Figure 1.
Procedure for inclusion in the study, starting with the 1056 cases provided by the AFA insurance company to the 24 patients tested.
Figure 1.
Procedure for inclusion in the study, starting with the 1056 cases provided by the AFA insurance company to the 24 patients tested.
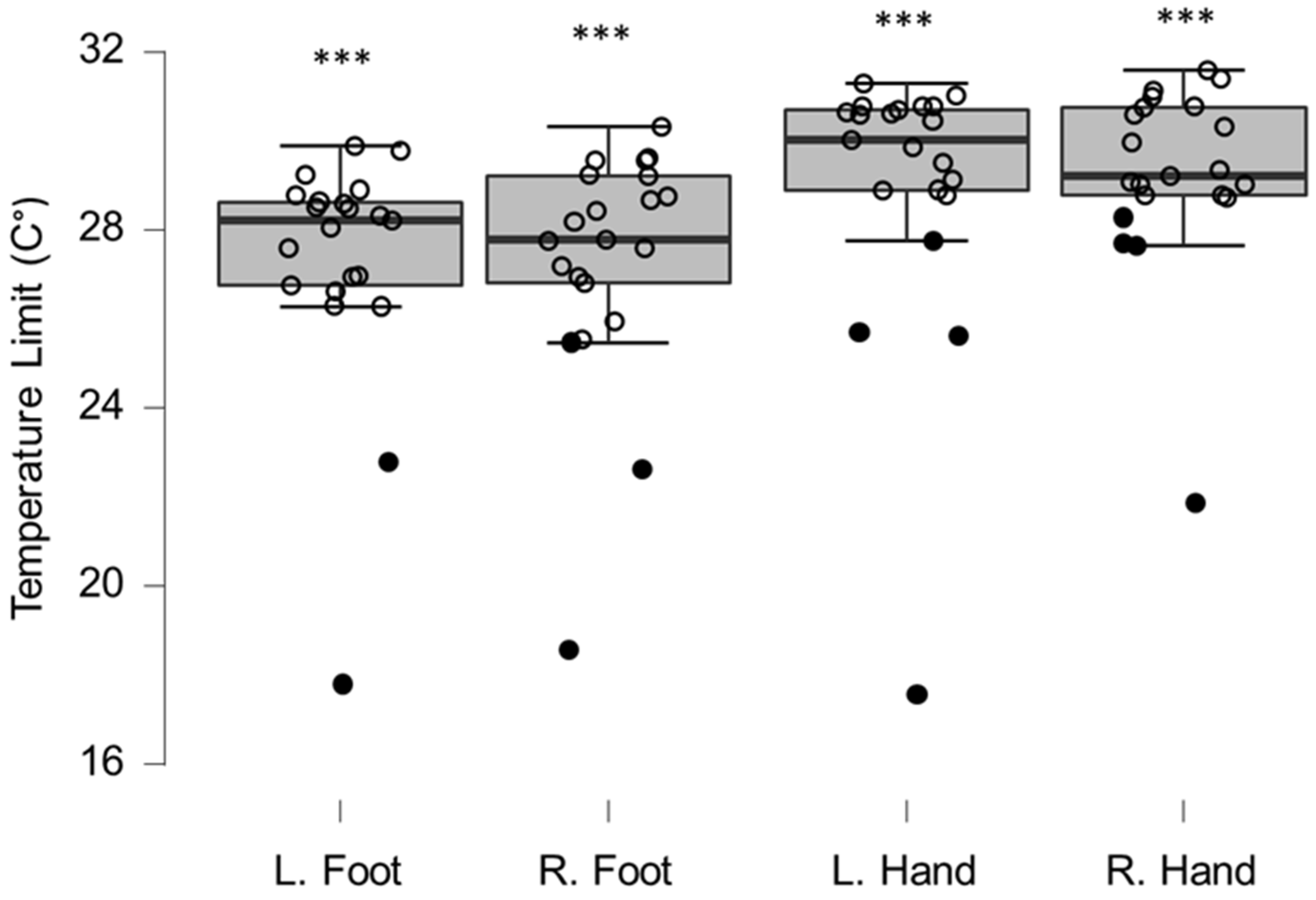
Figure 2.
Box-plots for cold sensation limits. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively. The line inside the box represents the median and the lower and upper whiskers represent the 10th and 90th percentiles. Filled circles represent values ≥ two standard deviations outside reference values. *** indicates a statistically significantly lower median values compared to the reference material, with a Bonferroni-corrected p-value corresponding to 0.001.
Figure 2.
Box-plots for cold sensation limits. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively. The line inside the box represents the median and the lower and upper whiskers represent the 10th and 90th percentiles. Filled circles represent values ≥ two standard deviations outside reference values. *** indicates a statistically significantly lower median values compared to the reference material, with a Bonferroni-corrected p-value corresponding to 0.001.
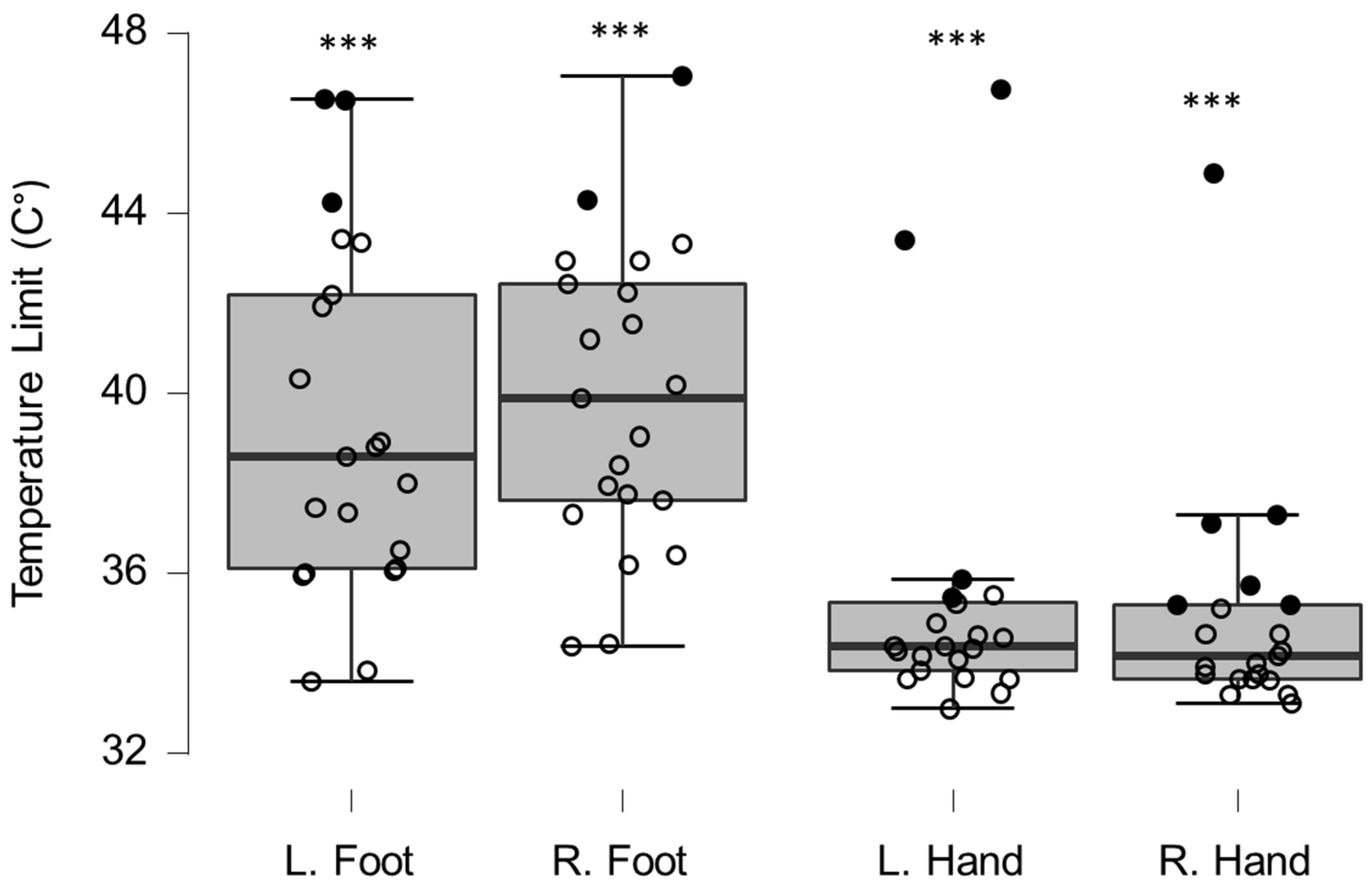
Figure 3.
