Music and Visual Art Training Increase Auditory-Evoked Theta Oscillations in Older Adults
Abstract
1. Introduction
1.1. Predictive Coding Theory and Auditory Segmentation
1.2. Neural Oscillations in Aging
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Description of Training Programs
2.4. ERP Data Collection
2.5. EEG Time-Frequency Preprocessing
2.6. Statistical Analyses
3. Results
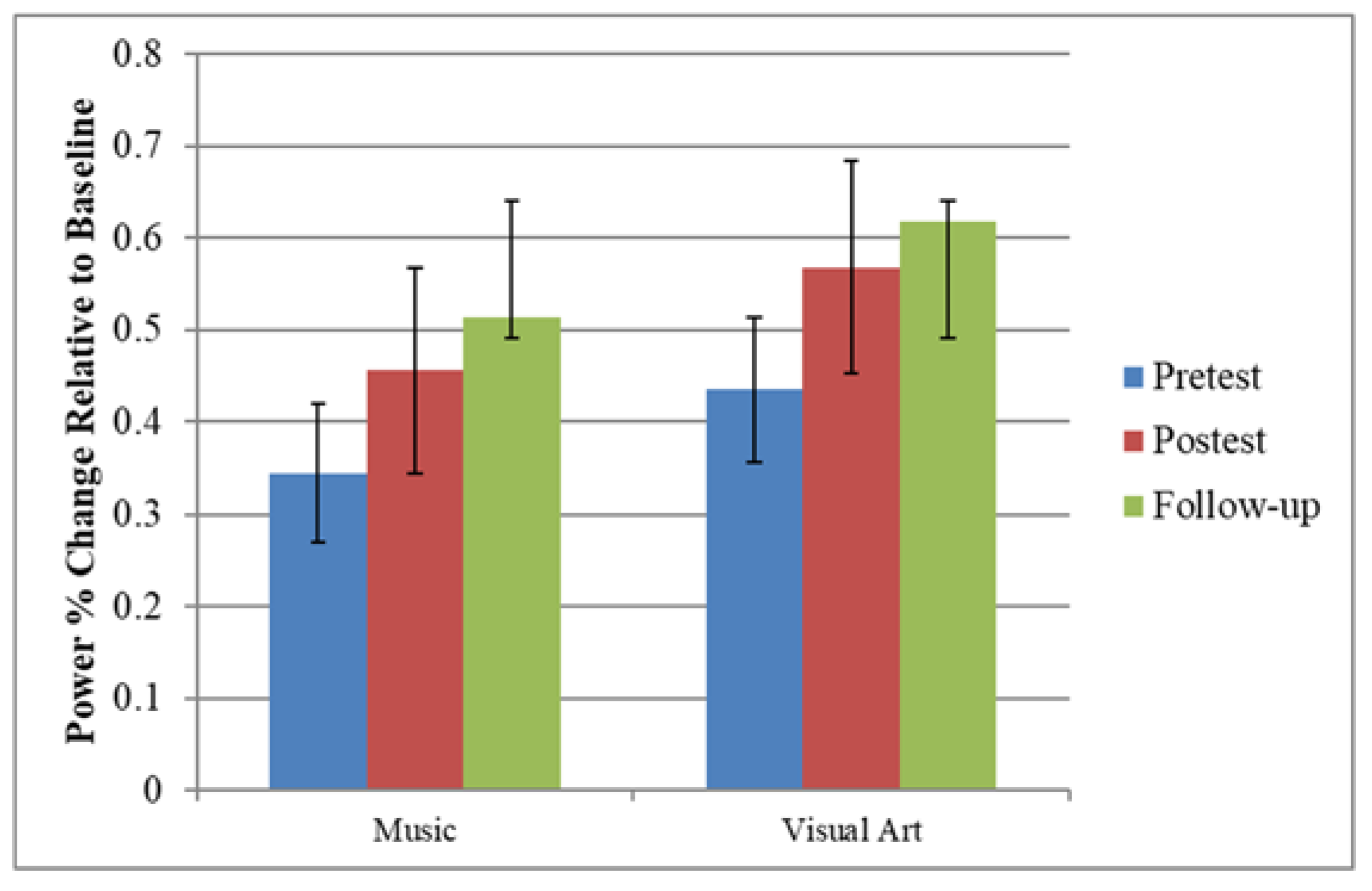
3.1. Effects of Training on Oscillatory Activity Indexing Auditory Processing
3.2. Brain Oscillations Indexing Automatic Deviance Detection: Pre-Training
3.3. Music Oddball Paradigm: Effects of Training on Deviance-Related Oscillatory Activity
3.4. Speech Oddball Paradigm: Effects of Training on Deviance-Related Oscillatory Activity
3.5. Follow-Up
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ERP | Event-related potentials |
| EEG | Electroencephalography |
| MMN | Mismatched Negativity |
| TSE | Temporal-Spectral Evolution |
References
- Kraus, N.; Chandrasekaran, B. Music training for the development of auditory skills. Nat. Rev. Neurosci. 2010, 11, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Sedley, W.; Gander, P.E.; Kumar, S.; Kovach, C.K.; Oya, H.; Kawasaki, H.; Howard, M.A.; Griffiths, T.D. Neural signatures of perceptual inference. eLife 2016, 5, e11476. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.H.; Koelsch, S.; Bhattacharya, J. Decrease in early right alpha band phase synchronization and late gamma band oscillations in processing syntax in music. Hum. Brain Mapp. 2008, 30, 1207–1225. [Google Scholar] [CrossRef] [PubMed]
- Yurgil, K.A.; Velasquez, M.A.; Winston, J.L.; Reichman, N.B.; Colombo, P.J. Music Training, Working Memory, and Neural Oscillations: A Review. Front. Psychol. 2020, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. A theory of cortical responses. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Vuust, P.; Witek, M.A.G. Rhythmic complexity and predictive coding: A novel approach to modeling rhythm and meter perception in music. Front. Psychol. 2014, 5, 1111. [Google Scholar] [CrossRef]
