Machine Learning Based on Event-Related EEG of Sustained Attention Differentiates Adults with Chronic High-Altitude Exposure from Healthy Controls
Abstract
:1. Introduction
2. Methods
2.1. Participants
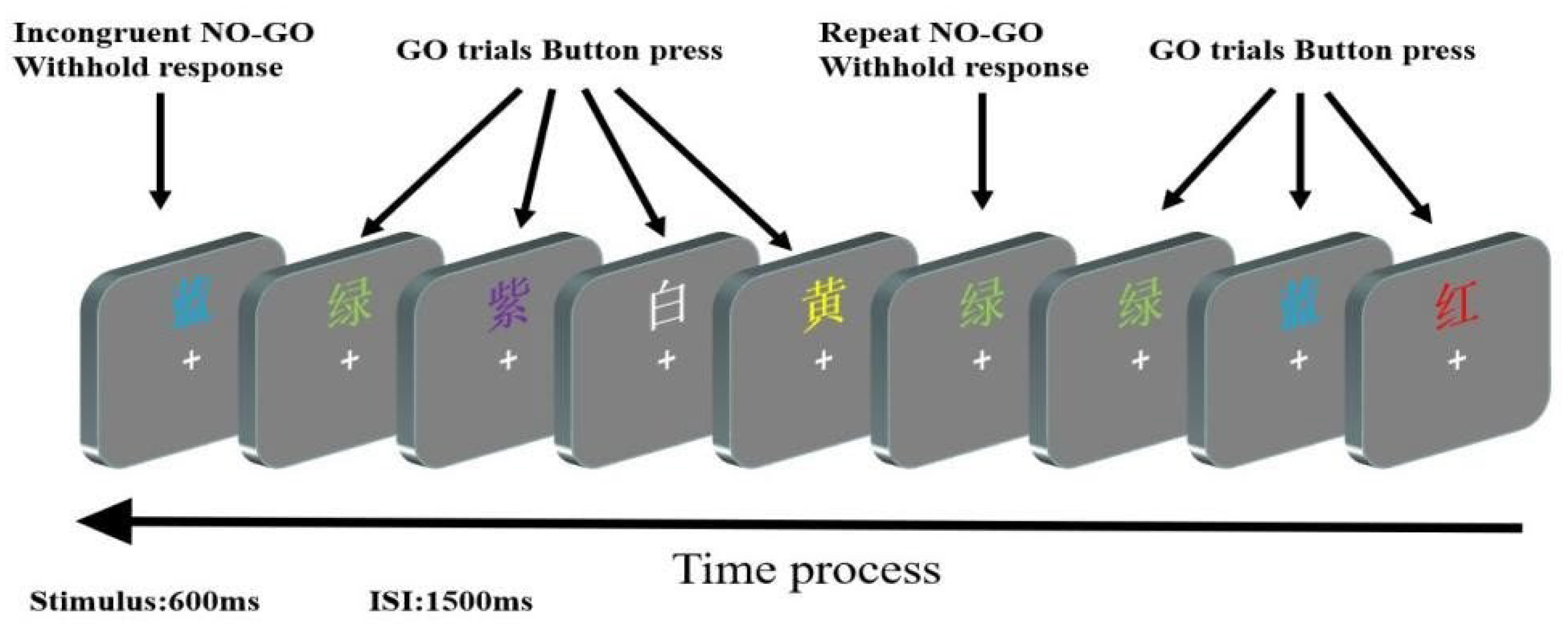
2.2. Experimental Procedure
2.3. EEG Recording and Analysis
2.3.1. Time Domain Analysis
2.3.2. Time-Frequency Analysis
2.4. Statistical Analysis
2.4.1. Analysis of Demographic Data
2.4.2. Behavioral Data
2.4.3. Time-Domain and Time-Frequency Analysis
2.5. Classification Methodology
2.5.1. Feature Generation
Time-Domain Features
Time-Frequency Domain Features
2.5.2. Feature Selection
2.5.3. Classifiers
2.5.4. ROC Analysis
3. Results
3.1. Demography Data
3.2. Behavior Performance
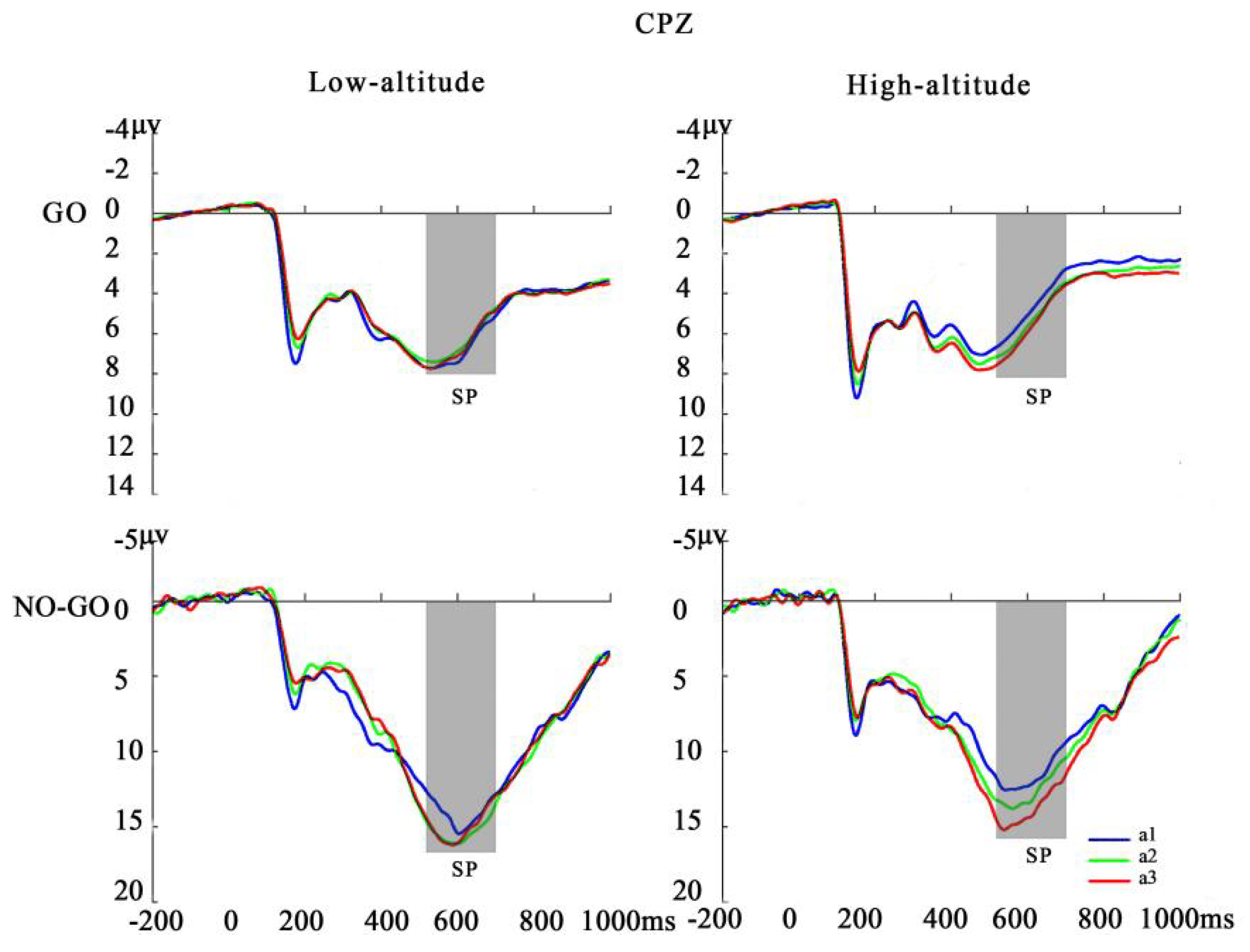
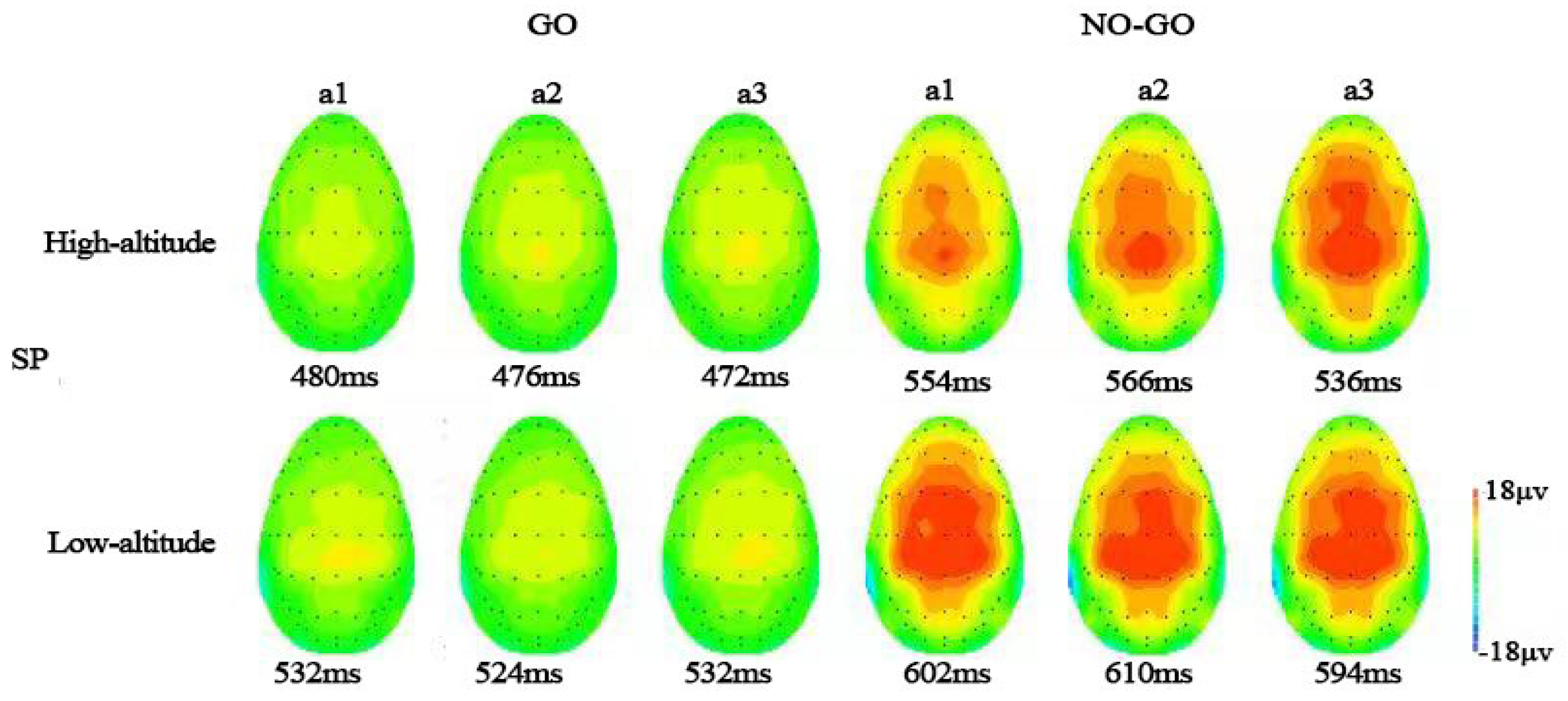
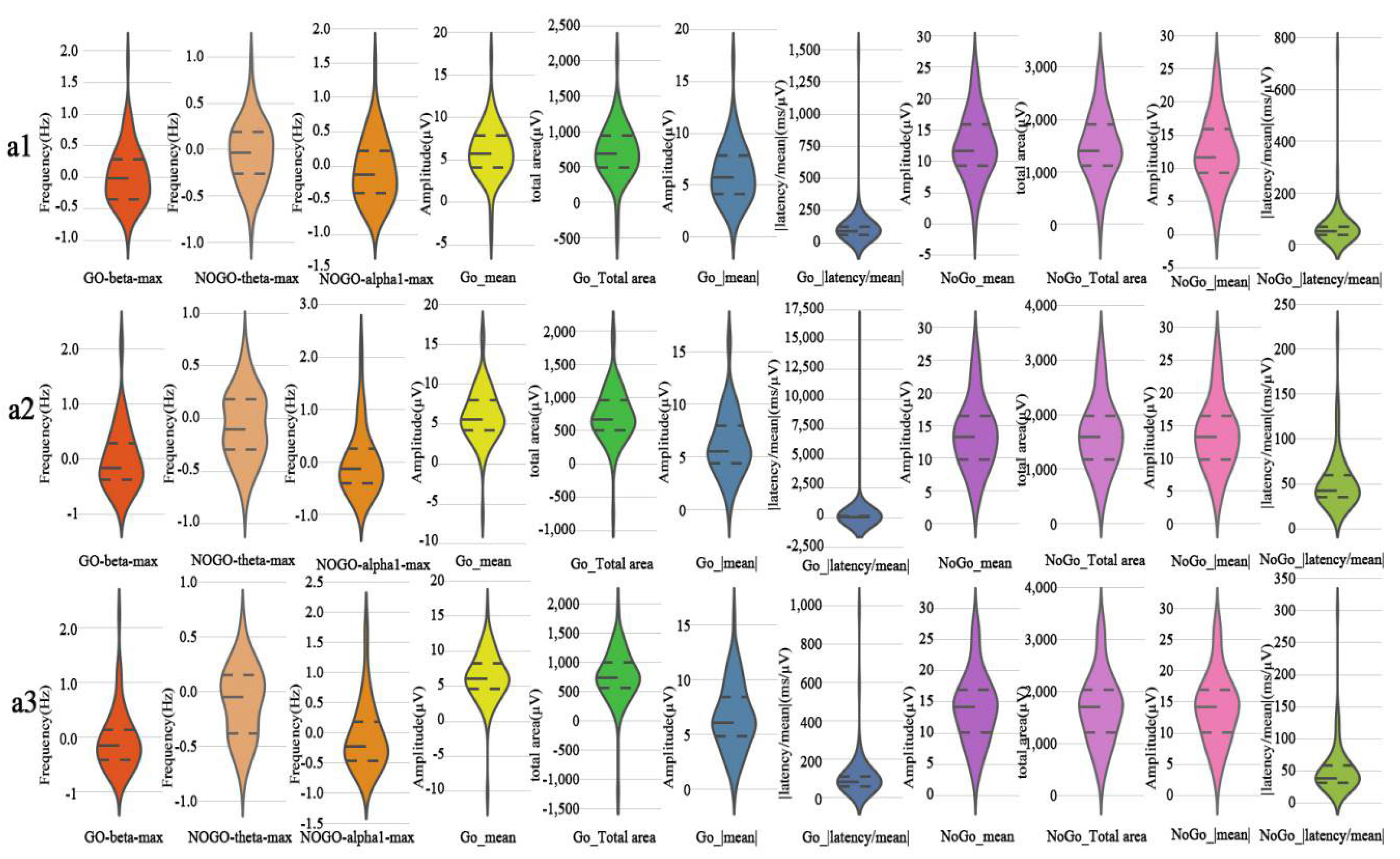
3.3. Time-Domain Analysis
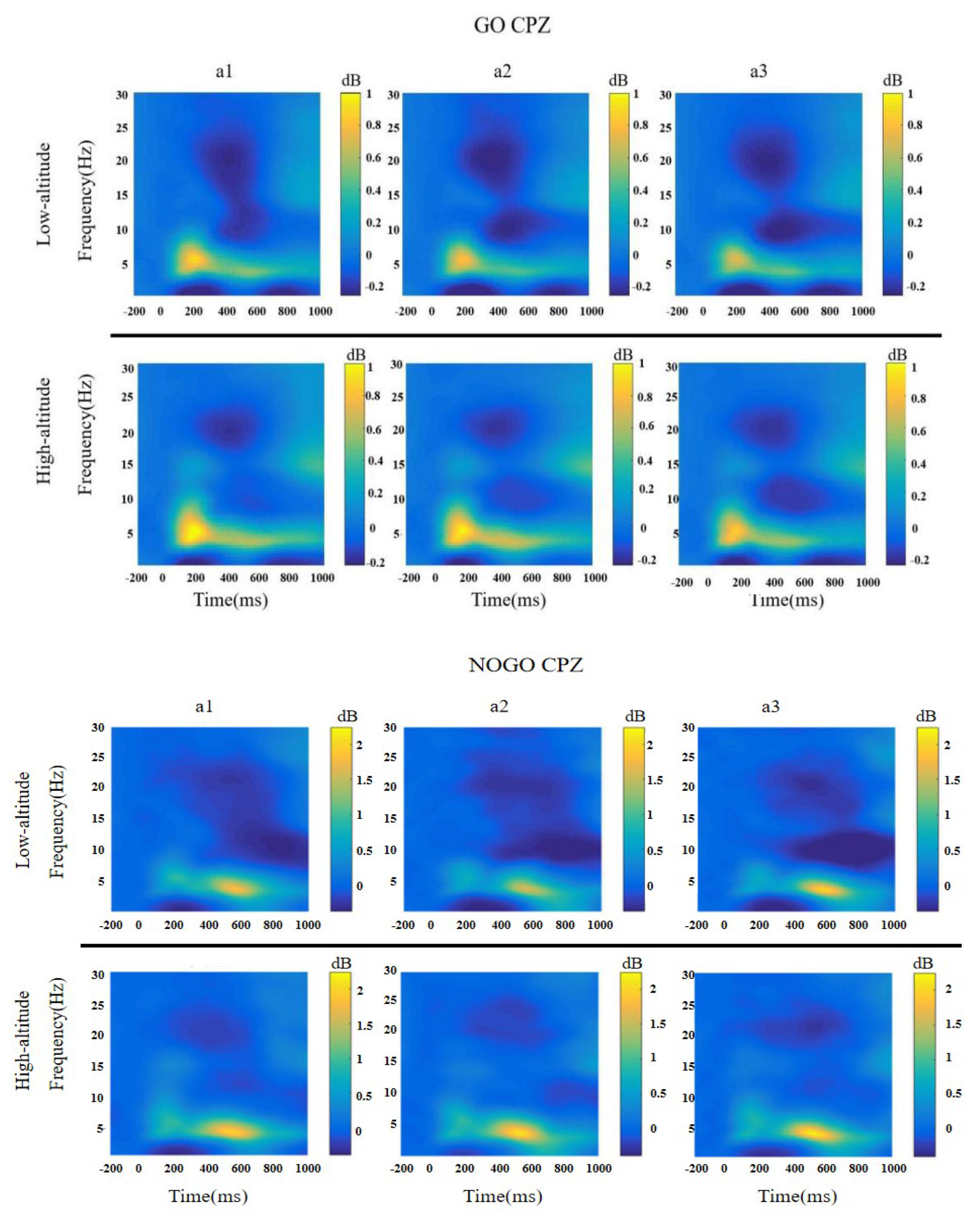
3.4. Time-Frequency Analysis
3.4.1. Theta
3.4.2. Slow Alpha
3.4.3. Fast Alpha
3.4.4. Beta
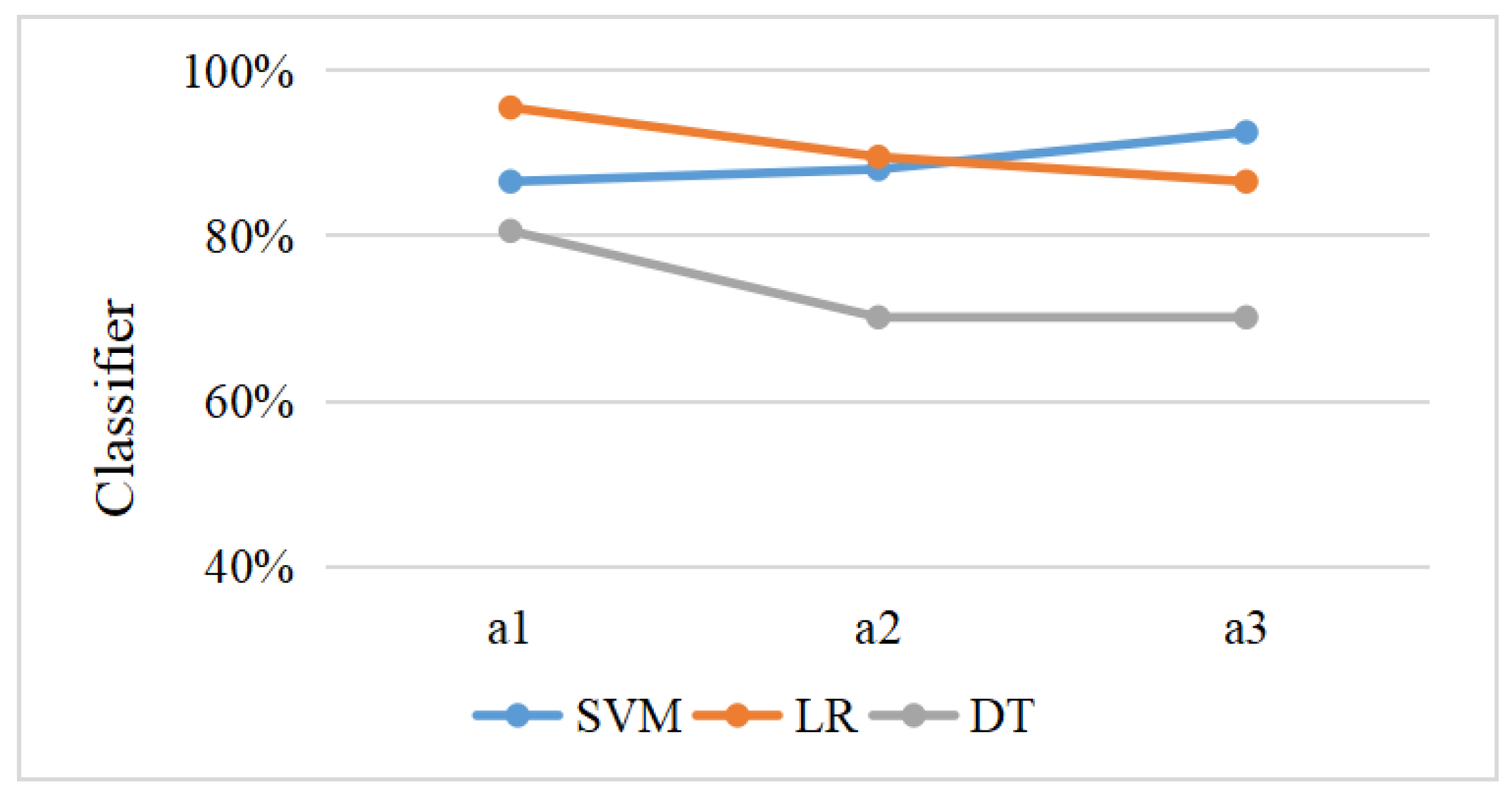
3.5. Machine Learning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, H.; Zhang, X.; Wang, Y.; Ma, H.; Cheng, Y.; Zhang, F.; Liu, M.; Zhang, D. Overactive alerting attention function in immigrants to high-altitude Tibet. Stress Brain 2021, 1, 76–95. [Google Scholar] [CrossRef]
- Su, R.; Wang, C.; Liu, W.; Han, C.; Fan, J.; Ma, H.; Li, H.; Zhang, D. Intensity-dependent acute aerobic exercise: Effect on reactive control of attentional functions in acclimatized lowlanders at high altitude. Physiol. Behav. 2022, 250, 113785. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Qin, S.; Zhang, R.; Wu, H.; Sun, G.; Liu, L.; Hou, X. The Effects of High-Altitude Environment on Brain Function in a Seizure Model of Young-Aged Rats. Front. Pediatr. 2020, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.J.; Su, R.; Li, Z.F.; Bu, X.O.; Dang, P.; Yu, S.F.; Wang, Z.X.; Chen, D.M.; Zeng, T.A.; Liu, M.; et al. Oxygen Metabolism-induced Stress Response Underlies Heart-brain Interaction Governing Human Consciousness-breaking and Attention. Neurosci. Bull. 2022, 38, 166–180. [Google Scholar] [CrossRef]
- Cohen, R.A.; Sparling-Cohen, Y.A.; O’Donnell, B.F. The Neuropsychology of Attention; Plenum Press: New York, NY, USA, 1993. [Google Scholar]
- Parasuraman, R. Vigilance, Monitoring, and Search. In Handbook of Perception and Human Performance; Cognitive Processes and Performance; John Wiley & Sons: Hoboken, NJ, USA, 1986; Volume 2, pp. 1–39. [Google Scholar]
- O’Connell, R.G.; Dockree, P.M.; Bellgrove, M.A.; Turin, A.; Ward, S.; Foxe, J.J.; Robertson, I.H. Two types of action error: Electrophysiological evidence for separable inhibitory and sustained attention neural mechanisms producing error on Go/No-Go tasks. J. Cogn. Neurosci. 2009, 21, 93–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeb, M.; Davies, D.R.; Parasuraman, R. The Psychology of Vigilance. Am. J. Psychol. 1984, 97, 466. [Google Scholar] [CrossRef]