Box-plots for warm sensation limits. Filled circles represent values ≥ two standard deviations outside reference values. *** indicates a statistically significantly lower median values compared to the reference material, with a Bonferroni-corrected p-value corresponding to 0.001.
Figure 3.
Box-plots for warm sensation limits. Filled circles represent values ≥ two standard deviations outside reference values. *** indicates a statistically significantly lower median values compared to the reference material, with a Bonferroni-corrected p-value corresponding to 0.001.
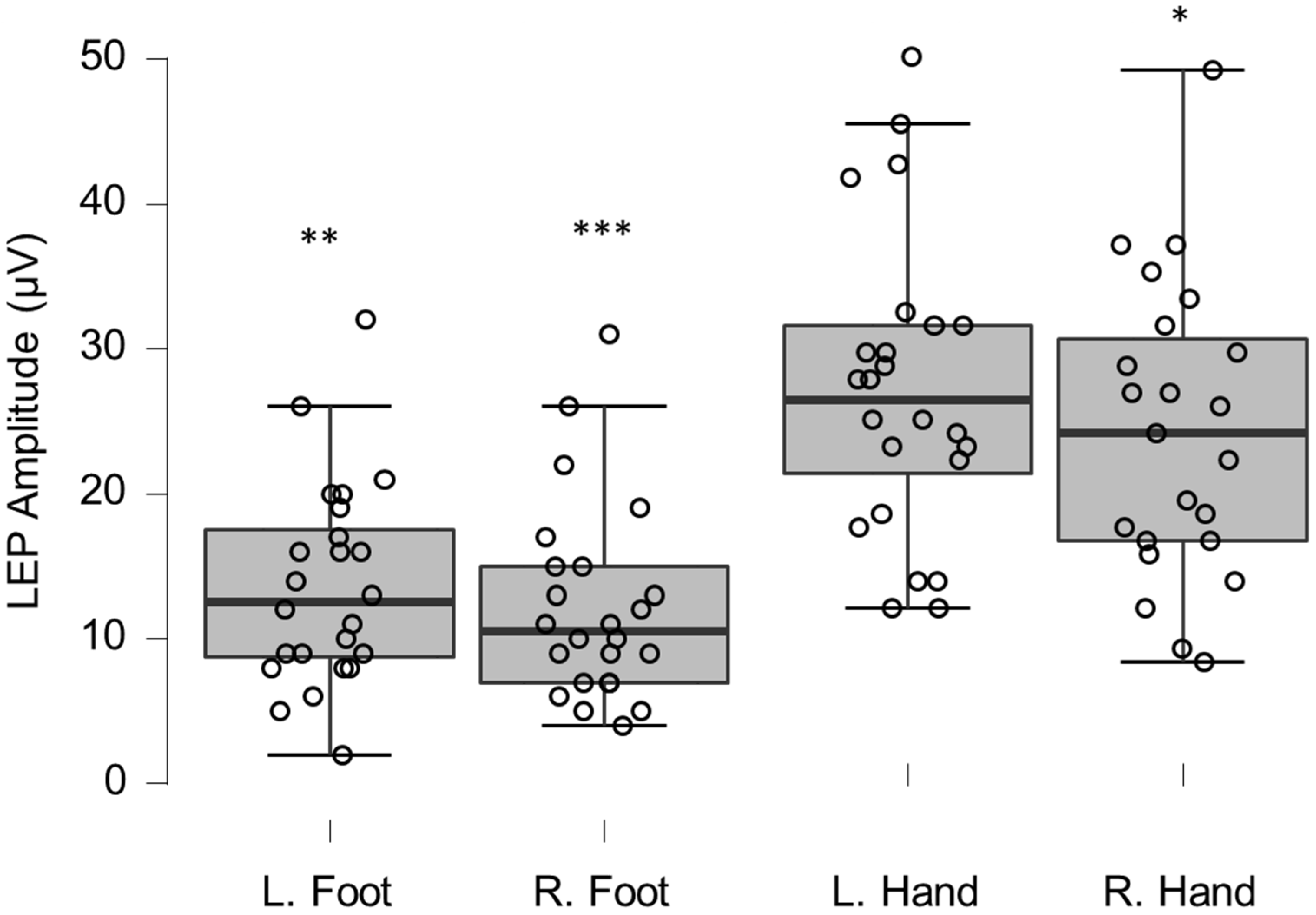
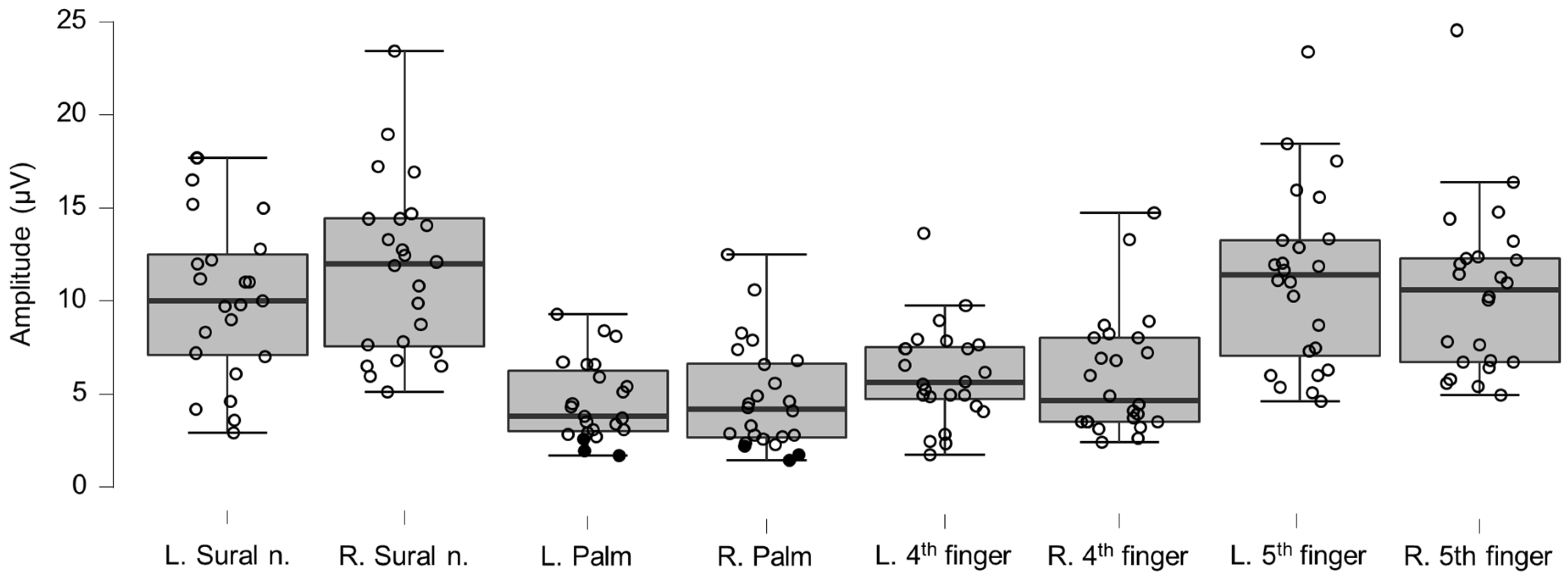
Figure 4.
Box-plots for LEP amplitudes. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively. The line inside the box represents the median and the lower and upper whiskers represent the 10th