Sleep and Neuroimmunomodulation for Maintenance of Optimum Brain Function: Role of Noradrenaline
Abstract
1. Introduction
2. Relationship between Sleep and Immune Function
3. Modulatory Role of Neurotransmitters on Immune Response
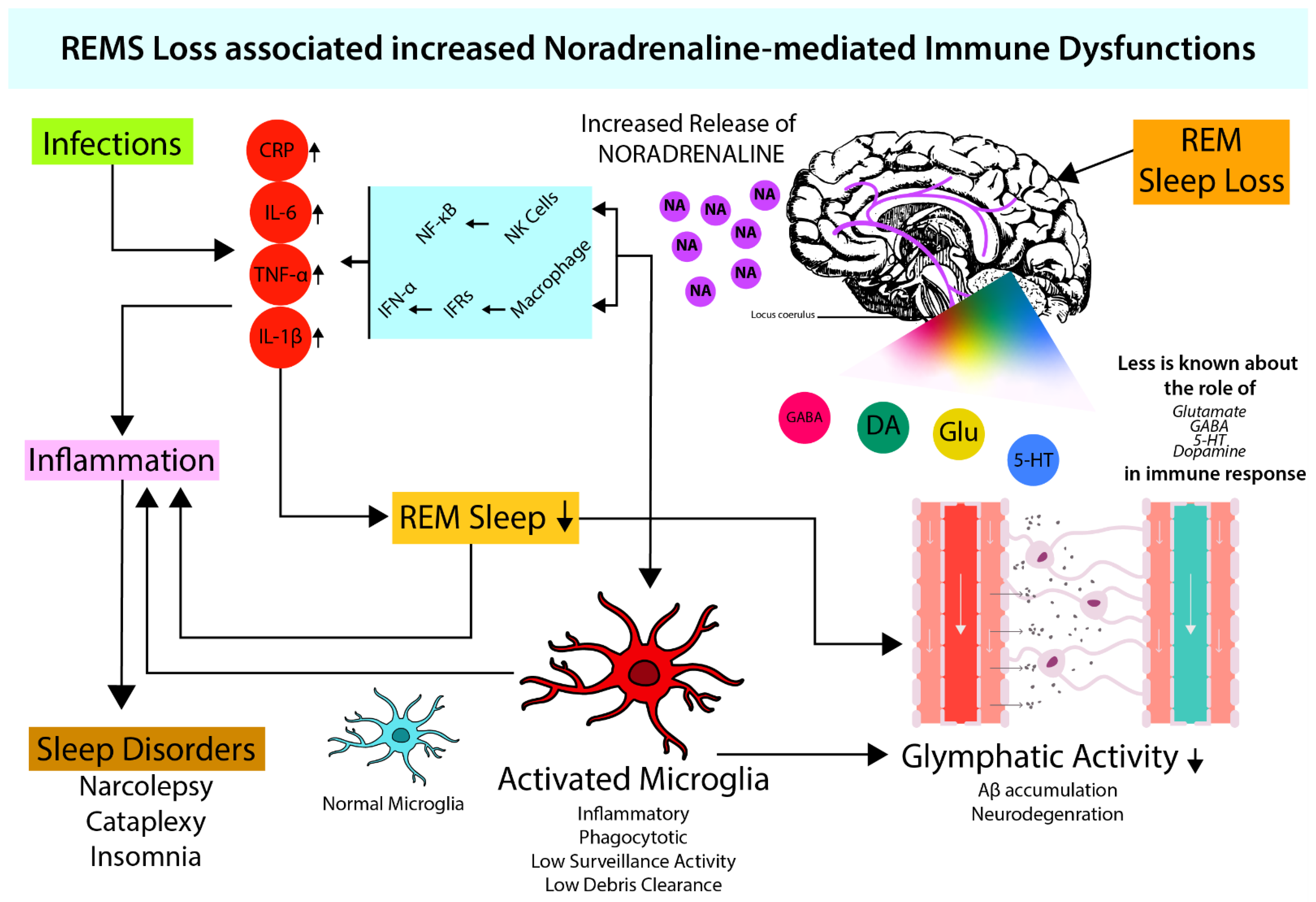
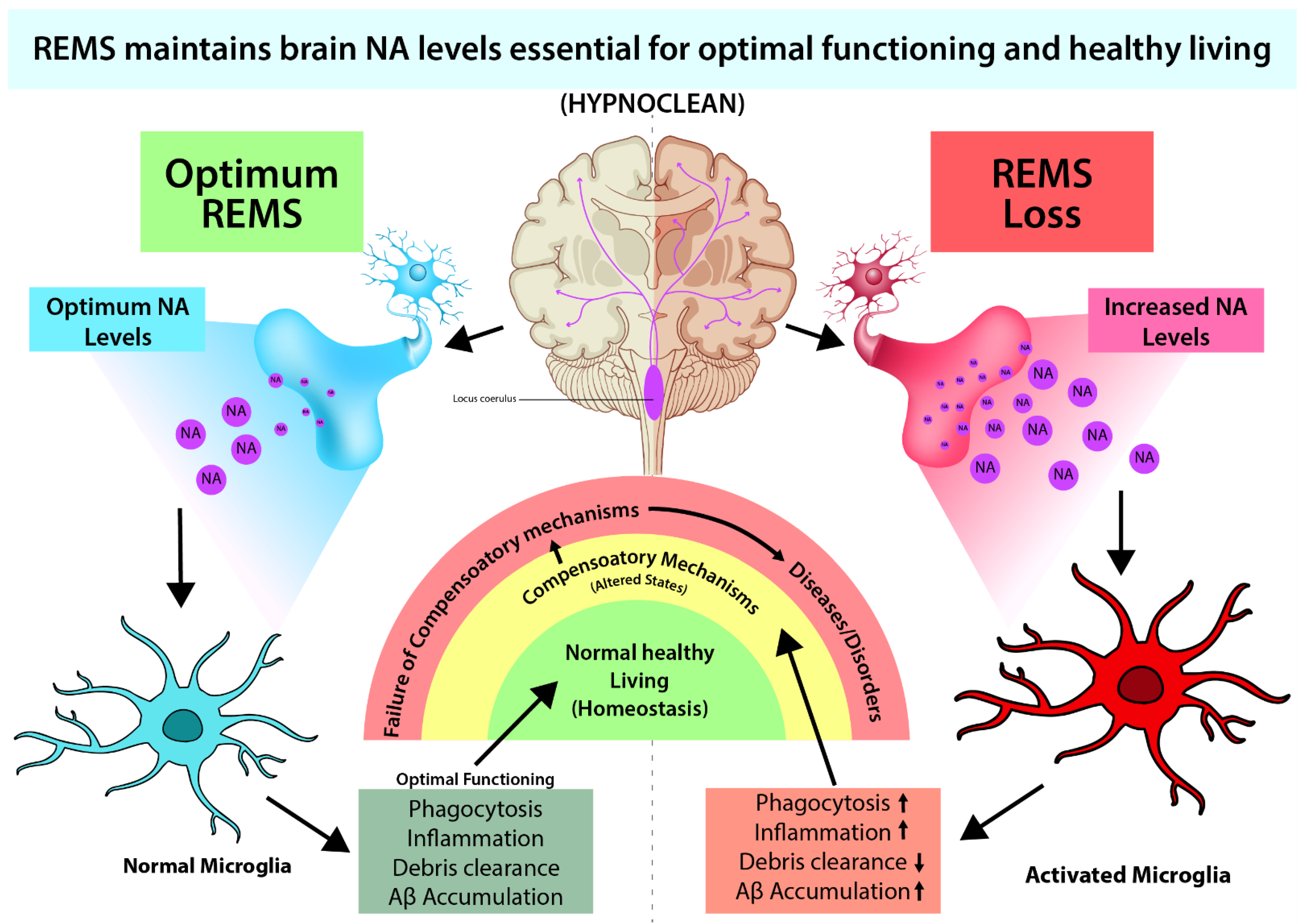
4. Microglia Activation and Noradrenergic System
4.1. Role of NA in Microglial Activation
4.2. Possible Effect of NA on the Glymphatic System through Microglia
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARs | adrenergic receptors |
| AD | Alzheimer’s disease |
| AQP-4 | aquaporin-4 |
| CRP | C-reactive protein |
| GABA | gamma-amino butyric acid |
| ILs | Interleukins |
| IL-6 | interleukin-6 |
| IL-1β | interleukin-1 β |
| NA | Noradrenaline |
| NF-κB | nuclear factor-κB |
| NREMS | non-rapid eye movement sleep |
| REMS | rapid eye movement sleep |
| TNF-α | tumor necrosis factor-α |
References
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2003, 111, S442–S459. [Google Scholar] [CrossRef] [PubMed]
- Hilleman, M.R. Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. 2), 14560–14566. [Google Scholar] [CrossRef]
- Konig, R.; Fleury, S.; Germain, R.N. The structural basis of CD4-MHC class II interactions: Coreceptor contributions to T cell receptor antigen recognition and oligomerization-dependent signal transduction. Curr. Top. Microbiol. Immunol. 1996, 205, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef] [PubMed]
- Blalock, J.E. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol. Rev. 1989, 69, 1–32. [Google Scholar] [CrossRef]
- Madden, K.S.; Felten, D.L. Experimental basis for neural-immune interactions. Physiol. Rev. 1995, 75, 77–106. [Google Scholar] [CrossRef]
- Pons, V.; Rivest, S. Targeting Systemic Innate Immune Cells as a Therapeutic Avenue for Alzheimer Disease. Pharmacol. Rev. 2022, 74, 1–17. [Google Scholar] [CrossRef]
- Besedovsky, H.; Sorkin, E. Network of immune-neuroendocrine interactions. Clin. Exp. Immunol. 1977, 28, 196. [Google Scholar]
- Morimoto, K.; Nakajima, K. Role of the Immune System in the Development of the Central Nervous System. Front. Neurosci. 2019, 13, 916. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Turnbull, A.V.; Rivier, C.L. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: Actions and mechanisms of action. Physiol. Rev. 1999, 79, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Nadjar, A.; Wigren, H.M.; Tremblay, M.E. Roles of Microglial Phagocytosis and Inflammatory Mediators in the Pathophysiology of Sleep Disorders. Front. Cell. Neurosci. 2017, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Singh, A.; Mallick, B.N. Disciplined sleep for healthy living: Role of noradrenaline. World J. Neurol. 2017, 7, 6–23. [Google Scholar] [CrossRef]