- Teng, X.; Tian, X.; Doelling, K.; Poeppel, D. Theta band oscillations reflect more than entrainment: Behavioral and neural evidence demonstrates an active chunking process. Eur. J. Neurosci. 2017, 48, 2770–2782. [Google Scholar] [CrossRef]
- Buzsáki, G.; Draguhn, A. Neuronal Oscillations in Cortical Networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef]
- Boutorabi, S.Y.; Sheikhani, A. Evaluation of electroencephalogram signals of the professional pianists during iconic memory and working memory tests using spectral coherence. J. Med. Signals Sens. 2018, 8, 87–94. [Google Scholar]
- Sharma, V.V.; Thaut, M.; Russo, F.; Alain, C. Absolute Pitch and Musical Expertise Modulate Neuro-Electric and Behavioral Responses in an Auditory Stroop Paradigm. Front. Neurosci. 2019, 13, 932. [Google Scholar] [CrossRef]
- Carpentier, S.M.; Moreno, S.; McIntosh, A.R. Short-term Music Training Enhances Complex, Distributed Neural Communication during Music and Linguistic Tasks. J. Cogn. Neurosci. 2016, 28, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.; Hornickel, J.; Strait, D.L.; Slater, J.; Thompson, E. Engagement in community music classes sparks neuroplasticity and language development in children from disadvantaged backgrounds. Front. Psychol. 2014, 5, 1403. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Lee, Y.; Janus, M.; Bialystok, E. Short-Term Second Language and Music Training Induces Lasting Functional Brain Changes in Early Childhood. Child Dev. 2014, 86, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.T.; Krizman, J.; Kraus, N. Music training alters the course of adolescent auditory development. Proc. Natl. Acad. Sci. USA 2015, 112, 10062–10067. [Google Scholar] [CrossRef]
- Tavor, I.; Botvinik-Nezer, R.; Bernstein-Eliav, M.; Tsarfaty, G.; Assaf, Y. Short-term plasticity following motor sequence learning revealed by diffusion magnetic resonance imaging. Hum. Brain Mapp. 2019, 41, 442–452. [Google Scholar] [CrossRef]
