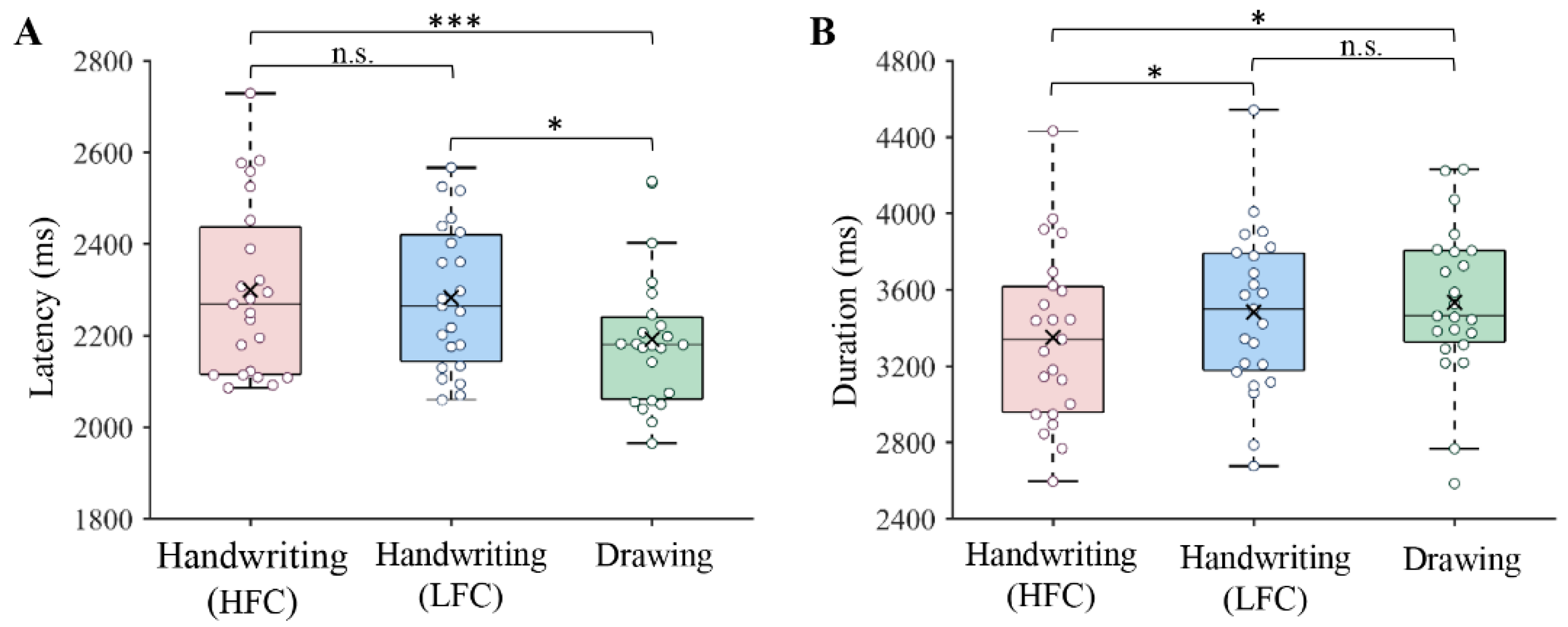
3.1. Behavioral Results
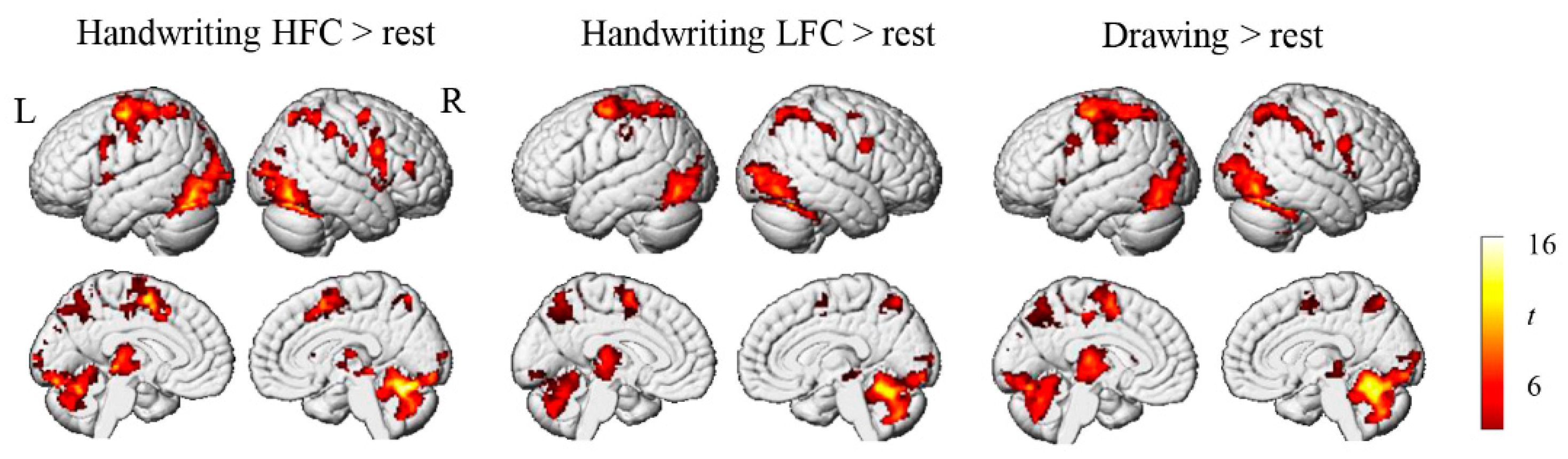
3.2. Brain Activation Results
3.3. Functional Lateralization Results
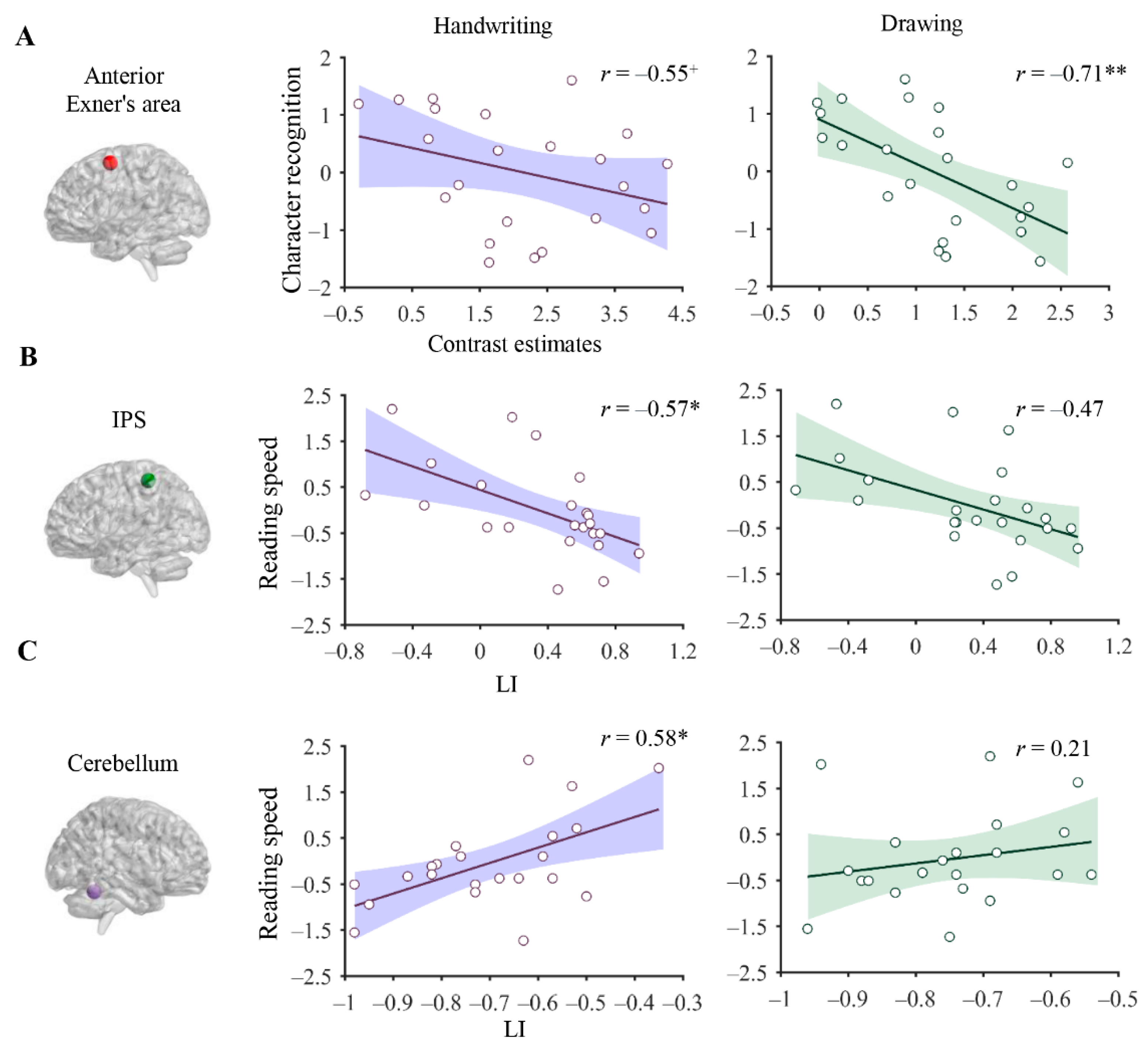
3.4. Association between Brain Correlates of Handwriting and Reading
4. Discussion
4.1. Brain Activation during Chinese Handwriting in Children
4.2. Brain Lateralization during Chinese Handwriting in Children
4.3. The Relationship between Neural Correlates of Handwriting and Reading Skills
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dinehart, L.H. Handwriting in early childhood education: Current research and future implications. J. Early Child. Lit. 2015, 15, 97–118. [Google Scholar] [CrossRef]
- James, K.H. The importance of handwriting experience on the development of the literate brain. Curr. Dir. Psychol. Sci. 2017, 26, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zuo, Z.; Tam, F.; Graham, S.J.; Li, J.; Ji, Y.; Meng, Z.; Gu, C.; Bi, H.Y.; Ou, J. The brain basis of handwriting deficits in Chinese children with developmental dyslexia. Dev. Sci. 2022, 25, e13161. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.; Fishman, E.J.; Reid, R.; Hebert, M. Writing characteristics of students with attention deficit hyperactive disorder: A meta-analysis. Learn. Disabil. Res. Pract. 2016, 31, 75–89. [Google Scholar] [CrossRef]
- Kushki, A.; Chau, T.; Anagnostou, E. Handwriting difficulties in children with autism spectrum disorders: A scoping review. J. Autism Dev. Disord. 2011, 41, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
- Palmis, S.; Danna, J.; Velay, J.-L.; Longcamp, M. Motor control of handwriting in the developing brain: A review. Cogn. Neuropsychol. 2017, 34, 187–204. [Google Scholar] [CrossRef]
- Planton, S.; Jucla, M.; Roux, F.-E.; Démonet, J.-F. The “handwriting brain”: A meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex 2013, 49, 2772–2787. [Google Scholar] [CrossRef]
- Roux, F.E.; Dufor, O.; Giussani, C.; Wamain, Y.; Draper, L.; Longcamp, M.; Démonet, J.F. The graphemic/motor frontal area Exner’s area revisited. Ann. Neurol. 2009, 66, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Planton, S.; Longcamp, M.; Péran, P.; Démonet, J.-F.; Jucla, M. How specialized are writing-specific brain regions? An fMRI study of writing, drawing and oral spelling. Cortex 2017, 88, 66–80. [Google Scholar] [CrossRef]