- Roffwarg, H.P.; Muzio, J.N.; Dement, W.C. Ontogenetic development of the human sleep-dream cycle. Science 1966, 152, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Mallick, B.N.; Singh, A.; Khanday, M.A. Activation of inactivation process initiates rapid eye movement sleep. Prog. Neurobiol. 2012, 97, 259–276. [Google Scholar] [CrossRef]
- Khanday, M.A.; Somarajan, B.I.; Mehta, R.; Mallick, B.N. Noradrenaline from Locus Coeruleus Neurons Acts on Pedunculo-Pontine Neurons to Prevent REM Sleep and Induces Its Loss-Associated Effects in Rats. eNeuro 2016, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mallick, B.N.; Singh, A. REM sleep loss increases brain excitability: Role of noradrenalin and its mechanism of action. Sleep Med. Rev. 2011, 15, 165–178. [Google Scholar] [CrossRef]
- Frank, M.G.; Heller, H.C. The ontogeny of mammalian sleep: A reappraisal of alternative hypotheses. J. Sleep Res. 2003, 12, 25–34. [Google Scholar] [CrossRef]
- Mehta, R.; Singh, S.; Khanday, M.A.; Mallick, B.N. Reciprocal changes in noradrenaline and GABA levels in discrete brain regions upon rapid eye movement sleep deprivation in rats. Neurochem. Int. 2017, 108, 190–198. [Google Scholar] [CrossRef]
- Mehta, R.; Giri, S.; Mallick, B.N. REM sleep loss-induced elevated noradrenaline could predispose an individual to psychosomatic disorders: A review focused on proposal for prediction, prevention, and personalized treatment. EPMA J. 2020, 11, 529–549. [Google Scholar] [CrossRef]
- Singh, A.; Das, G.; Kaur, M.; Mallick, B.N. Noradrenaline Acting on Alpha1 Adrenoceptor as well as by Chelating Iron Reduces Oxidative Burden on the Brain: Implications with Rapid Eye Movement Sleep. Front. Mol. Neurosci. 2019, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Somarajan, B.I.; Khanday, M.A.; Mallick, B.N. Rapid Eye Movement Sleep Deprivation Induces Neuronal Apoptosis by Noradrenaline Acting on Alpha1 Adrenoceptor and by Triggering Mitochondrial Intrinsic Pathway. Front. Neurol. 2016, 7, 25. [Google Scholar] [CrossRef]
- Majumdar, S.; Mallick, B.N. Increased levels of tyrosine hydroxylase and glutamic acid decarboxylase in locus coeruleus neurons after rapid eye movement sleep deprivation in rats. Neurosci. Lett. 2003, 338, 193–196. [Google Scholar] [CrossRef]
- Giri, S.; Ranjan, A.; Kumar, A.; Amar, M.; Mallick, B.N. Rapid eye movement sleep deprivation impairs neuronal plasticity and reduces hippocampal neuronal arborization in male albino rats: Noradrenaline is involved in the process. J. Neurosci. Res. 2021, 99, 1815–1834. [Google Scholar] [CrossRef] [PubMed]
- Porkkaheiskanen, T.; Smith, S.E.; Taira, T.; Urban, J.H.; Levine, J.E.; Turek, F.W.; Stenberg, D. Noradrenergic Activity in Rat-Brain during Rapid Eye-Movement Sleep-Deprivation and Rebound Sleep. Am. J. Physiol.-Reg. Integr. Comp. Physiol. 1995, 268, R1456–R1463. [Google Scholar] [CrossRef]