- Olszewska, A.M.; Gaca, M.; Herman, A.M.; Jednoróg, K.; Marchewka, A. How Musical Training Shapes the Adult Brain: Predispositions and Neuroplasticity. Front. Neurosci. 2021, 15, 630829. [Google Scholar] [CrossRef]
- Bidelman, G.M. Amplified induced neural oscillatory activity predicts musicians’ benefits in categorical speech perception. Neuroscience 2017, 348, 107–113. [Google Scholar] [CrossRef]
- Shahin, A.J.; Roberts, L.E.; Chau, W.; Trainor, L.J.; Miller, L.M. Music training leads to the development of timbre-specific gamma band activity. NeuroImage 2008, 41, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Trainor, L.J.; Shahin, A.J.; Roberts, L.E. Understanding the benefits of musical training: Effects on oscillatory brain activity. Ann. N. Y. Acad. Sci. 2009, 1169, 133–142. [Google Scholar] [CrossRef]
- Jacobson, T.K.; Schmidt, B.; Hinman, J.R.; Escabí, M.A.; Markus, E.J. Age-related decrease in theta and gamma coherence across dorsal ca1 pyramidale and radiatum layers. Hippocampus 2015, 25, 1327–1335. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Freunberger, R.; Sauseng, P.; Gruber, W. A short review of slow phase synchronization and memory: Evidence for control processes in different memory systems? Brain Res. 2008, 1235, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Winterling, S.L.; Shields, S.M.; Rose, M. Reduced memory-related ongoing oscillatory activity in healthy older adults. Neurobiol. Aging 2019, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Strunk, J.; James, T.; Arndt, J.; Duarte, A. Age-related changes in neural oscillations supporting context memory retrieval. Cortex 2017, 91, 40–55. [Google Scholar] [CrossRef]
- Strunk, J.; Duarte, A. Prestimulus and poststimulus oscillatory activity predicts successful episodic encoding for both young and older adults. Neurobiol. Aging 2019, 77, 1–12. [Google Scholar] [CrossRef]
- Hsieh, L.-T.; Ekstrom, A.; Ranganath, C. Neural Oscillations Associated with Item and Temporal Order Maintenance in Working Memory. J. Neurosci. 2011, 31, 10803–10810. [Google Scholar] [CrossRef]