- Rapp, B.; Dufor, O. The Neurotopography of Written Word Production: An fMRI Investigation of the Distribution of Sensitivity to Length and Frequency. J. Cogn. Neurosci. 2011, 23, 4067–4081. [Google Scholar] [CrossRef]
- Purcell, J.J.; Jiang, X.; Eden, G.F. Shared orthographic neuronal representations for spelling and reading. Neuroimage 2017, 147, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Rapp, B.; Lipka, K. The literate brain: The relationship between spelling and reading. J. Cogn. Neurosci. 2011, 23, 1180–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longcamp, M.; Anton, J.-L.; Roth, M.; Velay, J.-L. Premotor activations in response to visually presented single letters depend on the hand used to write: A study on left-handers. Neuropsychologia 2005, 43, 1801–1809. [Google Scholar] [CrossRef]
- Palmis, S.; Velay, J.L.; Habib, M.; Anton, J.l.; Nazarian, B.; Sein, J.; Longcamp, M. The handwriting brain in middle childhood. Dev. Sci. 2020, 24, e13046. [Google Scholar] [CrossRef]
- Vinci-Booher, S.; James, K.H. Protracted Neural Development of Dorsal Motor Systems During Handwriting and the Relation to Early Literacy Skills. Front. Psychol. 2021, 12, 750559. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.H.; Spinks, J.A.; Eden, G.F.; Perfetti, C.A.; Siok, W.T.; Desimone, R. Reading Depends on Writing, in Chinese. Proc. Natl. Acad. Sci. USA 2005, 102, 8781–8785. [Google Scholar] [CrossRef] [Green Version]
- Berninger, V.W.; Abbott, R.D.; Abbott, S.P.; Graham, S.; Richards, T. Writing and reading: Connections between language by hand and language by eye. J. Learn. Disabil. 2002, 35, 39–56. [Google Scholar] [CrossRef]
- Meng, Z.-L.; Wydell, T.N.; Bi, H.-Y. Visual-motor integration and reading Chinese in children with/without dyslexia. Read. Writ. 2019, 32, 493–510. [Google Scholar] [CrossRef] [Green Version]
- Longcamp, M.; Zerbato-Poudou, M.-T.; Velay, J.-L. The influence of writing practice on letter recognition in preschool children: A comparison between handwriting and typing. Acta Psychol. 2005, 119, 67–79. [Google Scholar] [CrossRef]