- Cottrell, G.A. Occurrence of dopamine and noradrenaline in the nervous tissue of some invertebrate species. Br. J. Pharmacol. Chemother. 1967, 29, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.P.; Accordi, F.; Chimenti, C.; Civinini, A.; Crivellato, E. Catecholaminergic System of Invertebrates: Comparative and Evolutionary Aspects in Comparison with the Octopaminergic System. Int. Rev. Cell Mol. Biol. 2016, 322, 363–394. [Google Scholar] [CrossRef]
- Shein-Idelson, M.; Ondracek, J.M.; Liaw, H.P.; Reiter, S.; Laurent, G. Slow waves, sharp waves, ripples, and REM in sleeping dragons. Science 2016, 352, 590–595. [Google Scholar] [CrossRef]
- Aserinsky, E.; Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953, 118, 273–274. [Google Scholar] [CrossRef]
- Iglesias, T.L.; Boal, J.G.; Frank, M.G.; Zeil, J.; Hanlon, R.T. Cyclic nature of the REM sleep-like state in the cuttlefish Sepia officinalis. J. Exp. Biol. 2019, 222, jeb174862. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, S.L.S.; Paiva, M.M.M.; Lopes, P.H.; Blanco, W.; Lima, F.D.; Oliveira, J.B.C.; Medeiros, I.G.; Sequerra, E.B.; de Souza, S.; Leite, T.S.; et al. Cyclic alternation of quiet and active sleep states in the octopus. iScience 2021, 24, 102223. [Google Scholar] [CrossRef] [PubMed]
- Khanday, M.A.; Mallick, B.N. REM sleep modulation by perifornical orexinergic inputs to the pedunculo-pontine tegmental neurons in rats. Neuroscience 2015, 308, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M.; Karnovsky, M.L. Sleep and the immune response. Ann. N. Y. Acad. Sci. 1987, 496, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Opp, M.R. Sleeping to fuel the immune system: Mammalian sleep and resistance to parasites. BMC Evol. Biol. 2009, 9, 8. [Google Scholar] [CrossRef]
- Kohm, A.P.; Sanders, V.M. Norepinephrine: A messenger from the brain to the immune system. Immunol. Today 2000, 21, 539–542. [Google Scholar] [CrossRef]
- Rommelfanger, K.S.; Weinshenker, D. Norepinephrine: The redheaded stepchild of Parkinson’s disease. Biochem. Pharmacol. 2007, 74, 177–190. [Google Scholar] [CrossRef]
- Beckman, D.; Bonillas, A.; Diniz, G.B.; Ott, S.; Roh, J.W.; Elizaldi, S.R.; Schmidt, B.A.; Sammak, R.L.; Van Rompay, K.K.A.; Iyer, S.S.; et al. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Rep. 2022, 41, 111573. [Google Scholar] [CrossRef] [PubMed]
- Ahnach, M.; Zbiri, S.; Nejjari, S.; Ousti, F.; Elkettani, C. C-reactive protein as an early predictor of COVID-19 severity. J. Med. Biochem. 2020, 39, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Santa Cruz, A.; Mendes-Frias, A.; Oliveira, A.I.; Dias, L.; Matos, A.R.; Carvalho, A.; Capela, C.; Pedrosa, J.; Castro, A.G.; Silvestre, R. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front. Immunol. 2021, 12, 613422. [Google Scholar] [CrossRef]