and 90th percentiles. Filled circles represent values ≥ two standard deviations outside reference values. *, **, and *** indicate statistically significantly lower median values compared to the reference material, with Bonferroni-corrected p-values corresponding to 0.05, 0.01, and 0.001, respectively.
Figure 4.
Box-plots for LEP amplitudes. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively. The line inside the box represents the median and the lower and upper whiskers represent the 10th and 90th percentiles. Filled circles represent values ≥ two standard deviations outside reference values. *, **, and *** indicate statistically significantly lower median values compared to the reference material, with Bonferroni-corrected p-values corresponding to 0.05, 0.01, and 0.001, respectively.
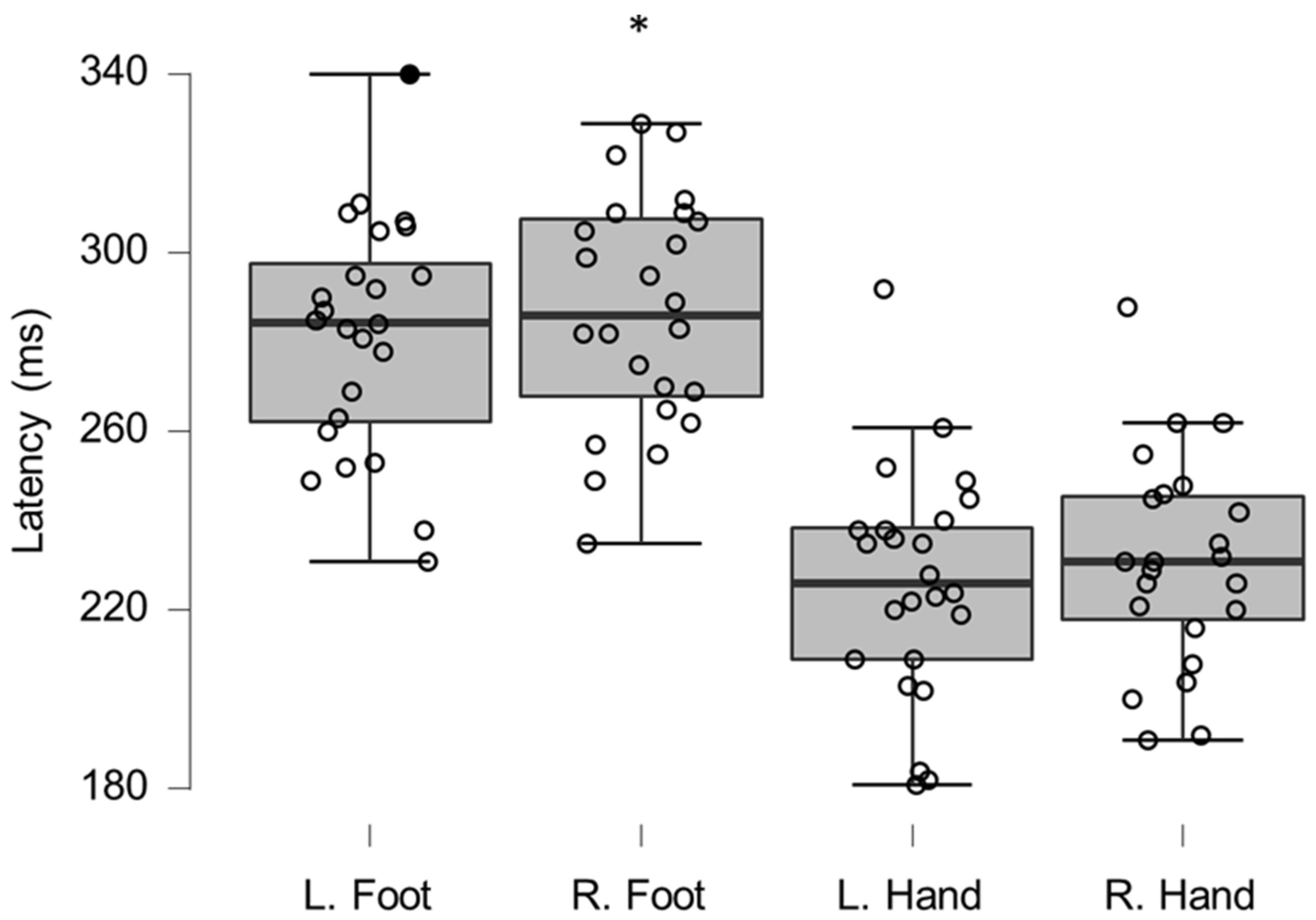
Figure 5.