- Doelling, K.B.; Poeppel, D. Cortical entrainment to music and its modulation by expertise. Proc. Natl. Acad. Sci. USA 2015, 112, E6233–E6242. [Google Scholar] [CrossRef]
- Chang, A.; Bosnyak, D.J.; Trainor, L.J. Beta oscillatory power modulation reflects the predictability of pitch change. Cortex 2018, 106, 248–260. [Google Scholar] [CrossRef]
- Chang, A.; Bosnyak, D.J.; Trainor, L.J. Rhythmicity facilitates pitch discrimination: Differential roles of low and high frequency neural oscillations. NeuroImage 2019, 198, 31–43. [Google Scholar] [CrossRef]
- Cheung, M.; Chan, A.S.; Liu, Y.; Law, D.; Wong, C.W.Y. Music training is associated with cortical synchronization reflected in EEG coherence during verbal memory encoding. PLoS ONE 2017, 12, e0174906. [Google Scholar] [CrossRef]
- Ruiz, M.H.; Maess, B.; Altenmüller, E.; Curio, G.; Nikulin, V. Cingulate and cerebellar beta oscillations are engaged in the acquisition of auditory-motor sequences. Hum. Brain Mapp. 2017, 38, 5161–5179. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, Y.; Stitt, I.M.; Zhou, Z.C.; Sellers, K.K.; Frohlich, F. Theta Oscillations Organize Spiking Activity in Higher-Order Visual Thalamus during Sustained Attention. Eneuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Moran, R.J.; Campa, P.; Maestu, F.; Reilly, R.B.; Dolan, R.J.; Strange, B.A. Peak frequency in the theta and alpha bands correlates with human working memory capacity. Front. Hum. Neurosci. 2010, 4, 200. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Huizeling, E.; Wang, H.; Holland, C.; Kessler, K. Changes in theta and alpha oscillatory signatures of attentional control in older and middle age. Eur. J. Neurosci. 2021, 54, 4314–4337. [Google Scholar] [CrossRef]
- Nowak, K.; Costa-Faidella, J.; Dacewicz, A.; Escera, C.; Szelag, E. Altered event-related potentials and theta oscillations index auditory working memory deficits in healthy aging. Neurobiol. Aging 2021, 108, 1–15. [Google Scholar] [CrossRef]