- Kaushal, K.; Kaur, H.; Sarma, P.; Bhattacharyya, A.; Sharma, D.J.; Prajapat, M.; Pathak, M.; Kothari, A.; Kumar, S.; Rana, S.; et al. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J. Crit. Care 2022, 67, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Hackler, J.; Heller, R.A.; Sun, Q.; Schwarzer, M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relation of Serum Copper Status to Survival in COVID-19. Nutrients 2021, 13, 1898. [Google Scholar] [CrossRef] [PubMed]
- Guihur, A.; Rebeaud, M.E.; Fauvet, B.; Tiwari, S.; Weiss, Y.G.; Goloubinoff, P. Moderate Fever Cycles as a Potential Mechanism to Protect the Respiratory System in COVID-19 Patients. Front. Med. 2020, 7, 564170. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.G.; Benveniste, E.N. Interleukin-6 production by astrocytes: Induction by the neurotransmitter norepinephrine. J. Neuroimmunol. 1993, 45, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- Tapryal, N.; Vivek, G.V.; Mukhopadhyay, C.K. Catecholamine stress hormones regulate cellular iron homeostasis by a posttranscriptional mechanism mediated by iron regulatory protein: Implication in energy homeostasis. J. Biol. Chem. 2015, 290, 7634–7646. [Google Scholar] [CrossRef]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef]
- Lutsenko, S.; Washington-Hughes, C.; Ralle, M.; Schmidt, K. Copper and the brain noradrenergic system. J. Biol. Inorg. Chem. 2019, 24, 1179–1188. [Google Scholar] [CrossRef]
- Almeida, M.C.; Steiner, A.A.; Coimbra, N.C.; Branco, L.G. Thermoeffector neuronal pathways in fever: A study in rats showing a new role of the locus coeruleus. J. Physiol. 2004, 558, 283–294. [Google Scholar] [CrossRef]
- Fitzgerald, P.J. Serious infection may systemically increase noradrenergic signaling and produce psychological effects. Med. Hypotheses 2020, 139, 109692. [Google Scholar] [CrossRef]
- Giarman, N.J.; Tanaka, C.; Mooney, J.; Atkins, E. Serotonin, norepinephrine, and fever. Adv. Pharmacol. 1968, 6, 307–317. [Google Scholar] [CrossRef]
- Guo, P.; Benito Ballesteros, A.; Yeung, S.P.; Liu, R.; Saha, A.; Curtis, L.; Kaser, M.; Haggard, M.P.; Cheke, L.G. COVCOG 2: Cognitive and Memory Deficits in Long COVID: A Second Publication From the COVID and Cognition Study. Front. Aging Neurosci. 2022, 14, 804937. [Google Scholar] [CrossRef] [PubMed]
- Jahrami, H.; BaHammam, A.S.; Bragazzi, N.L.; Saif, Z.; Faris, M.; Vitiello, M.V. Sleep problems during the COVID-19 pandemic by population: A systematic review and meta-analysis. J. Clin. Sleep Med. 2021, 17, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.M. Sleep viewed as a state of adaptive inactivity. Nat. Rev. Neurosci. 2009, 10, 747–753. [Google Scholar] [CrossRef]