- Demeter, E.; Hernandez-Garcia, L.; Sarter, M.; Lustig, C. Challenges to attention: A continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. NeuroImage 2011, 54, 1518–1529. [Google Scholar] [CrossRef] [Green Version]
- Staub, B.; Doignon-Camus, N.; Bacon, É.; Bonnefond, A. The effects of aging on sustained attention ability: An ERP study. Psychol. Aging 2014, 29, 684. [Google Scholar] [CrossRef]
- Dovis, S.; Van Der Oord, S.; Wiers, R.; Prins, P.J.M. Can Motivation Normalize Working Memory and Task Persistence in Children with Attention-Deficit/Hyperactivity Disorder? The Effects of Money and Computer-Gaming. J. Abnorm. Child Psychol. 2011, 40, 669–681. [Google Scholar] [CrossRef] [Green Version]
- Casey, B.; Durston, S.; Fossella, J.A. Evidence for a mechanistic model of cognitive control. Clin. Neurosci. Res. 2001, 1, 267–282. [Google Scholar] [CrossRef]
- Nigg, J.T. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychol. Bull. 2000, 126, 220. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.E.; Jonides, J. Storage and Executive Processes in the Frontal Lobes. Science 1999, 283, 1657–1661. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, M.P.; Schilhab, T.; Bentsen, P. Attention Restoration Theory II: A systematic review to clarify attention processes affected by exposure to natural environments. J. Toxicol. Environ. Health Part B 2018, 21, 227–268. [Google Scholar] [CrossRef]
- Malhotra, P.A. Impairments of attention in Alzheimer’s disease. Curr. Opin. Psychol. 2019, 29, 41–48. [Google Scholar] [CrossRef]
- Huang-Pollock, C.; Ratcliff, R.; McKoon, G.; Roule, A.; Warner, T.; Feldman, J.; Wise, S. A diffusion model analysis of sustained attention in children with attention deficit hyperactivity disorder. Neuropsychology 2020, 34, 641–653. [Google Scholar] [CrossRef]
- Johannsen, P.; Jakobsen, J.; Bruhn, P.; Gjedde, A. Cortical responses to sustained and divided attention in Alzheimer’s disease. Neuroimage 1999, 10, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Wald, E.L.A.F.; Klinkenberg, S.; Voncken, T.P.C.; Ebus, S.C.M.; Aldenkamp, A.P.; Vles, J.S.H.; Vermeulen, R.J.; Hendriksen, J.G.M.; Hall, M.H.J.A.D.-V. Cognitive development in absence epilepsy during long-term follow-up. Child Neuropsychol. 2019, 25, 1003–1021. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A.V. Selective sustained attention: A developmental foundation for cognition. Curr. Opin. Psychol. 2019, 29, 248–253. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y. Effects of Long-Term Exposure to High Altitude Hypoxia on Cognitive Function and Its Mechanism: A Narrative Review. Brain Sci. 2022, 12, 808. [Google Scholar] [CrossRef]