Box-plots for LEP latencies. Filled circles represent values ≥ two standard deviations outside reference values. * indicates a statistically significantly lower median values compared to the reference material, with a Bonferroni-corrected p-value corresponding to 0.05.
Figure 5.
Box-plots for LEP latencies. Filled circles represent values ≥ two standard deviations outside reference values. * indicates a statistically significantly lower median values compared to the reference material, with a Bonferroni-corrected p-value corresponding to 0.05.
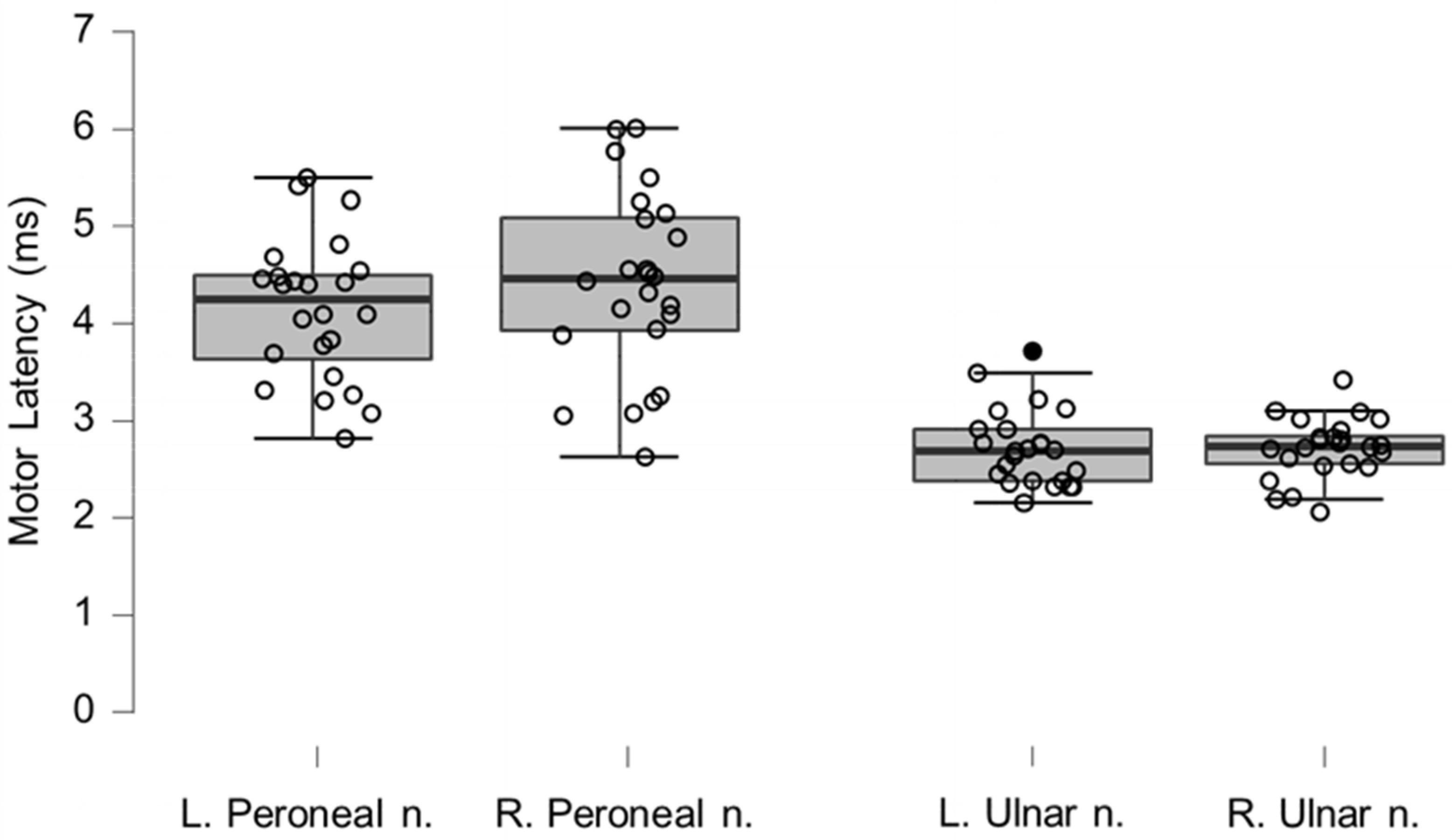
Figure 6.
Box-plots for motor neurography latencies. For box plots summary and explanation of symbols, see
Figure 2.
Figure 6.
Box-plots for motor neurography latencies. For box plots summary and explanation of symbols, see
Figure 2.
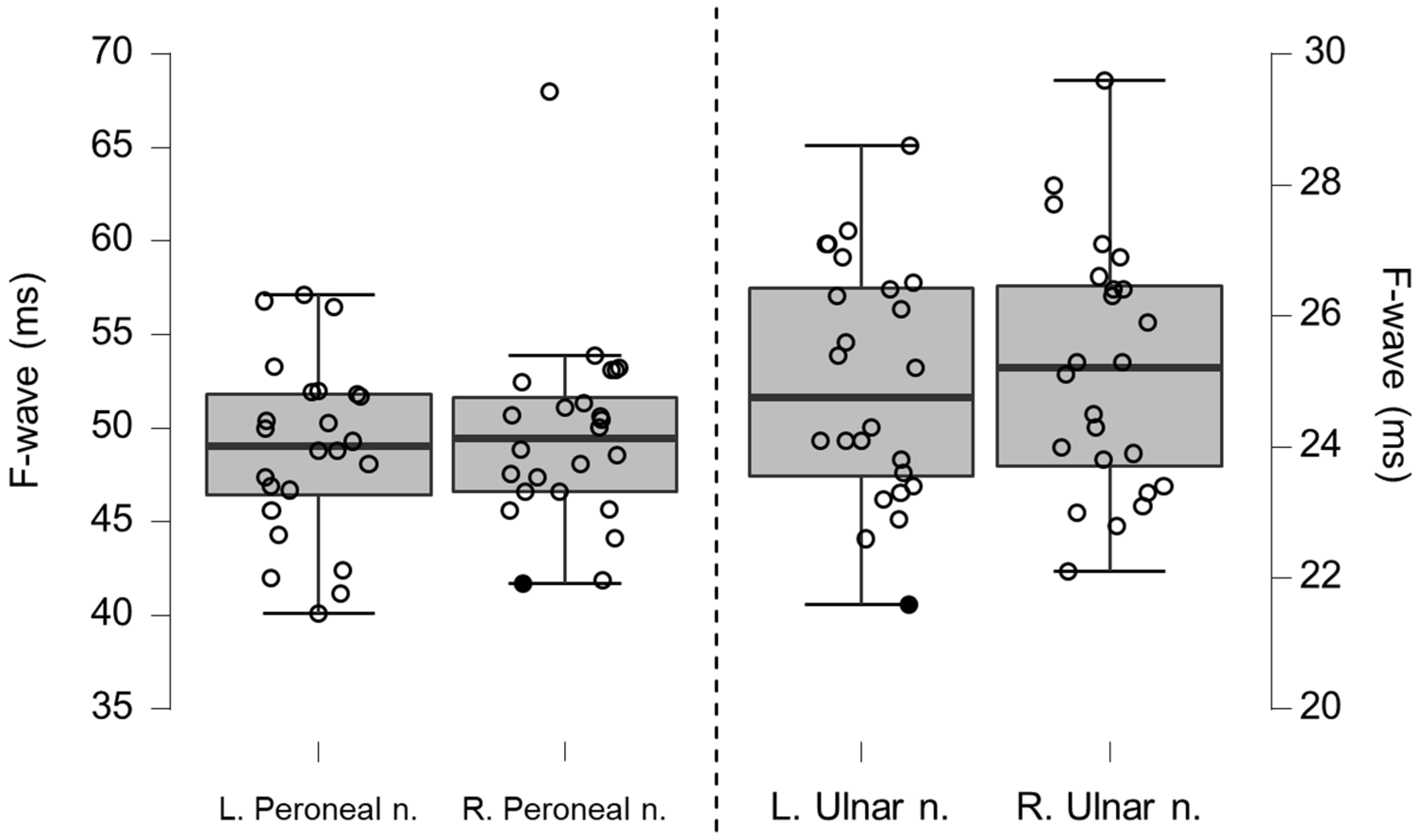
Figure 7.