- Rechtschaffen, A.; Bergmann, B.M.; Everson, C.A.; Kushida, C.A.; Gilliland, M.A. Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep 1989, 12, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Imeri, L.; Opp, M.R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009, 10, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Redwine, L.; Hauger, R.L.; Gillin, J.C.; Irwin, M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J. Clin. Endocrinol. Metab. 2000, 85, 3597–3603. [Google Scholar] [CrossRef]
- Bhusan-Mishra, B. Serum Proteins Affected by Rapid Eye Movement Sleep Deprivation and Neurophysiological Studies of Its Effects on Sleep-Wakefulness in Rats’; Jawaharlal Nehru University: New Delhi, India, 2004. [Google Scholar]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Lotsikas, A.; Zachman, K.; Kales, A.; Prolo, P.; Wong, M.L.; Licinio, J.; Gold, P.W.; et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J. Clin. Endocrinol. Metab. 1999, 84, 2603–2607. [Google Scholar] [CrossRef]
- Papanicolaou, D.A.; Vgontzas, A.N. Interleukin-6: The endocrine cytokine. J. Clin. Endocrinol. Metab. 2000, 85, 1331–1333. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef]
- Rockstrom, M.D.; Chen, L.; Taishi, P.; Nguyen, J.T.; Gibbons, C.M.; Veasey, S.C.; Krueger, J.M. Tumor necrosis factor alpha in sleep regulation. Sleep Med. Rev. 2018, 40, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Opp, M.R.; Krueger, J.M. Anti-interleukin-1 beta reduces sleep and sleep rebound after sleep deprivation in rats. Am. J. Physiol. 1994, 266, R688–R695. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; McGinty, D.; Bashir, T.; Kumar, S.; Imeri, L.; Opp, M.R.; Szymusiak, R. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: Role in sleep regulation. Eur. J. Neurosci. 2004, 20, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kopitar-Jerala, N. The Role of Interferons in Inflammation and Inflammasome Activation. Front. Immunol. 2017, 8, 873. [Google Scholar] [CrossRef]
- Opp, M.R.; Kapas, L.; Toth, L.A. Cytokine involvement in the regulation of sleep. Proc. Soc. Exp. Biol. Med. 1992, 201, 16–27. [Google Scholar] [CrossRef]
- Krueger, J.M.; Dinarello, C.A.; Shoham, S.; Davenne, D.; Walter, J.; Kubillus, S. Interferon alpha-2 enhances slow-wave sleep in rabbits. Int. J. Immunopharmacol. 1987, 9, 23–30. [Google Scholar] [CrossRef]
- Wen, X.M.; Zhang, Y.L.; Liu, X.M.; Guo, S.X.; Wang, H. Immune responses in mice to arecoline mediated by lymphocyte muscarinic acetylcholine receptor. Cell Biol. Int. 2006, 30, 1048–1053. [Google Scholar] [CrossRef]
- Lee, M.; McGeer, E.G.; McGeer, P.L. Mechanisms of GABA release from human astrocytes. Glia 2011, 59, 1600–1611. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Parrish, W.R.; Rosas-Ballina, M.; Ochani, M.; Puerta, M.; Ochani, K.; Chavan, S.; Al-Abed, Y.; Tracey, K.J. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2009, 23, 41–45. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Kang, J.; Sheng, J.G.; Barger, S.W.; Mrak, R.E.; Griffin, W.S. Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 149–155. [Google Scholar] [CrossRef]