- Pena, E.; El Alam, S.; Siques, P.; Brito, J. Oxidative Stress and Diseases Associated with High-Altitude Exposure. Antioxidants 2022, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.K. Hypoxia. 3. Hypoxia and neurotransmitter synthesis. Am. J. Physiol. Cell Physiol. 2011, 300, C743–C751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, B. Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biol. Psychiatry 2002, 51, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, L.; Wang, Z.; Wu, X.; Ma, N.; Zhang, T.; Chen, K.; Biswal, B.B.; Yang, Q.; Ma, H. Alteration of Behavioral Inhibitory Control in High-Altitude Immigrants. Front. Behav. Neurosci. 2021, 15, 712278. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, S.F.; Rexhaj, E.; Duplain, H.; Urben, S.; Billieux, J.; Allemann, Y.; Romero, C.; Ayaviri, A.; Salinas, C.; Villena, M.; et al. Acute and chronic altitude-induced cognitive dysfunction in children and adolescents. J. Pediatr. 2016, 169, 238–243. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.; Burns, K.; Fennell, C.; Kim, J.-H.; Gunstad, J.; Glickman, E.; McDaniel, J. The Influence of Exercise on Cognitive Performance in Normobaric Hypoxia. High Alt. Med. Biol. 2015, 16, 298–305. [Google Scholar] [CrossRef]
- Virues-Ortega, J.; Garrido, E.; Javierre, C.; Kloezeman, K.C. Human behaviour and development under high-altitude conditions. Dev. Sci. 2006, 9, 400–410. [Google Scholar] [CrossRef]
- Forster, S.; Elizalde, A.O.N.; Castle, E.; Bishop, S.J. Unraveling the Anxious Mind: Anxiety, Worry, and Frontal Engagement in Sustained Attention Versus Off-Task Processing. Cereb. Cortex 2013, 25, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Van Puyvelde, M.; Neyt, X.; McGlone, F.; Pattyn, N. Voice stress analysis: A new framework for voice and effort in human performance. Front. Psychol. 2018, 9, 1994. [Google Scholar] [CrossRef]
- Braver, T.S.; Gray, J.R.; Burgess, G.C. Explaining the Many Varieties of Working Memory Variation: Dual Mechanisms of Cognitive Control. Var. Work. Mem. 2008, 76, 106. [Google Scholar] [CrossRef]
- Staub, B.; Doignon-Camus, N.; Bacon, É.; Bonnefond, A. Age-related differences in the recruitment of proactive and reactive control in a situation of sustained attention. Biol. Psychol. 2014, 103, 38–47. [Google Scholar] [CrossRef]
- Wei, X.; Ni, X.; Zhao, S.; Chi, A. Influence of Exposure at Different Altitudes on the Executive Function of Plateau Soldiers—Evidence from ERPs and Neural Oscillations. Front. Physiol. 2021, 12, 632058. [Google Scholar] [CrossRef]
- Roxburgh, A.D.; White, D.J.; Cornwell, B.R. Anxious arousal alters prefrontal cortical control of stopping. Eur. J. Neurosci. 2022, 55, 2529–2541. [Google Scholar] [CrossRef]
- Carlin, M.T.; Costello, M.S.; Flansburg, M.A.; Darden, A. Reconsideration of the type I error rate for psychological science in the era of replication. Psychol. Methods 2022. [Google Scholar] [CrossRef]
- Doan, A.E. Type I and Type II Error. Encycl. Soc. Meas. 2005, 3, 883–888. [Google Scholar]
- Libbrecht, M.W.; Noble, W.S. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015, 16, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Craik, A.; He, Y.; Contreras-Vidal, J.L. Deep learning for electroencephalogram (EEG) classification tasks: A review. J. Neural Eng. 2019, 16, 031001. [Google Scholar] [CrossRef]
