Association of Exercise with Inhibitory Control and Prefrontal Brain Activity Under Acute Psychosocial Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
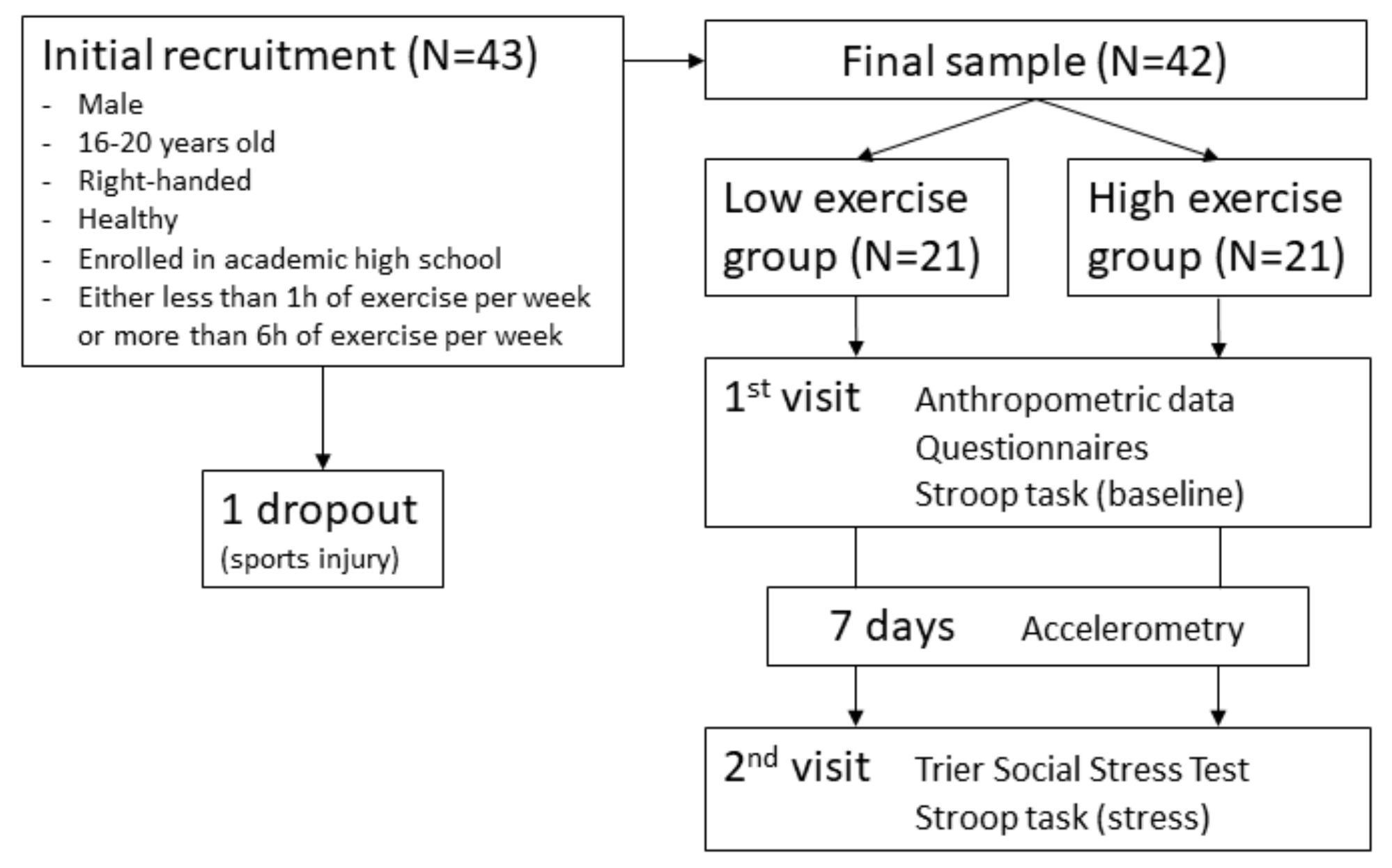
2.3. Procedure
2.4. Accelerometry
2.5. Cognitive Task and Prefrontal Brain Activity
2.6. Stress Induction and Measurement of Stress Reactivity
2.7. Statistical Analysis
3. Results
3.1. Sample Description
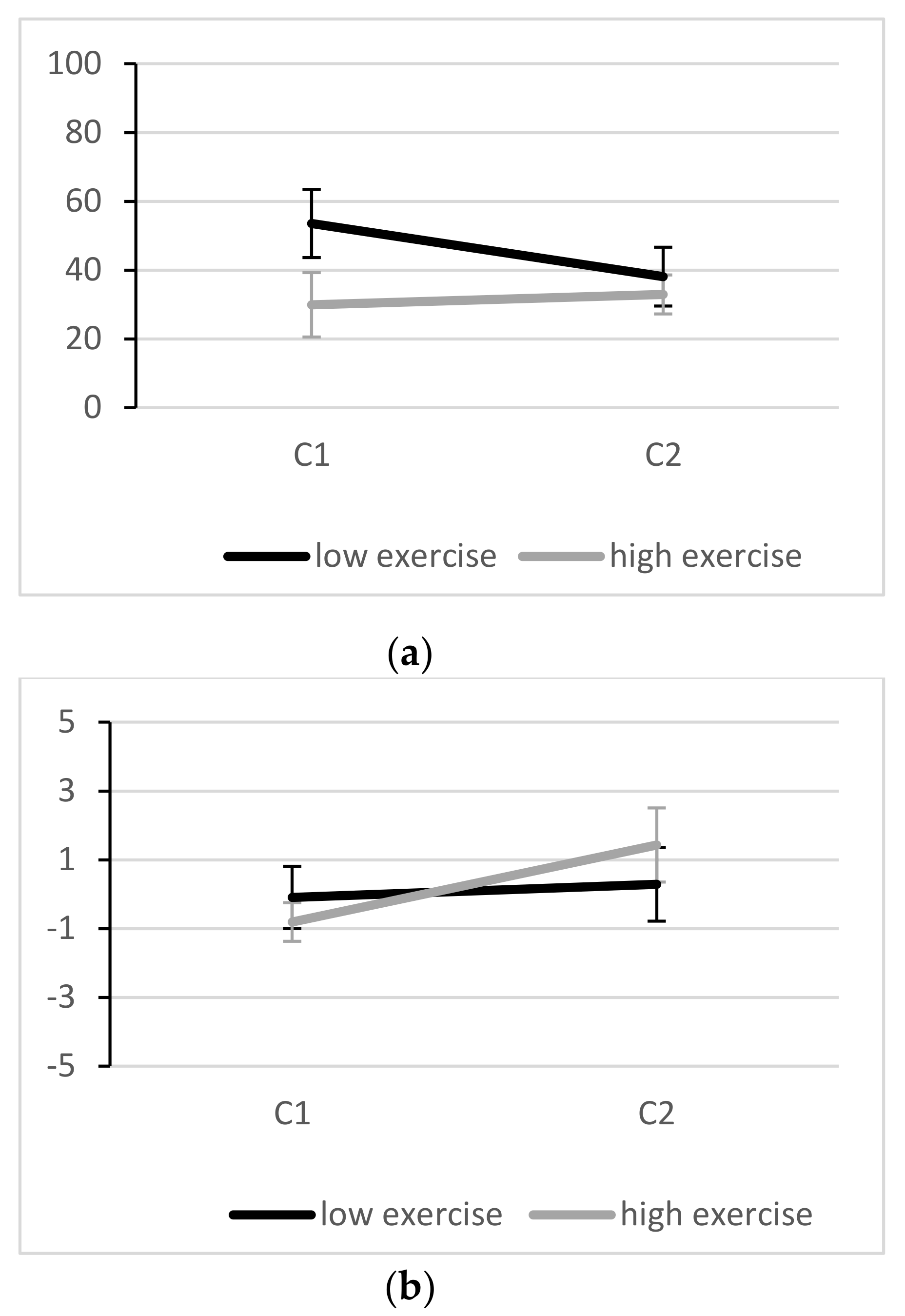
3.2. Effectiveness of the Stressor
3.3. Inhibitory Performance
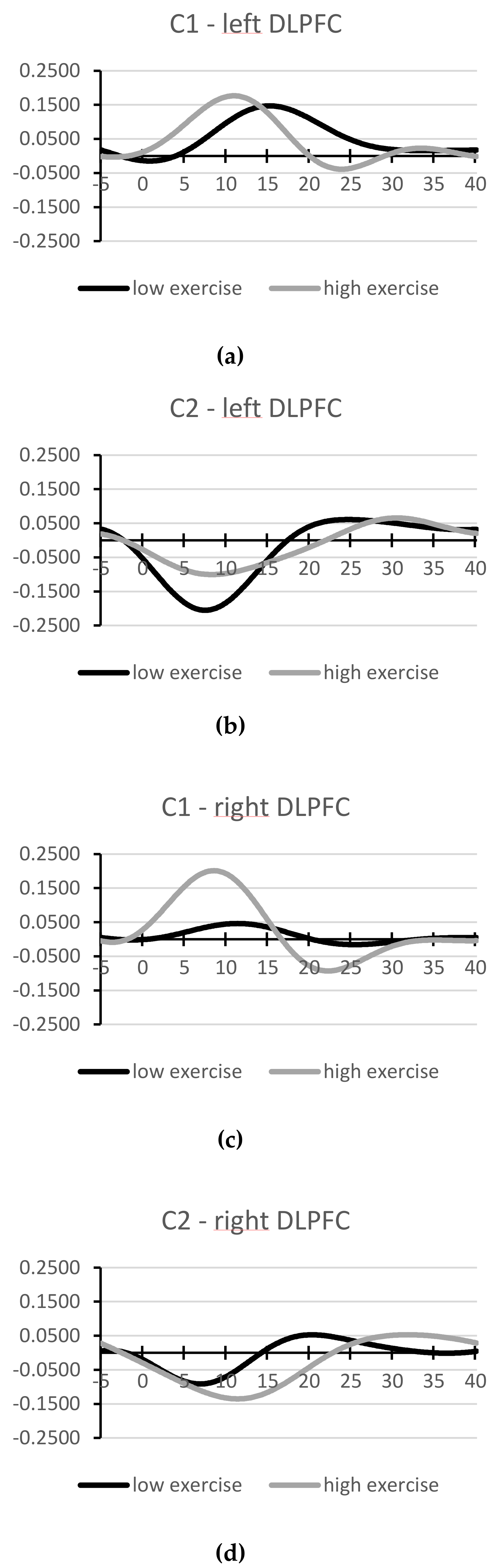
3.4. Oxygenation of Left and Right DLPFC
4. Discussion
4.1. Associations with Exercise
4.2. Inhibitory Control Under Stress
4.3. Strengths and Limitations
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jex, S.M. Stress and job performance. Theory, research, and implications for managerial practice; Sage Publications: Thousand Oaks, CA, USA, 1998; ISBN 978-0761909248. [Google Scholar]
- Muse, L.A.; Harris, S.G.; Feild, H.S. Has the Inverted-U Theory of Stress and Job Performance Had a Fair Test? Hum. Perform. 2003, 16, 349–364. [Google Scholar] [CrossRef]
- Landsbergis, P.A.; Dobson, M.; LaMontagne, A.D.; Choi, B.K.; Schnall, P.; Baker, D.B. Occupational Stress. In Occupational and Environmental Health: Recognizing and Preventing Disease and Injury, 7th ed.; Levy, B.S., Wegman, D.H., Baron, S., Sokas, R.K., McStowe, H.L., Eds.; Oxford University Press: New York, NY, 2018; ISBN 9780190662691. [Google Scholar]