- Miller, A.H.; Haroon, E.; Raison, C.L.; Felger, J.C. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress. Anxiety 2013, 30, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J.; Wang, J.; Ando, T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv. Exp. Med. Biol. 1999, 461, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Shintani, F.; Kanba, S.; Nakaki, T.; Nibuya, M.; Kinoshita, N.; Suzuki, E.; Yagi, G.; Kato, R.; Asai, M. Interleukin-1 beta augments release of norepinephrine, dopamine, and serotonin in the rat anterior hypothalamus. J. Neurosci. Off. J. Soc. Neurosci. 1993, 13, 3574–3581. [Google Scholar] [CrossRef]
- Opp, M.R. Cytokines and sleep. Sleep Med. Rev. 2005, 9, 355–364. [Google Scholar] [CrossRef]
- Krueger, J.M.; Obal, F.J.; Fang, J.; Kubota, T.; Taishi, P. The role of cytokines in physiological sleep regulation. Ann. N. Y. Acad. Sci. 2001, 933, 211–221. [Google Scholar] [CrossRef]
- Brambilla, D.; Franciosi, S.; Opp, M.R.; Imeri, L. Interleukin-1 inhibits firing of serotonergic neurons in the dorsal raphe nucleus and enhances GABAergic inhibitory post-synaptic potentials. Eur. J. Neurosci. 2007, 26, 1862–1869. [Google Scholar] [CrossRef]
- Gemma, C.; Imeri, L.; Opp, M.R. Serotonergic activation stimulates the pituitary-adrenal axis and alters interleukin-1 mRNA expression in rat brain. Psychoneuroendocrinology 2003, 28, 875–884. [Google Scholar] [CrossRef]
- Katsuura, G.; Gottschall, P.E.; Arimura, A. Identification of a high-affinity receptor for interleukin-1 beta in rat brain. Biochem. Biophys. Res. Commun. 1988, 156, 61–67. [Google Scholar] [CrossRef]
- Ovadia, H.; Abramsky, O.; Barak, V.; Conforti, N.; Saphier, D.; Weidenfeld, J. Effect of interleukin-1 on adrenocortical activity in intact and hypothalamic deafferentated male rats. Exp. Brain Res. 1989, 76, 246–249. [Google Scholar] [CrossRef]
- Melia, K.R.; Duman, R.S. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc. Natl. Acad. Sci. USA 1991, 88, 8382–8386. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.L.; Giese, S.; Lin, L.; Einen, M.; Mignot, E.; Coussons-Read, M.E. Exploring the cytokine and endocrine involvement in narcolepsy. Brain Behav. Immun. 2004, 18, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Opp, M.R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology 2017, 42, 129–155. [Google Scholar] [CrossRef]
- Fang, J.; Sanborn, C.K.; Renegar, K.B.; Majde, J.A.; Krueger, J.M. Influenza viral infections enhance sleep in mice. Proc. Soc. Exp. Biol. Med. 1995, 210, 242–252. [Google Scholar] [CrossRef]
- Walsh, K.B.; Teijaro, J.R.; Wilker, P.R.; Jatzek, A.; Fremgen, D.M.; Das, S.C.; Watanabe, T.; Hatta, M.; Shinya, K.; Suresh, M.; et al. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc. Natl. Acad. Sci. USA 2011, 108, 12018–12023. [Google Scholar] [CrossRef]