- Rajaguru, H.; Prabhakar, S.K. Non linear ICA and logistic regression for classification of epilepsy from EEG signals. In Proceedings of the 2017 International Conference of Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, 20–22 April 2017. [Google Scholar]
- Mumtaz, W.; Saad, M.N.B.M.; Kamel, N.; Ali, S.S.A.; Malik, A.S. An EEG-based functional connectivity measure for automatic detection of alcohol use disorder. Artif. Intell. Med. 2018, 84, 79–89. [Google Scholar] [CrossRef]
- Jakaite, L.; Schetinin, V.; Maple, C.; Schult, J. Bayesian decision trees for EEG assessment of newborn brain maturity. In Proceedings of the 2010 UK Workshop on Computational Intelligence (UKCI), Colchester, UK, 8–10 September 2010. [Google Scholar]
- Wang, Y.; Chen, W.; Huang, K.; Gu, Q. Classification of neonatal amplitude-integrated EEG using random forest model with combined feature. In Proceedings of the 2013 IEEE International Conference on Bioinformatics and Biomedicine, Shanghai, China, 18–21 December 2013. [Google Scholar]
- Hosseini, M.-P.; Hosseini, A.; Ahi, K. A Review on Machine Learning for EEG Signal Processing in Bioengineering. IEEE Rev. Biomed. Eng. 2020, 14, 204–218. [Google Scholar] [CrossRef]
- Snider, D.H.; Linnville, S.E.; Phillips, J.B.; Rice, G.M. Predicting hypoxic hypoxia using machine learning and wearable sensors. Biomed. Signal Process. Control 2021, 71, 103110. [Google Scholar] [CrossRef]
- Stuss, D.; Shallice, T.; Alexander, M.; Picton, T. A multidimensional approach to anterior attention functions. Ann. N. Y. Acad. Sci. 1995, 769, 191211. [Google Scholar] [CrossRef] [PubMed]
- Clayton, M.S.; Yeung, N.; Kadosh, R.C. The roles of cortical oscillations in sustained attention. Trends Cogn. Sci. 2015, 19, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, K.; Zheng, X.; Li, J.; Fan, B.; Yao, X.; Li, Z. Linear and nonlinear analyses of normal and fatigue heart rate variability signals for miners in high-altitude and cold areas. Comput. Methods Programs Biomed. 2020, 196, 105667. [Google Scholar] [CrossRef] [PubMed]
- Barańczuk, U. The Five-Factor Model of personality and generalized self efficacy: A meta-analysis. J. Individ. Differ. 2021, 42, 183. [Google Scholar] [CrossRef]
- Raven, J. The Raven’s 2 Progressive Matrices Tests and Manual Pearson; Western Psychological Services: Los Angeles, CA, USA, 2018; pp. 1–26. [Google Scholar]
- Liu, D.; Kahathuduwa, C.; Vazsonyi, A.T. The Pittsburgh Sleep Quality Index (PSQI): Psychometric and clinical risk score applications among college students. Psychol. Assess. 2021, 33, 816–826. [Google Scholar] [CrossRef]
- Chen, X.; Deng, X. Differences in Emotional Conflict Processing between High and Low Mindfulness Adolescents: An ERP Study. Int. J. Environ. Res. Public Health 2022, 19, 2891. [Google Scholar] [CrossRef]
- Jia, L.X.; Qin, X.J.; Cui, J.F.; Shi, H.S.; Ye, J.Y.; Yang, T.X.; Wang, Y.; Chan, R.C. Dissociation of proactive and reactive cognitive control in individuals with schizotypy: An event-related potential study. J. Int. Neuropsychol. Soc. 2021, 27, 981–991. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef]