Box-plots for motor neurography F-wave latencies. Filled circles represent values ≥ two standard deviations outside reference values.
Figure 7.
Box-plots for motor neurography F-wave latencies. Filled circles represent values ≥ two standard deviations outside reference values.
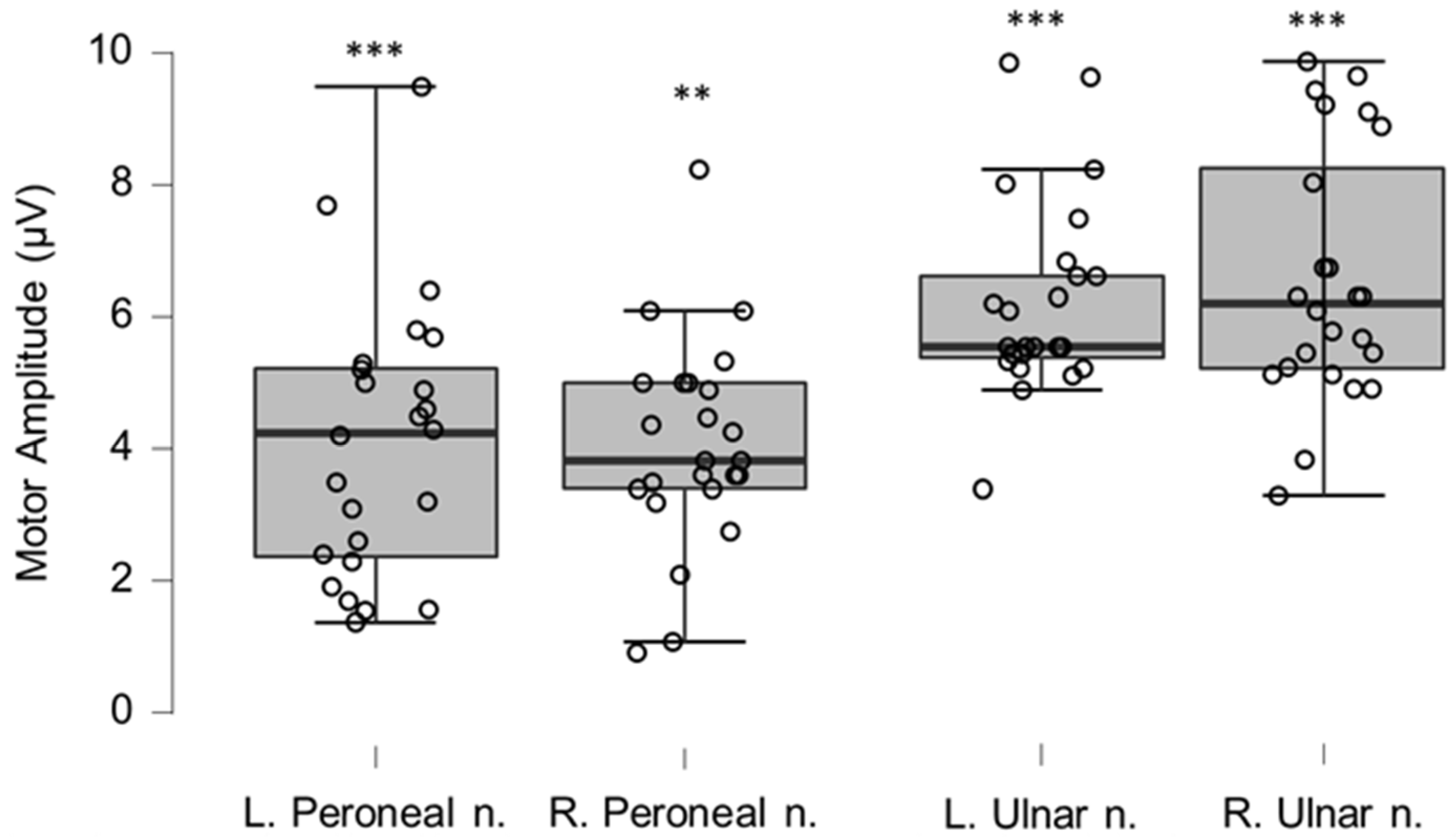
Figure 8.
Box-plots for motor neurography amplitudes. For box plots summary and explanation of symbols, see
Figure 2. ** and *** indicate statistically significantly lower median values compared to the reference material, with Bonferroni-corrected
p-values corresponding to 0.01 and 0.001, respectively.
Figure 8.
Box-plots for motor neurography amplitudes. For box plots summary and explanation of symbols, see
Figure 2. ** and *** indicate statistically significantly lower median values compared to the reference material, with Bonferroni-corrected
p-values corresponding to 0.01 and 0.001, respectively.