- Gyoneva, S.; Traynelis, S.F. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J. Biol. Chem. 2013, 288, 15291–15302. [Google Scholar] [CrossRef]
- Mori, K.; Ozaki, E.; Zhang, B.; Yang, L.; Yokoyama, A.; Takeda, I.; Maeda, N.; Sakanaka, M.; Tanaka, J. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology 2002, 43, 1026–1034. [Google Scholar] [CrossRef]
- Sugama, S.; Kakinuma, Y. Noradrenaline as a key neurotransmitter in modulating microglial activation in stress response. Neurochem. Int. 2021, 143, 104943. [Google Scholar] [CrossRef]
- Sugama, S.; Takenouchi, T.; Hashimoto, M.; Ohata, H.; Takenaka, Y.; Kakinuma, Y. Stress-induced microglial activation occurs through beta-adrenergic receptor: Noradrenaline as a key neurotransmitter in microglial activation. J. Neuroinflamm. 2019, 16, 266. [Google Scholar] [CrossRef]
- Liu, Y.U.; Ying, Y.; Li, Y.; Eyo, U.B.; Chen, T.; Zheng, J.; Umpierre, A.D.; Zhu, J.; Bosco, D.B.; Dong, H.; et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 2019, 22, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Landreth, G.E. The role of microglia in amyloid clearance from the AD brain. J. Neural. Transm. 2010, 117, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Jardanhazi-Kurutz, D.; Kummer, M.P.; Terwel, D.; Vogel, K.; Thiele, A.; Heneka, M.T. Distinct adrenergic system changes and neuroinflammation in response to induced locus ceruleus degeneration in APP/PS1 transgenic mice. Neuroscience 2011, 176, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Reddy, O.C.; van der Werf, Y.D. The Sleeping Brain: Harnessing the Power of the Glymphatic System through Lifestyle Choices. Brain. Sci. 2020, 10, 868. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2019, 65, 106–119. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, R.; Bhattacharya, R.; Mallick, B.N. Sleep and Neuroimmunomodulation for Maintenance of Optimum Brain Function: Role of Noradrenaline. Brain Sci. 2022, 12, 1725. https://doi.org/10.3390/brainsci12121725
Mehta R, Bhattacharya R, Mallick BN. Sleep and Neuroimmunomodulation for Maintenance of Optimum Brain Function: Role of Noradrenaline. Brain Sciences. 2022; 12(12):1725. https://doi.org/10.3390/brainsci12121725
Chicago/Turabian StyleMehta, Rachna, Rohosen Bhattacharya, and Birendra Nath Mallick. 2022. "Sleep and Neuroimmunomodulation for Maintenance of Optimum Brain Function: Role of Noradrenaline" Brain Sciences 12, no. 12: 1725. https://doi.org/10.3390/brainsci12121725
APA StyleMehta, R., Bhattacharya, R., & Mallick, B. N. (2022). Sleep and Neuroimmunomodulation for Maintenance of Optimum Brain Function: Role of Noradrenaline. Brain Sciences, 12(12), 1725. https://doi.org/10.3390/brainsci12121725