- Li, J.X.; James, K.H. Handwriting generates variable visual input to facilitate symbol learning. J. Exp. Psychol. Gen. 2016, 145, 298–313. [Google Scholar] [CrossRef]
- Nakamura, K.; Kuo, W.-J.; Pegado, F.; Cohen, L.; Tzeng, O.J.; Dehaene, S. Universal brain systems for recognizing word shapes and handwriting gestures during reading. Proc. Natl. Acad. Sci. USA 2012, 109, 20762–20767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, C.Q.; Liu, Y.; Chan, D.H.L.; Ye, F.F.; Perfetti, C.A. Writing Strengthens Orthography and Alphabetic-Coding Strengthens Phonology in Learning to Read Chinese. J. Educ. Psychol. 2011, 103, 509–522. [Google Scholar] [CrossRef]
- Longcamp, M.; Boucard, C.; Gilhodes, J.-C.; Anton, J.-L.; Roth, M.; Nazarian, B.; Velay, J.-L. Learning through Hand- or Typewriting Influences Visual Recognition of New Graphic Shapes: Behavioral and Functional Imaging Evidence. J. Cogn. Neurosci. 2008, 20, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Longcamp, M.; Anton, J.-L.; Roth, M.; Velay, J.-L. Visual presentation of single letters activates a premotor area involved in writing. Neuroimage 2003, 19, 1492–1500. [Google Scholar] [CrossRef]
- James, K.H.; Engelhardt, L. The effects of handwriting experience on functional brain development in pre-literate children. Trends Neurosci. Educ. 2012, 1, 32–42. [Google Scholar] [CrossRef] [Green Version]
- James, K.H.; Gauthier, I. Letter processing automatically recruits a sensory–motor brain network. Neuropsychologia 2006, 44, 2937–2949. [Google Scholar] [CrossRef]
- Vinci-Booher, S.; James, T.W.; James, K.H. Visual-motor functional connectivity in preschool children emerges after handwriting experience. Trends Neurosci. Educ. 2016, 5, 107–120. [Google Scholar] [CrossRef]
- James, K.H.; Atwood, T.P. The role of sensorimotor learning in the perception of letter-like forms: Tracking the causes of neural specialization for letters. Cogn. Neuropsychol. 2009, 26, 91–110. [Google Scholar] [CrossRef]
- Cao, F.; Vu, M.; Chan, D.H.L.; Lawrence, J.M.; Harris, L.N.; Guan, Q.; Xu, Y.; Perfetti, C.A. Writing affects the brain network of reading in Chinese: A functional magnetic resonance imaging study. Hum. Brain Mapp. 2013, 34, 1670–1684. [Google Scholar] [CrossRef] [Green Version]