- Crasta, J.E.; Thaut, M.H.; Anderson, C.W.; Davies, P.L.; Gavin, W.J. Auditory priming improves neural synchronization in auditory-motor entrainment. Neuropsychologia 2018, 117, 102–112. [Google Scholar] [CrossRef]
- Large, E.W.; Herrera, J.A.; Velasco, M.J. Neural Networks for Beat Perception in Musical Rhythm. Front. Syst. Neurosci. 2015, 9, 159. [Google Scholar] [CrossRef]
- Fujioka, T.; Ween, J.E.; Jamali, S.; Stuss, D.T.; Ross, B. Changes in neuromagnetic beta-band oscillation after music-supported stroke rehabilitation. Ann. N. Y. Acad. Sci. 2012, 1252, 294–304. [Google Scholar] [CrossRef]
- Fujioka, T.; Ross, B. Beta-band oscillations during passive listening to metronome sounds reflect improved timing representation after short-term musical training in healthy older adults. Eur. J. Neurosci. 2017, 46, 2339–2354. [Google Scholar] [CrossRef]
- Chang, A.; Bosnyak, D.J.; Trainor, L.J. Unpredicted Pitch Modulates Beta Oscillatory Power during Rhythmic Entrainment to a Tone Sequence. Front. Psychol. 2016, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.; Fujioka, T.; Ross, B. Neuromagnetic beta and gamma oscillations in the somatosensory cortex after music training in healthy older adults and a chronic stroke patient. Clin. Neurophysiol. 2014, 125, 1213–1222. [Google Scholar] [CrossRef]
- Alain, C.; Moussard, A.; Singer, J.; Lee, Y.; Bidelman, G.M.; Moreno, S. Music and visual art training modulate brain activity in older adults. Front. Neurosci. 2019, 13, 182. [Google Scholar] [CrossRef]
- Galuske, R.A.W.; Munk, M.H.J.; Singer, W. Relation between gamma oscillations and neuronal plasticity in the visual cortex. Proc. Natl. Acad. Sci. USA 2019, 116, 23317–23325. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, M.; Nielsen, A.H.; Friis-Olivarius, M.; Vuust, C.; Vuust, P. The Musical Ear Test, a new reliable test for measuring musical competence. Learn. Individ. Differ. 2010, 20, 188–196. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence, 2nd ed.; NCS Pearson: San Antonio, TX, USA, 2011. [Google Scholar] [CrossRef]