- Güntzer, A. Jugendliche in der Schweiz leiden unter Leistungsdruck und Stress. Schweiz. Z. Für Heilpädagogik 2017, 23, 38–44. [Google Scholar]
- Eppelmann, L.; Parzer, P.; Lenzen, C.; Bürger, A.; Haffner, J.; Resch, F.; Kaess, M. Stress, coping and emotional and behavioral problems among German high school students. Ment. Health Prev. 2016, 4, 81–87. [Google Scholar] [CrossRef]
- Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef] [Green Version]
- Kirschbaum, C.; Pirke, K.M.; Hellhammer, D.H. The ‘Trier Social Stress Test’: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef]
- Campbell, J.; Ehlert, U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology 2012, 37, 1111–1134. [Google Scholar] [CrossRef]
- Foley, P.; Kirschbaum, C. Human hypothalamus–pituitary–adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci. Biobehav. Rev. 2010, 35, 91–96. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [Green Version]
- Arnsten, A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Sandi, C. Stress and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 245–261. [Google Scholar] [CrossRef]
- Shields, G.S.; Sazma, M.A.; Yonelinas, A.P. The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neurosci. Biobehav. Rev. 2016, 68, 651–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batouli, S.A.H.; Saba, V. At least eighty percent of brain grey matter is modifiable by physical activity: A review study. Behav. Brain Res. 2017, 332, 204–217. [Google Scholar] [CrossRef]