- Cao, F.; Perfetti, C.A. Neural Signatures of the Reading-Writing Connection: Greater Involvement of Writing in Chinese Reading than English Reading. PLoS ONE 2016, 11, e0168414. [Google Scholar] [CrossRef]
- Yang, Y.; Zuo, Z.; Tam, F.; Graham, S.J.; Tao, R.; Wang, N.; Bi, H.-Y. Brain activation and functional connectivity during Chinese writing: An fMRI study. J. Neurolinguistics 2019, 51, 199–211. [Google Scholar] [CrossRef]
- Yang, Y.; Tam, F.; Graham, S.J.; Sun, G.; Li, J.; Gu, C.; Tao, R.; Wang, N.; Bi, H.Y.; Zuo, Z. Men and women differ in the neural basis of handwriting. Hum. Brain Mapp. 2020, 41, 2642–2655. [Google Scholar] [CrossRef] [PubMed]
- Xinchun, W.U.; Wenling, L.I.; Anderson, R.C. Reading instruction in China. J. Curric. Stud. 1999, 31, 571–586. [Google Scholar] [CrossRef]
- Karimpoor, M.; Churchill, N.W.; Tam, F.; Fischer, C.E.; Schweizer, T.A.; Graham, S.J. Functional MRI of handwriting tasks: A study of healthy young adults interacting with a novel touch-sensitive tablet. Front. Hum. Neurosci. 2018, 12, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, P.J.; Harris, L.J. Handedness, Sex, Familial Sinistrality Effects on Spatial Tasks. Cortex 1993, 29, 115–134. [Google Scholar] [CrossRef]
- Gimenez, P.; Bugescu, N.; Black, J.M.; Hancock, R.; Pugh, K.; Nagamine, M.; Kutner, E.; Mazaika, P.; Hendren, R.; McCandliss, B.D.; et al. Neuroimaging correlates of handwriting quality as children learn to read and write. Front. Hum. Neurosci. 2014, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tao, B. Chinese Character Recognition Test Battery and Assessment Scale for Primary School Children: Shanghai, China; Shanghai Education Press: Shanghai, China, 1993. [Google Scholar]
- Shu, H.; McBride-Chang, C.; Wu, S.; Liu, H. Understanding Chinese Developmental Dyslexia: Morphological Awareness as a Core Cognitive Construct. J. Educ. Psychol. 2006, 98, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Qi, T.; Gu, B.; Ding, G.; Gong, G.; Lu, C.; Peng, D.; Malins, J.G.; Liu, L. More bilateral, more anterior: Alterations of brain organization in the large-scale structural network in Chinese dyslexia. NeuroImage 2016, 124, 63–74. [Google Scholar] [CrossRef]
- Tam, F.; Churchill, N.W.; Strother, S.C.; Graham, S.J. A new tablet for writing and drawing during functional MRI. Hum. Brain Mapp. 2011, 32, 240–248. [Google Scholar] [CrossRef]