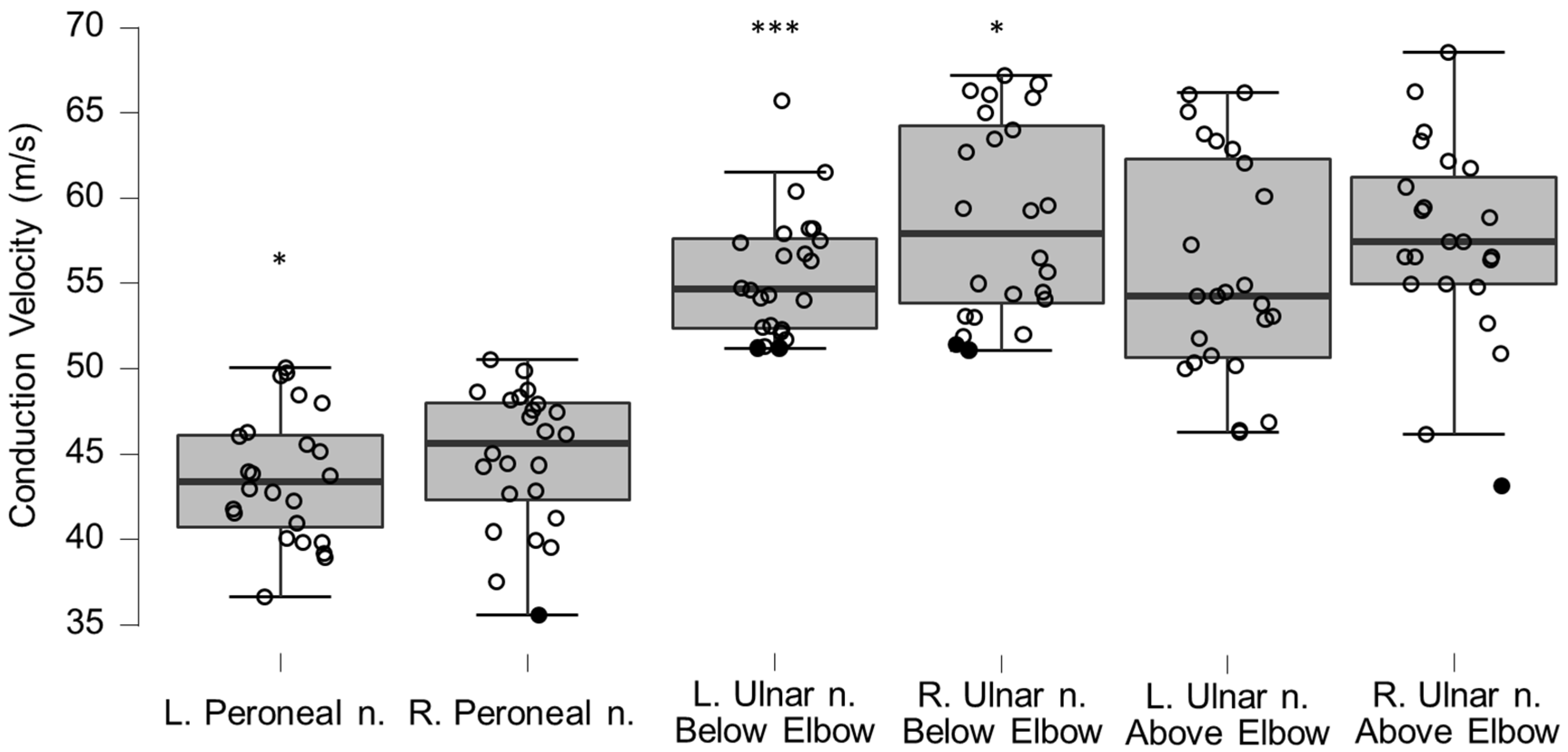
Figure 9.
Box-plots for motor neurography conduction velocities. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively. The line inside the box represents the median and the lower and upper whiskers represent the 10th and 90th percentiles. Filled circles represent values ≥ two standard deviations outside reference values. * and *** indicate statistically significantly lower median values compared to the reference material, with Bonferroni-corrected p-values corresponding to 0.05 and 0.001, respectively.
Figure 9.
Box-plots for motor neurography conduction velocities. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively. The line inside the box represents the median and the lower and upper whiskers represent the 10th and 90th percentiles. Filled circles represent values ≥ two standard deviations outside reference values. * and *** indicate statistically significantly lower median values compared to the reference material, with Bonferroni-corrected p-values corresponding to 0.05 and 0.001, respectively.
Figure 10.
Box-plots for sensory neurography amplitudes. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively. The line inside the box represents the median and the lower and upper whiskers represent the 10th and 90th percentiles. Filled circles represent values ≥ two standard deviations outside reference values.
Figure 10.
Box-plots for sensory neurography amplitudes. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively. The line inside the box represents the median and the lower and upper whiskers represent the 10th and 90th percentiles. Filled circles represent values ≥ two standard deviations outside reference values.
Figure 11.
Box-plots for sensory neurography conduction velocities. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively. The line inside the box represents the median and the lower and upper whiskers represent the 10th and 90th percentiles. Filled circles represent values ≥ two standard deviations outside reference values. ** and *** indicate statistically significantly lower median values compared to the reference material, with Bonferroni-corrected p-values corresponding to 0.01 and 0.001, respectively.
Figure 11.
Box-plots for sensory neurography conduction velocities. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively. The line inside the box represents the median and the lower and upper whiskers represent the 10th and 90th percentiles. Filled circles represent values ≥ two standard deviations outside reference values. ** and *** indicate statistically significantly lower median values compared to the reference material, with Bonferroni-corrected p-values corresponding to 0.01 and 0.001, respectively.