- Harper, J.; Malone, S.M.; Bernat, E.M. Theta and delta band activity explain N2 and P3 ERP component activity in a go/no-go task. Clin. Neurophysiol. 2013, 125, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.; Malone, S.M.; Bachman, M.D.; Bernat, E.M. Stimulus sequence context differentially modulates inhibition-related theta and delta band activity in a go/no-go task. Psychophysiology 2016, 53, 712–722. [Google Scholar] [CrossRef] [Green Version]
- Schmiedt-Fehr, C.; Mathes, B.; Kedilaya, S.; Krauss, J.; Basar-Eroglu, C. Aging differentially affects alpha and beta sensorimotor rhythms in a go/nogo task. Clin. Neurophysiol. 2016, 127, 3234–3242. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kamarajan, C.; Manz, N.; Chorlian, D.B.; Stimus, A.; Porjesz, B. Delta, theta, and alpha event-related oscillations in alcoholics during Go/NoGo task: Neurocognitive deficits in execution, inhibition, and attention processing. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 65, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Kirmizi-Alsan, E.; Bayraktaroglu, Z.; Gurvit, H.; Keskin, Y.H.; Emre, M.; Demiralp, T. Comparative analysis of event-related potentials during Go/NoGo and CPT: Decomposition of electrophysiological markers of response inhibition and sustained attention. Brain Res. 2006, 1104, 114–128. [Google Scholar] [CrossRef]
- Del Campo-Vera, R.M.; Tang, A.M.; Gogia, A.S.; Chen, K.H.; Sebastian, R.; Gilbert, Z.D.; Nune, G.; Liu, C.Y.; Kellis, S.; Lee, B. Neuromodulation in Beta-Band Power between Movement Execution and Inhibition in the Human Hippocampus. Neuromodul. Technol. Neural Interface 2022, 25, 232–244. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Hoch, C.C.; Yeager, A.L.; Kupfer, D.J. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 1991, 14, 331–338. [Google Scholar]
- Raven, J.C.; Court, J.H. Raven’s Progressive Matrices; Western Psychological Services: Los Angeles, CA, USA, 1938. [Google Scholar]
- Grouper, H.; Eisenberg, E.; Pud, D. More Insight on the Role of Personality Traits and Sensitivity to Experimental Pain. J. Pain Res. 2021, 14, 1837–1844. [Google Scholar] [CrossRef]
- Robnik-Šikonja, M.; Kononenko, I. Theoretical and Empirical Analysis of ReliefF and RReliefF. Mach. Learn. 2003, 53, 23–69. [Google Scholar] [CrossRef] [Green Version]
- Nakata, H.; Miyamoto, T.; Ogoh, S.; Kakigi, R.; Shibasaki, M. Effects of acute hypoxia on human cognitive processing: A study using ERPs and SEPs. J. Appl. Physiol. 2017, 123, 1246–1255. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, S.; Wu, M.; Tang, C.-H. Adaptive Strategies for the Elderly in Inhibiting Irrelevant and Conflict No-Go Trials while Performing the Go/No-Go Task. Front. Aging Neurosci. 2016, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Kusztor, A.; Raud, L.; Juel, B.E.; Nilsen, A.S.; Storm, J.F.; Huster, R.J. Sleep deprivation differentially affects subcomponents of cognitive control. Sleep 2019, 42, zsz016. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Wang, Y.; Wu, J.; Liu, H.; Luo, P.; Han, B. Overactive Performance Monitoring Resulting from Chronic Exposure to High Altitude. Aerosp. Med. Hum. Perform. 2015, 86, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, Y.; Wu, J.; Luo, P.; Han, B. Long-term exposure to high altitude affects response inhibition in the conflict-monitoring stage. Sci. Rep. 2015, 5, 13600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, H.; Huang, J.; Zhang, X.; Ma, H.; Liu, M. Exploring the impact of chronic high-altitude exposure on visual spatial attention using the ERP approach. Brain Behav. 2018, 8, e00944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Pornpattananangkul, N.; Nusslock, R. Executive control- and reward-related neural processes associated with the opportunity to engage in voluntary dishonest moral decision making. Cogn. Affect. Behav. Neurosci. 2015, 15, 475–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cespón, J.; Carreiras, M. Is there electrophysiological evidence for a bilingual advantage in neural processes related to executive functions? Neurosci. Biobehav. Rev. 2020, 118, 315–330. [Google Scholar] [CrossRef]
- Lanteaume, L.; Cassé-Perrot, C.; Lefebvre, M.N.; Audebert, C.; Deguil, J.; Auffret, A.; Otten, L.; Bartrés-Faz, D.; Blin, O.; Bordet, R.; et al. Neurobehavioral and cognitive changes induced by hypoxia in healthy volunteers. CNS Neurol. Disord. Drug Targets 2016, 15, 816–822. [Google Scholar] [CrossRef]
- Gustafsson, C.; Gennser, M.; Ornhagen, H.; Derefeldt, G. Effects of normobaric hypoxic confinement on visual and motor performance. Aviat. Space Environ. Med. 1997, 68, 985–992. [Google Scholar]
- Dehaene, S.; Changeux, J.-P. Experimental and Theoretical Approaches to Conscious Processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef] [Green Version]
- Benedek, M.; Schickel, R.J.; Jauk, E.; Fink, A.; Neubauer, A.C. Alpha power increases in right parietal cortex reflects focused internal attention. Neuropsychologia 2014, 56, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Sander, M.C.; Werkle-Bergner, M.; Lindenberger, U. Amplitude modulations and inter-trial phase stability of alpha-oscillations differentially reflect working memory constraints across the lifespan. NeuroImage 2012, 59, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Klimesch, W. Memory processes, brain oscillations and EEG synchronization. Int. J. Psychophysiol. 1996, 24, 61–100. [Google Scholar] [CrossRef]
- Klimesch, W.; Schimke, H.; Schwaiger, J. Episodic and semantic memory: An analysis in the EEG theta and alpha band. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 428–441. [Google Scholar] [CrossRef]
- Craig, A.; Tran, Y.; Wijesuriya, N.; Nguyen, H. Regional brain wave activity changes associated with fatigue. Psychophysiology 2012, 49, 574–582. [Google Scholar] [CrossRef]