- Moeller, S.; Yacoub, E.; Olman, C.A.; Auerbach, E.; Strupp, J.; Harel, N.; Ugurbil, K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010, 63, 1144–1153. [Google Scholar] [CrossRef]
- Wilke, M.; Lidzba, K. LI-tool: A new toolbox to assess lateralization in functional MR-data. J. Neurosci. Methods 2007, 163, 128–136. [Google Scholar] [CrossRef]
- Seghier, M.L. Laterality index in functional MRI: Methodological issues. Magn. Reson. Imaging 2008, 26, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Palmis, S.; Velay, J.-L.; Fabiani, E.; Nazarian, B.; Anton, J.-L.; Habib, M.; Kandel, S.; Longcamp, M. The impact of spelling regularity on handwriting production: A coupled fMRI and kinematics study. Cortex 2019, 113, 111–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Brown, S. Drawing and writing: An ALE meta-analysis of sensorimotor activations. Brain Cogn. 2015, 98, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, J.; Meng, Z.-l.; Qin, L.; Liu, Y.; Bi, H.-Y. Neural correlates of orthographic access in Mandarin Chinese writing: An fMRI study of the word-frequency effect. Front. Behav. Neurosci. 2018, 12, 288. [Google Scholar] [CrossRef] [Green Version]
- Bonin, P.; Laroche, B.; Perret, C. Locus of word frequency effects in spelling to dictation: Still at the orthographic level! J. Exp. Psychol. Learn. Mem. Cogn. 2016, 42, 1814. [Google Scholar] [CrossRef]
- Toga, A.W.; Thompson, P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003, 4, 37–48. [Google Scholar] [CrossRef]
- Serrien, D.J.; Ivry, R.B.; Swinnen, S.P. Dynamics of hemispheric specialization and integration in the context of motor control. Nat. Rev. Neurosci. 2006, 7, 160–166. [Google Scholar] [CrossRef]
- Hu, D.; Shen, H.; Zhou, Z. Functional asymmetry in the cerebellum: A brief review. Cerebellum 2008, 7, 304–313. [Google Scholar] [CrossRef]
- Purcell, J.; Turkeltaub, P.E.; Eden, G.F.; Rapp, B. Examining the central and peripheral processes of written word production through meta-analysis. Front. Psychol. 