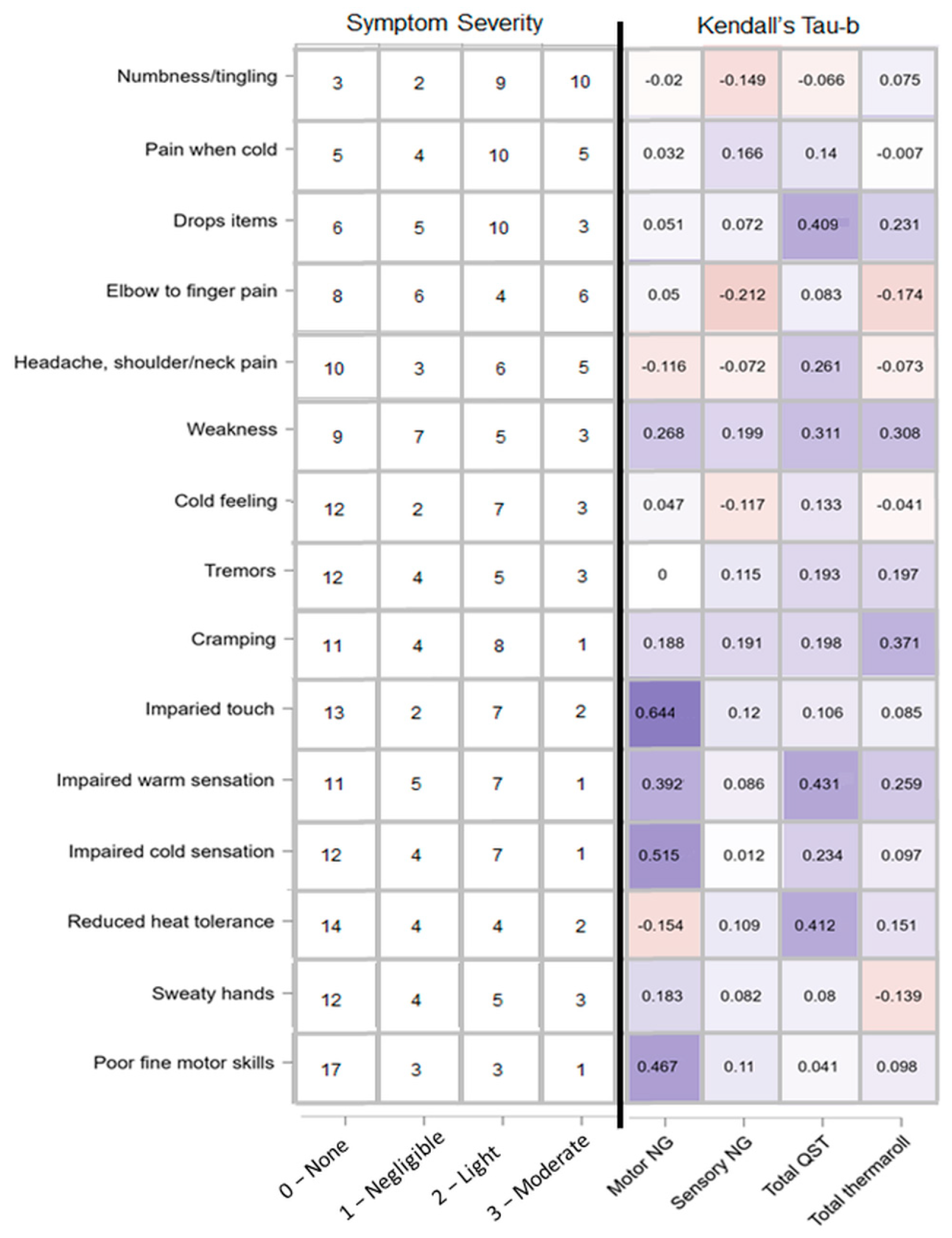
Figure 12.
Reported symptom severity and correlation with amount of clinically relevant cases per test paradigm. Left: self-reported, ranked (most prevalent at the top, least at the bottom) symptoms severity adapted from the HAVS questionnaire. Numbers represent number of patients who chose a given intensity option ranging from none to moderate. Right: heatmap of Kendall tau-b correlations representing patients’ self-reported symptoms and amount of clinically relevant cases for test paradigms motor NG (motor neurography), sensory NG, total QST (quantitative sensory testing), and total temperature roller cases.
Figure 12.
Reported symptom severity and correlation with amount of clinically relevant cases per test paradigm. Left: self-reported, ranked (most prevalent at the top, least at the bottom) symptoms severity adapted from the HAVS questionnaire. Numbers represent number of patients who chose a given intensity option ranging from none to moderate. Right: heatmap of Kendall tau-b correlations representing patients’ self-reported symptoms and amount of clinically relevant cases for test paradigms motor NG (motor neurography), sensory NG, total QST (quantitative sensory testing), and total temperature roller cases.
Table 1.
QST limit values for cold and warm sensations on the palm of the hand and arch of the foot. Reference values derived from manufacturers published data from healthy subject normative data. SD is standard deviation. *** indicates a statistically significant difference to reference material with p-values corresponding to Bonferroni corrected 0.001.
Table 1.
QST limit values for cold and warm sensations on the palm of the hand and arch of the foot. Reference values derived from manufacturers published data from healthy subject normative data. SD is standard deviation. *** indicates a statistically significant difference to reference material with p-values corresponding to Bonferroni corrected 0.001.
| QST Limits (°C) | Mean | n | SD | p-Value | |
|---|
| Left hand, cold | 30.04 | 18 | 0.98 | <0.00025 | *** |
| Reference hand, cold | 31.19 | 40 | 0.46 | | |
| Right hand, cold | 29.64 | 20 | 1.24 | <0.00025 | *** |
| Left foot, cold | 28.00 | 19 | 1.21 | <0.00025 | *** |
| Reference foot, cold | 30.25 | 40 | 1.59 | | |
| Right foot, cold | 27.74 | 20 | 1.87 | <0.00025 | *** |
| Left hand, warm | 34.38 | 19 | 0.78 | <0.00025 | *** |
| Reference hand, warm | 32.62 | 40 | 0.28 | | |
| Right hand, warm | 34.50 | 20 | 1.19 | <0.00025 | *** |
| Left foot, warm | 39.36 | 21 | 3.92 | <0.00025 | *** |
| Reference foot, warm | 34.81 | 40 | 2.24 | | |
| Right foot, warm | 39.97 | 21 | 3.43 | <0.00025 | *** |
Table 2.
LEP amplitudes in μV for evoked potentials on left and right dorsum of the hand or foot. Reference material derived from in-house, healthy subject normative data. *, **, and *** indicate statistically significant differences to reference material with p-values corresponding to Bonferroni corrected 0.05, 0.01, and 0.001, respectively.
Table 2.
LEP amplitudes in μV for evoked potentials on left and right dorsum of the hand or foot. Reference material derived from in-house, healthy subject normative data. *, **, and *** indicate statistically significant differences to reference material with p-values corresponding to Bonferroni corrected 0.05, 0.01, and 0.001, respectively.
| Amplitude (μV) | Mean | n | SD | p-Value | |
|---|
| Left hand | 24.13 | 23 | 9.99 | 0.0259 | |
| Reference hand | 29.05 | 105 | 13.37 | | |
| Right hand | 22.09 | 23 | 11.02 | 0.0061 | * |
| Left foot | 13.63 | 24 | 7.02 | 0.0006 | ** |
| Reference foot | 19.71 | 87 | 9.42 | | |
| Right foot | 11.39 | 23 | 5.61 | <0.0005 | *** |
Table 3.
LEP latencies in milliseconds for evoked potentials on left and right back of the hand or top of the foot. Reference material derived from in-house healthy subject normative data. * indicates a statistically significant differences to reference material with p-values corresponding to Bonferroni corrected 0.05.
Table 3.
LEP latencies in milliseconds for evoked potentials on left and right back of the hand or top of the foot. Reference material derived from in-house healthy subject normative data. * indicates a statistically significant differences to reference material with p-values corresponding to Bonferroni corrected 0.05.
| Latency (ms) | Mean | n | SD | p-Value | |
|---|
| Left hand | 223.26 | 23 | 22.25 | 0.1185 | |
| Reference hand | 227.19 | 105 | 19.93 | | |
| Right hand | 230.87 | 23 | 23.77 | 0.2475 | |
| Left foot | 281.79 | 24 | 26.05 | 0.1088 | |
| Reference foot | 274.08 | 87 | 28.89 | | |
| Right foot | 287.04 | 24 | 25.83 | 0.0189 | * |
Table 4.