- West, R.; Jakubek, K.; Wymbs, N.; Perry, M.; Moore, K. Neural correlates of conflict processing. Exp. Brain Res. 2005, 167, 38–48. [Google Scholar] [CrossRef]
- Larson, M.J.; Clayson, P.E.; Clawson, A. Making sense of all the conflict: A theoretical review and critique of conflict-related ERPs. Int. J. Psychophysiol. 2014, 93, 283–297. [Google Scholar] [CrossRef]
- Saqib, K.; Khan, A.F.; Butt, Z.A. Machine Learning Methods for Predicting Postpartum Depression: Scoping Review. JMIR Ment. Health 2021, 8, e29838. [Google Scholar] [CrossRef]
- Lin, H.; Jian, C.; Cao, Y.; Ma, X.; Wang, H.; Miao, F.; Fan, X.; Yang, J.; Zhao, G.; Zhou, H. MDD-TSVM: A novel semisupervised-based method for major depressive disorder detection using electroencephalogram signals. Comput. Biol. Med. 2022, 140, 105039. [Google Scholar] [CrossRef]
- Harmony, T.; Fernández, T.; Silva, J.; Bernal, J.; Díaz-Comas, L.; Reyes, A.; Marosi, E.; Rodríguez, M.; Rodríguez, M. EEG delta activity: An indicator of attention to internal processing during performance of mental tasks. Int. J. Psychophysiol. 1996, 24, 161–171. [Google Scholar] [CrossRef]
- Eidelman-Rothman, M.; Ben-Simon, E.; Freche, D.; Keil, A.; Hendler, T.; Levit-Binnun, N. Sleepless and desynchronized: Impaired inter trial phase coherence of steady-state potentials following sleep deprivation. NeuroImage 2019, 202, 116055. [Google Scholar] [CrossRef]


| True Value | |||
|---|---|---|---|
| Positive | Negative | ||
| Predictive value | Positive | TP | FN |
| Negative | FP | TN | |
| High-Altitude (M ± SD) | Low-Altitude (M ± SD) | t | p | |
|---|---|---|---|---|
| Age (years) | 22.200 ± 1.699 | 21.933 ± 1.438 | 0.691 | 0.492 |
| Gender (male/female) | 19/16 | 17/15 | 0.009 | 0.924 |
| PSQI | 3.400 ± 2.291 | 3.281 ± 2.098 | 0.221 | 0.826 |
| SPM | 44.686 ± 15.976 | 47.250 ± 9.374 | −0.792 | 0.431 |
| NEO-FFI | 177.286 ± 56.098 | 194.469 ± 11.086 | −1.775 | 0.084 |
| Stimulus | Period | HA Group (n = 35) | LA Group (n = 32) |
|---|---|---|---|
| Go | a1 | 491.43 ± 10.26 | 540.56 ± 10.69 |
| a2 | 503.03 ± 9.48 | 537.69 ± 9.51 | |
| a3 | 491.94 ± 8.23 | 542.25 ± 8.44 | |
| No-Go | a1 | 571.60 ± 8.90 | 618.69 ± 8.54 |
| a2 | 574.11 ± 9.82 | 584.25 ± 10.77 | |
| a3 | 564.34 ± 11.13 | 589.69 ± 9.90 |
| Stimulus | Period | HA Group (n = 35) | LA Group (n = 32) |
|---|---|---|---|
| Go | a1 | 5.02 ± 0.58 | 6.89 ± 0.45 |
| a2 | 5.60 ± 0.61 | 6.56 ± 0.55 | |
| a3 | 5.84 ± 0.69 | 6.69 ± 0.55 | |
| No-Go | a1 | 11.39 ± 0.93 | 13.55 ± 0.87 |
| a2 | 12.52 ± 0.82 | 14.80 ± 1.04 | |
| a3 | 13.62 ± 1.00 | 14.54 ± 0.96 |
| Stimulus | Period | HA Group (n = 35) | LA Group (n = 32) |
|---|---|---|---|
| Go | a1 | 5.02 ± 0.58 | 6.89 ± 0.45 |
| a2 | 5.60 ± 0.61 | 6.56 ± 0.55 | |
| a3 | 5.84 ± 0.69 | 6.69 ± 0.55 | |
| No-Go | a1 | 11.39 ± 0.93 | 13.55 ± 0.87 |
| a2 | 12.52 ± 0.82 | 14.80 ± 1.04 | |
| a3 | 13.62 ± 1.00 | 14.54 ± 0.96 |
| Stimulus | Period | HA Group (n = 35) | LA Group (n = 32) | |
|---|---|---|---|---|
| theta | Go | a1 | 0.84 ± 0.15 | 0.84 ± 0.16 |