2011, 2, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olulade, O.A.; Seydell-Greenwald, A.; Chambers, C.E.; Turkeltaub, P.E.; Dromerick, A.W.; Berl, M.M.; Gaillard, W.D.; Newport, E.L. The neural basis of language development: Changes in lateralization over age. Proc. Natl. Acad. Sci. USA 2020, 117, 23477–23483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Perfetti, C.A. The time course of brain activity in reading English and Chinese: An ERP study of Chinese bilinguals. Hum. Brain Mapp. 2003, 18, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.H.; Laird, A.R.; Li, K.; Fox, P.T. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta-analysis. Hum. Brain Mapp. 2005, 25, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Jiang, K.; Li, H.; Wang, Z.; Perkins, K.; Cao, F. Convergent and divergent brain structural and functional abnormalities associated with developmental dyslexia. Elife 2021, 10, e69523. [Google Scholar] [CrossRef]
- Vinci-Booher, S.; James, T.W.; James, K.H. Visual-motor contingency during symbol production contributes to short-term changes in the functional connectivity during symbol perception and long-term gains in symbol recognition. NeuroImage 2021, 227, 117554. [Google Scholar] [CrossRef]
- Pattamadilok, C.; Ponz, A.; Planton, S.; Bonnard, M. Contribution of writing to reading: Dissociation between cognitive and motor process in the left dorsal premotor cortex: Contribution of Writing to Reading. Hum. Brain Mapp. 2016, 37, 1531–1543. [Google Scholar] [CrossRef]
- Ringo, J.L.; Doty, R.W.; Demeter, S.; Simard, P.Y. Time is of the essence: A conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb. Cortex 1994, 4, 331–343. [Google Scholar] [CrossRef]
- Gotts, S.J.; Jo, H.J.; Wallace, G.L.; Saad, Z.S.; Cox, R.W.; Martin, A. Two distinct forms of functional lateralization in the human brain. Proc. Natl. Acad. Sci. USA 2013, 110, E3435–E3444. [Google Scholar] [CrossRef] [Green Version]
- Everts, R.; Lidzba, K.; Wilke, M.; Kiefer, C.; Mordasini, M.; Schroth, G.; Perrig, W.; Steinlin, M. Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Hum. Brain Mapp. 2009, 30, 473–483. [Google Scholar] [CrossRef]
- Berl, M.M.; Mayo, J.; Parks, E.N.; Rosenberger, L.R.; VanMeter, J.; Ratner, N.B.; Vaidya, C.J.; Gaillard, W.D. Regional differences in the developmental trajectory of lateralization of the language network. Hum. Brain Mapp. 2014, 35, 270–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, T.A.; Fiez, J.A. Current perspectives on the cerebellum and reading