Motor neurography latencies for left and right peroneal and ulnar nerves.
Table 4.
Motor neurography latencies for left and right peroneal and ulnar nerves.
| Motor Neurography |
|---|
| Latency (ms) | Mean | n | SD | p-Value |
|---|
| Left peroneal nerve | 4.42 | 24 | 0.94 | 0.7147 |
| Reference peroneal nerve | 4.54 | 54 | 0.74 | |
| Right peroneal nerve | 4.15 | 24 | 0.73 | 0.9830 |
| Left ulnar nerve | 2.71 | 24 | 0.39 | 0.9821 |
| Reference ulnar nerve | 2.90 | 100 | 0.30 | |
| Right ulnar nerve | 2.69 | 23 | 0.49 | 0.9926 |
Table 5.
Motor neurography F-wave latencies for left and right peroneal and ulnar nerves.
Table 5.
Motor neurography F-wave latencies for left and right peroneal and ulnar nerves.
| F-Wave (ms) | Mean | n | SD | p-Value |
|---|
| Left peroneal nerve | 48.10 | 23 | 4.94 | 0.9248 |
| Reference peroneal nerve | 46.77 | 54 | 2.43 | |
| Right peroneal nerve | 47.06 | 23 | 3.79 | 0.6639 |
| Left ulnar nerve | 24.98 | 24 | 1.81 | 0.5949 |
| Reference ulnar nerve | 24.89 | 100 | 1.09 | |
| Right ulnar nerve | 25.20 | 24 | 1.92 | 0.7771 |
Table 6.
Motor neurography amplitudes for left and right peroneal and ulnar nerve. ** and *** indicate statistically significant differences to reference material with p-values corresponding to Bonferroni corrected 0.01, and 0.001, respectively.
Table 6.
Motor neurography amplitudes for left and right peroneal and ulnar nerve. ** and *** indicate statistically significant differences to reference material with p-values corresponding to Bonferroni corrected 0.01, and 0.001, respectively.
| Amplitude (μV) | Mean | n | SD | p-Value | |
|---|
| Left peroneal nerve | 4.1 | 24 | 2.07 | 0.0003 | *** |
| Reference peroneal nerve | 6.08 | 54 | 2.46 | | |
| Right peroneal nerve | 4.48 | 22 | 1.12 | 0.0005 | ** |
| Left ulnar nerve | 6.30 | 22 | 1.17 | <0.0005 | *** |
| Reference ulnar nerve | 10.1 | 100 | 3.30 | | |
| Right ulnar nerve | 6.83 | 24 | 1.77 | <0.0005 | *** |
Table 7.
Motor neurography conduction velocities (CV) for left and right peroneal and ulnar nerves. * and *** indicate statistically significant differences to reference material with p-values corresponding to Bonferroni corrected 0.05 and 0.001, respectively.
Table 7.
Motor neurography conduction velocities (CV) for left and right peroneal and ulnar nerves. * and *** indicate statistically significant differences to reference material with p-values corresponding to Bonferroni corrected 0.05 and 0.001, respectively.
| Conduction Velocity (m/s) | Mean | n | SD | p-Value | |
|---|
| Left peroneal nerve | 43.57 | 24 | 3.76 | 0.0061 | * |
| Reference peroneal nerve | 45.96 | 54 | 3.68 | | |
| Right peroneal nerve | 44.73 | 24 | 4.05 | 0.1049 | |
| Left ulnar nerve, below elbow | 55.44 | 24 | 3.67 | 0.0008 | *** |
| Reference ulnar nerve | 61.60 | 100 | 5.30 | | |
| Right ulnar nerve, below elbow | 58.68 | 24 | 5.75 | 0.015 | * |
| Left ulnar nerve, above elbow | 56.45 | 24 | 7.07 | 0.5905 | |
| Reference ulnar nerve | 56.10 | 100 | 5.20 | | |
| Right ulnar nerve, above elbow | 58.10 | 22 | 5.09 | 0.9209 | |
Table 8.
Sensory neurography amplitudes for left and right sural and ulnar nerves.
Table 8.
Sensory neurography amplitudes for left and right sural and ulnar nerves.
| Sensory Neurography |
|---|
| Amplitude (μV) | Mean | n | SD | p-Value |
|---|
| Left sural nerve | 11.04 | 23 | 4.38 | 1 |
| Reference sural nerve | 2.41 | 165 | 0.58 | |
| Right sural nerve | 10.53 | 24 | 5.02 | 1 |
| Left ulnar palm | 4.49 | 24 | 2.18 | 0.9863 |
| Reference ulnar palm | 3.44 | 89 | 0.64 | |
| Right ulnar palm | 4.81 | 24 | 2.89 | 0.9848 |
| Left ulnar 4th finger | 5.65 | 23 | 2.17 | 1 |
| Reference ulnar 4th finger | 2.01 | 89 | 0.49 | |
| Right ulnar 4th finger | 5.96 | 24 | 3.25 | 1 |
| Left ulnar 5th finger | 10.06 | 24 | 5.39 | 1 |
| Reference ulnar 5th finger | 2.15 | 89 | 0.55 | |
| Right ulnar 5th finger | 8.53 | 23 | 3.67 | 1 |
Table 9.
Sensory neurography conduction velocities for left and right sural and ulnar nerves. ** and *** indicate statistically significant differences to reference material with p-values corresponding to Bonferroni corrected 0.01, and 0.001, respectively.
Table 9.
Sensory neurography conduction velocities for left and right sural and ulnar nerves. ** and *** indicate statistically significant differences to reference material with p-values corresponding to Bonferroni corrected 0.01, and 0.001, respectively.
| Conduction Velocity (m/s) | Mean | n | SD | p-Value | |
|---|
| Left sural nerve | 45.96 | 24 | 5.66 | 0.0006 | ** |
| Reference sural nerve | 50.43 | 165 | 6.03 | | |
| Right sural nerve | 49.40 | 24 | 5.94 | 0.2176 | |
| Left ulnar palm | 59.13 | 24 | 5.34 | 0.000167 | *** |
| Reference ulnar palm | 69.50 | 89 | 8.54 | | |
| Right ulnar palm | 58.91 | 24 | 5.29 | 0.000167 | *** |
| Left ulnar 4th finger | 58.23 | 24 | 5.27 | 0.0303 | |
| Reference ulnar 4th finger | 60.61 | 89 | 5.66 | | |
| Right ulnar 4th finger | 57.46 | 24 | 5.68 | 0.0104 | |
| Left ulnar 5th finger | 58.41 | 21 | 4.13 | 0.3385 | |
| Reference ulnar 5th finger | 60.10 | 89 | 5.81 | | |
| Right ulnar 5th finger | 60.00 | 24 | 5.93 | 0.4692 | |