| a2 | 1.07 ± 0.16 | 0.74 ± 0.16 | ||
| a3 | 1.44 ± 0.24 | 0.88 ± 0.25 | ||
| No-Go | a1 | −0.01 ± 0.07 | −0.19 ± 0.08 | |
| a2 | −0.03 ± 0.09 | −0.27 ± 0.09 | ||
| a3 | 0.13 ± 0.09 | −0.43 ± 0.09 | ||
| slow alpha | Go | a1 | −0.10 ± 0.05 | −0.14 ± 0.05 |
| a2 | −0.13 ± 0.05 | −0.17 ± 0.05 | ||
| a3 | −0.17 ± 0.06 | −0.20 ± 0.05 | ||
| No-Go | a1 | −0.01 ± 0.08 | −0.18 ± 0.07 | |
| a2 | −0.02 ± 0.11 | −0.27 ± 0.07 | ||
| a3 | −0.02 ± 0.10 | −0.42 ± 0.04 | ||
| fast alpha | Go | a1 | −0.09 ± 0.05 | −0.17 ± 0.06 |
| a2 | −0.12 ± 0.05 | −0.18 ± 0.06 | ||
| a3 | −0.15 ± 0.06 | −0.18 ± 0.06 | ||
| No-Go | a1 | −0.14 ± 0.07 | −0.28 ± 0.07 | |
| a2 | −0.03 ± 0.09 | −0.31 ± 0.09 | ||
| a3 | −0.11 ± 0.08 | −0.37 ± 0.08 | ||
| beta | Go | a1 | −0.04 ± 0.02 | −0.11 ± 0.02 |
| a2 | −0.03 ± 0.02 | −0.10 ± 0.02 | ||
| a3 | −0.05 ± 0.02 | −0.08 ± 0.02 | ||
| No-Go | a1 | −0.07 ± 0.03 | −0.17 ± 0.03 | |
| a2 | −0.05 ± 0.04 | −0.19 ± 0.04 | ||
| a3 | −0.09 ± 0.03 | −0.16 ± 0.03 |
| Classifiers | Periods | Accuracy (95% Confidence Interval) | F1 Score | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|
| SVM | a1 | 86.57% (78.41%, 94.73%) | 0.89 | 85.71% | 87.50% | 0.90 |
| a2 | 88.06% (80.3%, 95.82%) | 0.88 | 91.43% | 84.38% | 0.96 | |
| a3 | 92.54% (86.25%, 98.83%) | 0.93 | 91.43% | 93.75% | 0.97 | |
| LR | a1 | 95.52% (90.57%, 100.00%) | 0.96 | 91.43% | 100.00% | 0.94 |
| a2 | 89.55% (82.22%, 96.88%) | 0.90 | 91.43% | 87.50% | 0.93 | |
| a3 | 86.57% (78.41%, 94.73%) | 0.87 | 88.57% | 84.38% | 0.92 | |
| DT | a1 | 80.60% (71.13%, 90.07%) | 0.80 | 85.71% | 75.00% | 0.69 |
| a2 | 70.15% (59.19%, 81.11%) | 0.68 | 80.00% | 59.38% | 0.70 | |
| a3 | 70.15% (59.19%, 81.11%) | 0.70 | 65.71% | 75.00% | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Shi, R.; Liao, R.; Liu, Y.; Che, J.; Bai, Z.; Cheng, N.; Ma, H. Machine Learning Based on Event-Related EEG of Sustained Attention Differentiates Adults with Chronic High-Altitude Exposure from Healthy Controls. Brain Sci. 2022, 12, 1677. https://doi.org/10.3390/brainsci12121677
Liu H, Shi R, Liao R, Liu Y, Che J, Bai Z, Cheng N, Ma H. Machine Learning Based on Event-Related EEG of Sustained Attention Differentiates Adults with Chronic High-Altitude Exposure from Healthy Controls. Brain Sciences. 2022; 12(12):1677. https://doi.org/10.3390/brainsci12121677
Chicago/Turabian StyleLiu, Haining, Ruijuan Shi, Runchao Liao, Yanli Liu, Jiajun Che, Ziyu Bai, Nan Cheng, and Hailin Ma. 2022. "Machine Learning Based on Event-Related EEG of Sustained Attention Differentiates Adults with Chronic High-Altitude Exposure from Healthy Controls" Brain Sciences 12, no. 12: 1677. https://doi.org/10.3390/brainsci12121677
APA StyleLiu, H., Shi, R., Liao, R., Liu, Y., Che, J., Bai, Z., Cheng, N., & Ma, H. (2022). Machine Learning Based on Event-Related EEG of Sustained Attention Differentiates Adults with Chronic High-Altitude Exposure from Healthy Controls. Brain Sciences, 12(12), 1677. https://doi.org/10.3390/brainsci12121677