- Silverman, D. The Rationale and History of the 10-20 System of the International Federation. Am. J. EEG Technol. 1963, 3, 17–22. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Jabès, A.; Klencklen, G.; Ruggeri, P.; Antonietti, J.-P.; Lavenex, P.B.; Lavenex, P. Age-Related Differences in Resting-State EEG and Allocentric Spatial Working Memory Performance. Front. Aging Neurosci. 2021, 13, 704362. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Romei, V.; Gross, J.; Thut, G. On the Role of Prestimulus Alpha Rhythms over Occipito-Parietal Areas in Visual Input Regulation: Correlation or Causation? J. Neurosci. 2010, 30, 8692–8697. [Google Scholar] [CrossRef]
- Keller, A.S.; Payne, L.; Sekuler, R. Characterizing the roles of alpha and theta oscillations in multisensory attention. Neuropsychologia 2017, 99, 49–63. [Google Scholar] [CrossRef] [PubMed]
- VanRullen, R.; Busch, N.A.; Drewes, J.; Dubois, J. Ongoing EEG Phase as a Trial-by-Trial Predictor of Perceptual and Attentional Variability. Front. Psychol. 2011, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Reuter-Lorenz, P.A.; Park, D.C. How Does it STAC Up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychol. Rev. 2014, 24, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Roberts, B.M.; Ranganath, C. Neural oscillations during conditional associative learning. NeuroImage 2018, 174, 485–493. [Google Scholar] [CrossRef]
- Rondina Ii, R.; Olsen, R.K.; Li, L.; Meltzer, J.A.; Ryan, J.D. Age-related changes to oscillatory dynamics during maintenance and retrieval in a relational memory task. PLoS ONE 2019, 14, e0211851. [Google Scholar] [CrossRef]
- Lee, D.J.; Kulubya, E.; Goldin, P.; Goodarzi, A.; Girgis, F. Review of the Neural Oscillations Underlying Meditation. Front. Neurosci. 2018, 12, 178. [Google Scholar] [CrossRef]
- Kawamata, M.; Kirino, E.; Inoue, R.; Arai, H. Event-Related Desynchronization of Frontal-Midline Theta Rhythm during Preconscious Auditory Oddball Processing. Clin. EEG Neurosci. 2007, 38, 193–202. [Google Scholar] [CrossRef]