development. Neurosci. Biobehav. Rev. 2018, 92, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, L.; Zhang, M.; Yang, X.; Tian, M.; Xie, W.; Lu, Y.; Liu, L.; Bélanger, N.N.; Meng, X. Dyslexic Children Show Atypical Cerebellar Activation and Cerebro-Cerebellar Functional Connectivity in Orthographic and Phonological Processing. Cerebellum 2017, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bi, H.-Y.; Long, Z.-Y.; Tao, S. Evidence for cerebellar dysfunction in Chinese children with developmental dyslexia: An fMRI study. Int. J. Neurosci. 2013, 123, 300–310. [Google Scholar] [CrossRef]
- Caramazza, A. Some aspects of language processing revealed through the analysis of acquired aphasia: The lexical system. Annu. Rev. Neurosci. 1988, 11, 395–421. [Google Scholar] [CrossRef]

| Variable | Mean (Standard Deviation) |
|---|---|
| Age (years) | 10.40 (0.54) |
| Sex (male/female) | 15/21 |
| IQ | 111.81 (15.56) |
| Handwriting skills | |
| Writing quality | 25.55 (6.08) |
| Copying speed (in s) | 101.49 (27.18) |
| Writing fluency (sentences) | 27.64 (4.82) |
| Writing fluency (digits) | 58.50 (12.20) |
| Reading skills | |
| Character recognition | 2908.46 (261.60) |
| Reading speed (characters) | 100.58 (19.57) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Kang, L.; Li, J.; Li, Y.; Bi, H.; Yang, Y. Brain Correlates of Chinese Handwriting and Their Relation to Reading Development in Children: An fMRI Study. Brain Sci. 2022, 12, 1724. https://doi.org/10.3390/brainsci12121724
Zhang J, Kang L, Li J, Li Y, Bi H, Yang Y. Brain Correlates of Chinese Handwriting and Their Relation to Reading Development in Children: An fMRI Study. Brain Sciences. 2022; 12(12):1724. https://doi.org/10.3390/brainsci12121724
Chicago/Turabian StyleZhang, Jun, Liying Kang, Junjun Li, Yizhen Li, Hongyan Bi, and Yang Yang. 2022. "Brain Correlates of Chinese Handwriting and Their Relation to Reading Development in Children: An fMRI Study" Brain Sciences 12, no. 12: 1724. https://doi.org/10.3390/brainsci12121724
APA StyleZhang, J., Kang, L., Li, J., Li, Y., Bi, H., & Yang, Y. (2022). Brain Correlates of Chinese Handwriting and Their Relation to Reading Development in Children: An fMRI Study. Brain Sciences, 12(12), 1724. https://doi.org/10.3390/brainsci12121724