- Gazzaley, A.; Clapp, W.; Kelley, J.; McEvoy, K.; Knight, R.T.; D'Esposito, M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc. Natl. Acad. Sci. USA 2008, 105, 13122–13126. [Google Scholar] [CrossRef]
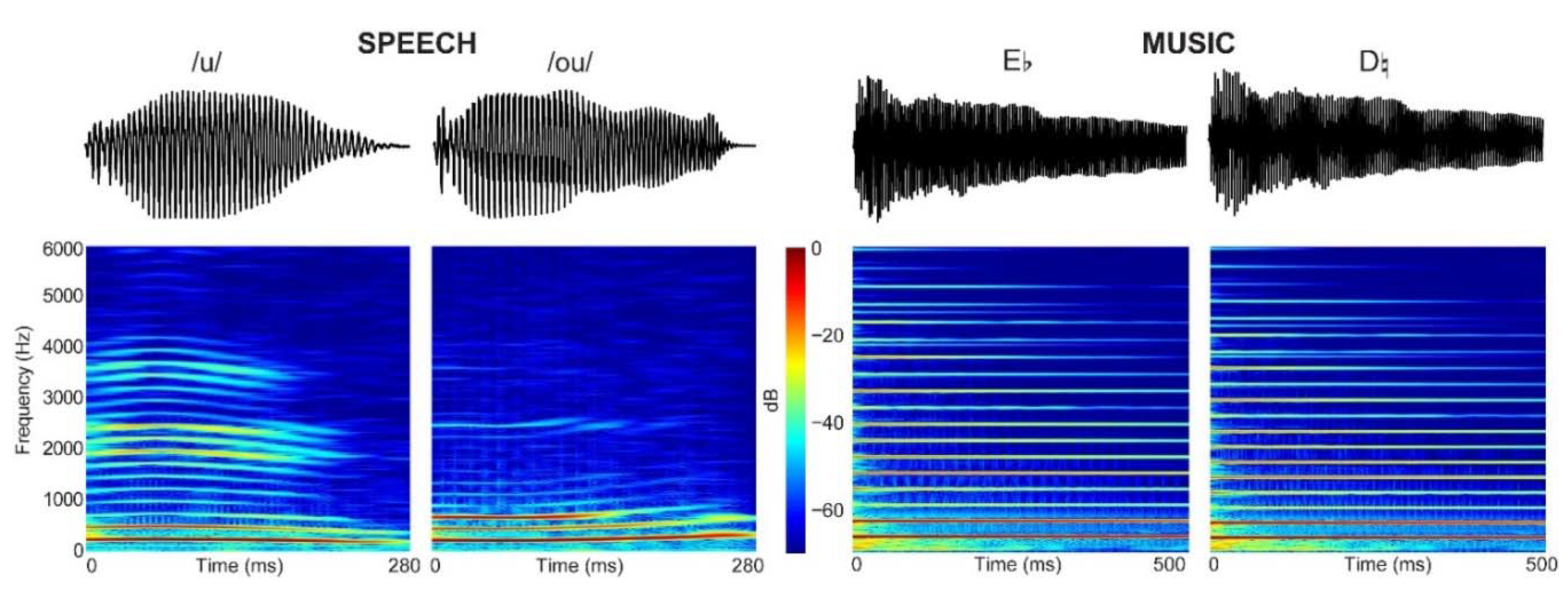
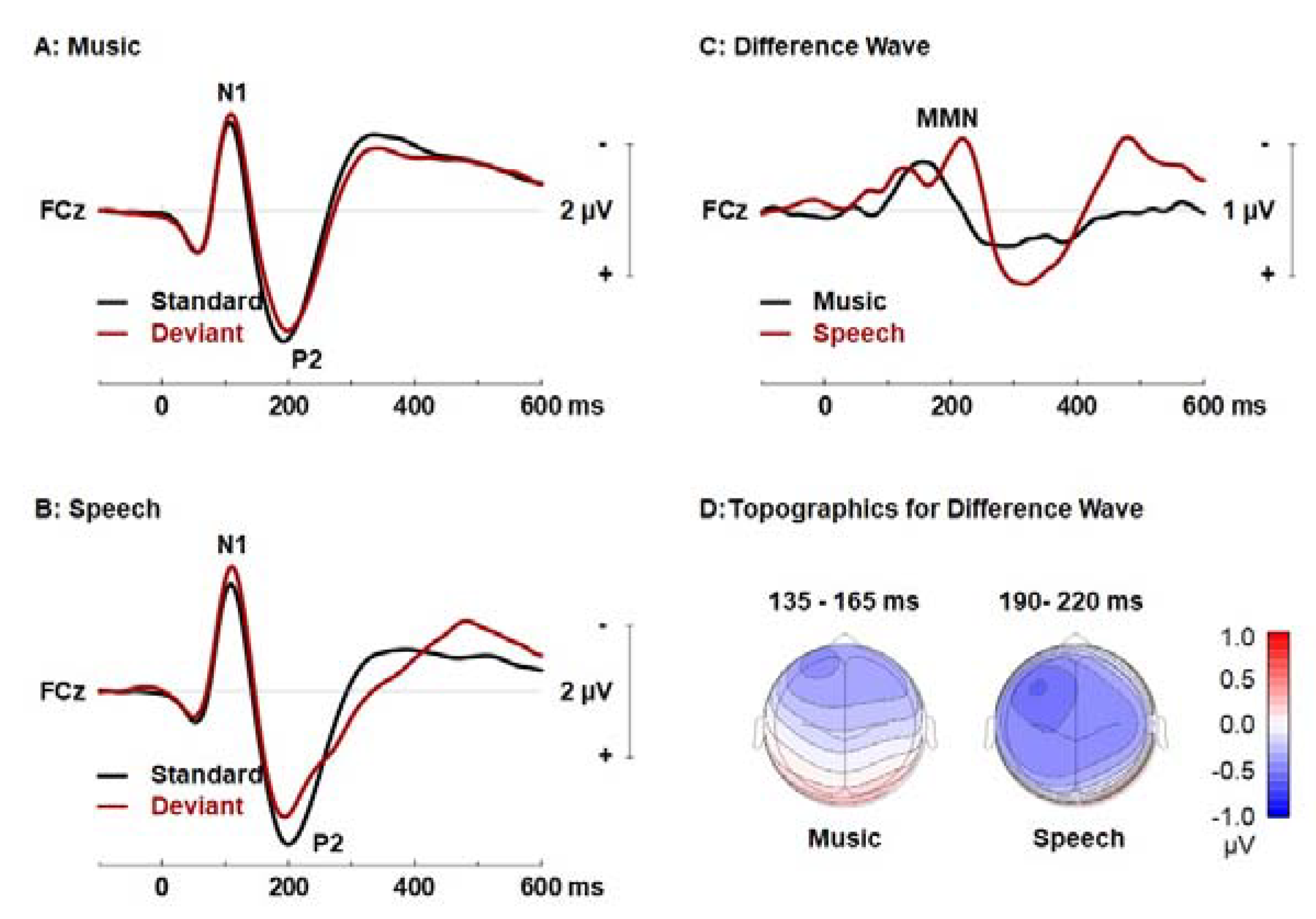
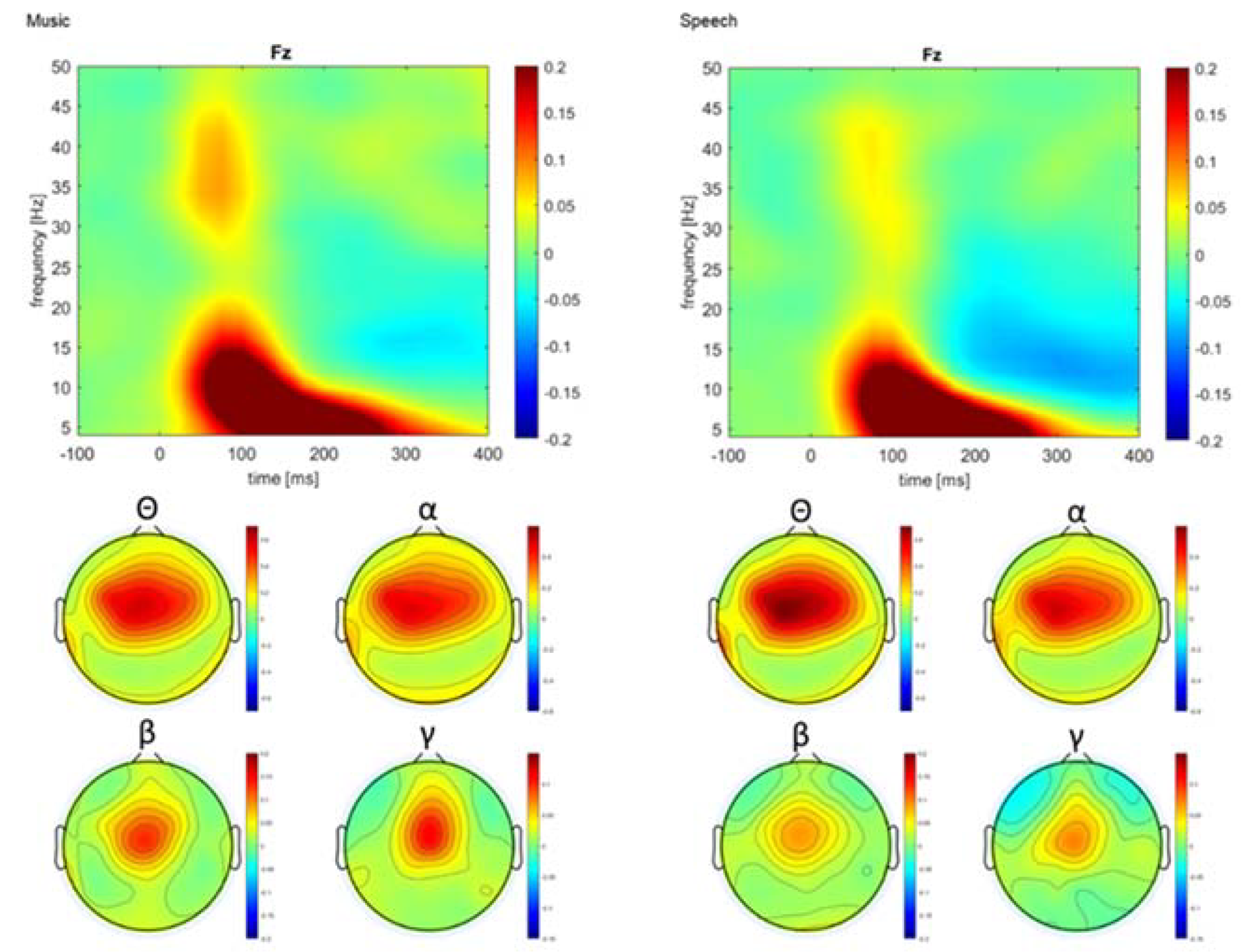
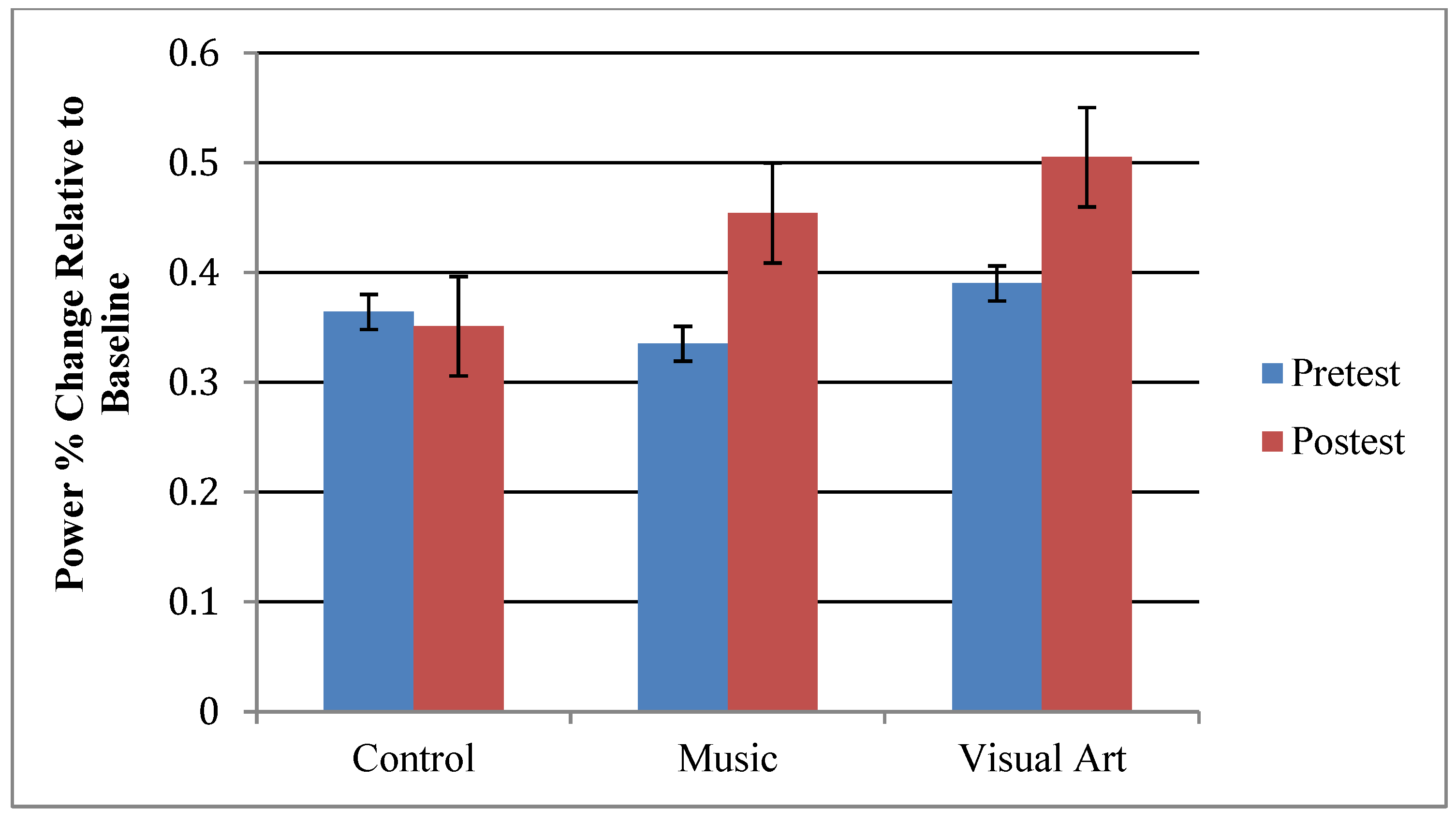

| Music (n = 16) | Visual Art (n = 17) | Control (n = 17) | |
|---|---|---|---|
| Age | 67.75 (6.02) | 68.47 (6.21) | 68.53 (5.99) |
| Sex (M/F) | 3/13 | 2/15 | 3/14 |
| Education | 16.38 (2.68) | 17.18 (2.40) | 16. 94 (1.48) |
| Paradigms | Cluster | Range; | Peak | Electrodes | p Values |
|---|---|---|---|---|---|
| Music | 1 | 90–265 ms | 145 ms, FC3 | Fp1, AF7, AF3, F1, F3, F5, F7, FT7, FC5, FC3, FC1, C1, T7, C5, C3, P5, P7, Fpz, AF8, AF4, AFz, Fz, F2, F4, F6, F8, FC6, FC4, FC2, FCz, Cz, C2, C4, C6, CP4 | <0.001 |
| 2 | 227–396 ms | 371 ms, C6 | F1, F3, FC3, FC1, C1, CP1, AF4, AFz, Fz, F2, F4, F6, F8, FT8, FC6, FC4, FC2, FCz, Cz, C2, C4, C6, T8, CP6, CP4, CP2 | <0.001 | |
| 3 | 57–230 ms | 156 ms, O1 | CP5, CP3, CP1, P1, P3, P7, PO7, PO3, O1, Oz, POz, CPz, Cz, C2, C6, T8, TP8, CP6, CP4, CP2, P2, P4, P6, P8, PO8, PO4, O2 | <0.001 | |
| 4 | 264–389 ms | 375 ms, Oz | P5, P7, PO7, PO3, O1, Oz, POz, PO4, O2 | <0.001 | |
| Speech | 1 | 47–332 ms | 213 ms, FC3 | Fp1, AF3, F1, F3, F5, FC5, FC3, FC1, C1, TP7, T7, C5, C3, CP5, CP3, CP1, P1, P3, P5, P7, PO7, PO3, O1, Oz, POz, Pz, CPz, Fpz, Fp2, AF8, AF4, AFz, Fz, F2, F4, F6, FC6, FC4, FC2, FCz, Cz, C2, C4, C6, T8, TP8, CP6, CP4, CP2, P2, P4, P6, P8, PO8, PO4, O2 | <0.001 |
| 2 | 225–400 ms | 359, TP7 | Fp1, AF7, AF3, F1, F5, F7, FT7, FC5, FC3, FC1, C1, TP7, T7, C5, C3, CP5, CP3, CP1, CPz, Fpz, Fp2, AF8, AF4, AFz, Fz, F2, F4, F6, F8, FT8, FC6, FC4, FC2, FCz, Cz, C2, C4, C6, CP2 | <0.001 | |
| 3 | 61–244 ms | 219, PO8 | P7, PO7, O1, Oz, F8, FT8, C6, T8, TP8, CP6, P6, P8, PO8, PO4, O2 | =0.002 |
| Paradigms | Frequency Band | Cluster Difference | Range | Peak | Electrodes | p Values |
|---|---|---|---|---|---|---|
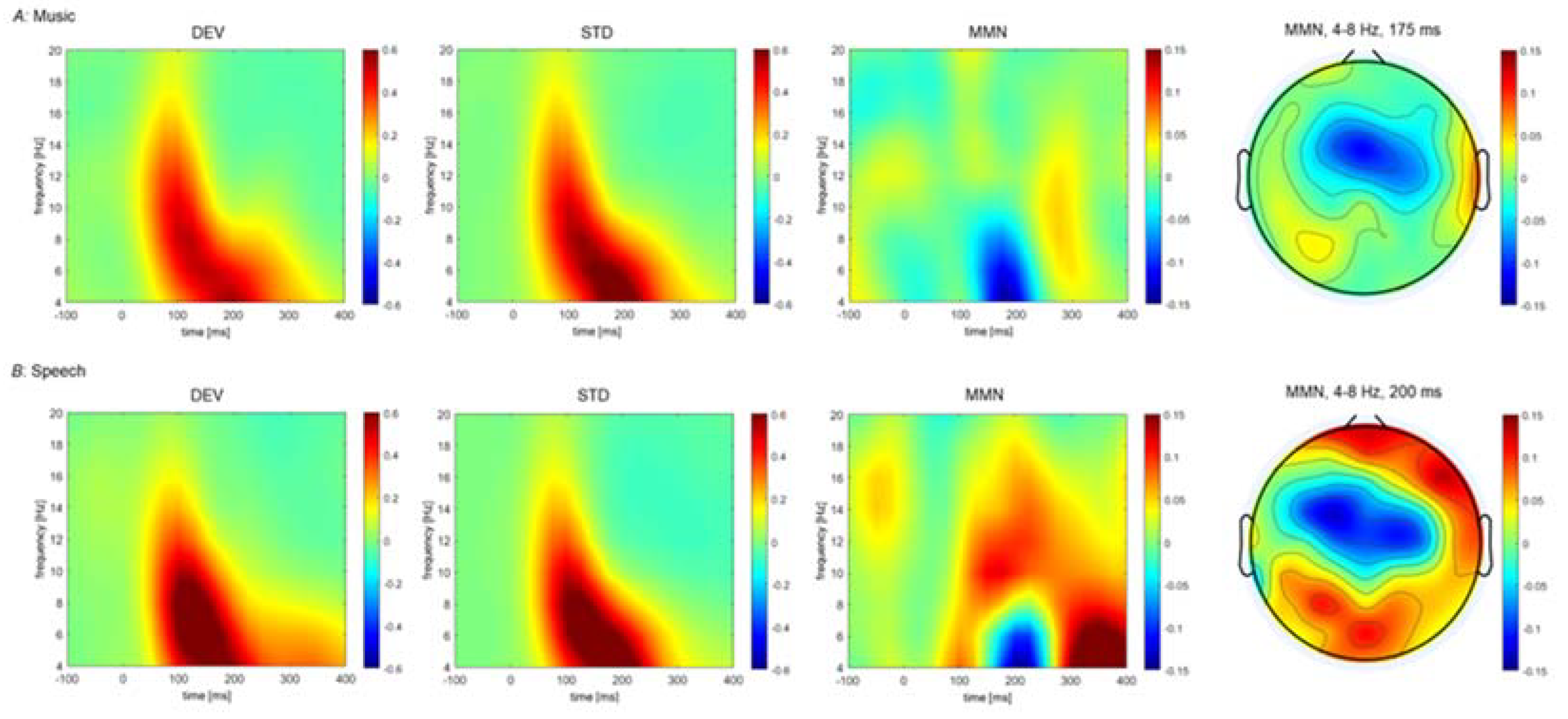
| Music | Theta | 1; D > S | 125–275 ms | 175 ms, T8 | FT8, T8, TP8 | =0.009 |
| 2; D < S | 150–200 ms | 175 ms, FCz | FC1, FC2, FCz | =0.032 | ||
| Alpha | ||||||
| Beta | 1 D < S | 150–275 ms | 225 ms, AFz | AF3, AF4, AFz, Fz, F2 | =0.014 | |
| Gamma | ||||||
| Speech | Theta | 1; D > S | 75–275 ms | 200 ms, POz | Fp1, AF7, AF3, F1, F3, F5, FC5, FC3, P1, P3, PO3, O1, Oz, POz, Fpz, Fp2, AF8, AF4, AFz, Fz, F2, F4, F6, F8, FT8, FC6, C6, T8, TP8, CP6, P2, P4, P6, P8, PO8, PO4, O2 | <0.001 |
| 2; D < S | 175–225 ms | 200 ms, FC1 | FC3, FC1, FC2, FCz, C2, C4 | =0.039 | ||
| Alpha | 1; D > S | 75–225 ms | 200 ms, POz | Fp1, AF7, AF3, F1, F3, F5, FC5, FC3, FC1, C1, TP7, T7, C5, C3, CP5, CP3, CP1, P1, P3, P5, P7, PO7, PO3, O1, Oz, POz, Fpz, Fp2, AF8, AF4, AFz, Fz, F2, F4, F6, F8, FT8, FC6, FC4, FC2, FCz, Cz, C2, C4, C6, T8, TP8, CP6, CP4, P4, P6, P8, PO8,PO4, O2 | <0.001 | |
| Beta | ||||||
| Gamma | 1; D < S | 75–225 ms | 150 ms, P7 | C3, CP3, CP1, P1, P3, P5, P7, POz, Pz | =0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugos, J.A.; Bidelman, G.M.; Moreno, S.; Shen, D.; Lu, J.; Alain, C. Music and Visual Art Training Increase Auditory-Evoked Theta Oscillations in Older Adults. Brain Sci. 2022, 12, 1300. https://doi.org/10.3390/brainsci12101300
Bugos JA, Bidelman GM, Moreno S, Shen D, Lu J, Alain C. Music and Visual Art Training Increase Auditory-Evoked Theta Oscillations in Older Adults. Brain Sciences. 2022; 12(10):1300. https://doi.org/10.3390/brainsci12101300
Chicago/Turabian StyleBugos, Jennifer A., Gavin M. Bidelman, Sylvain Moreno, Dawei Shen, Jing Lu, and Claude Alain. 2022. "Music and Visual Art Training Increase Auditory-Evoked Theta Oscillations in Older Adults" Brain Sciences 12, no. 10: 1300. https://doi.org/10.3390/brainsci12101300
APA StyleBugos, J. A., Bidelman, G. M., Moreno, S., Shen, D., Lu, J., & Alain, C. (2022). Music and Visual Art Training Increase Auditory-Evoked Theta Oscillations in Older Adults. Brain Sciences, 12(10), 1300. https://doi.org/10.3390/brainsci12101300