- Budde, H.; Wegner, M.; Soya, H.; Voelcker-Rehage, C.; McMorris, T. Neuroscience of Exercise: Neuroplasticity and Its Behavioral Consequences. Neural Plast. 2016, 2016, 3643879. [Google Scholar] [CrossRef]
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging 2014, 35 Suppl. S2, S20–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, G.E.; Cantelon, J.A.; Eddy, M.D.; Brunyé, T.T.; Urry, H.L.; Mahoney, C.R.; Kanarek, R.B. Habitual exercise is associated with cognitive control and cognitive reappraisal success. Exp. Brain Res. 2017, 235, 3785–3797. [Google Scholar] [CrossRef] [PubMed]
- Guiney, H.; Machado, L. Benefits of regular aerobic exercise for executive functioning in healthy populations. Psychon. Bull. Rev. 2013, 20, 73–86. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Pühse, U.; Looser, V.N.; Kamijo, K. Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nat. Hum. Behav. 2020. [Google Scholar] [CrossRef]
- Nigg, J.T. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychol. Bull. 2000, 126, 220–246. [Google Scholar] [CrossRef]
- Vanderhasselt, M.-A.; de Raedt, R.; Baeken, C. Dorsolateral prefrontal cortex and Stroop performance: Tackling the lateralization. Psychon. Bull. Rev. 2009, 16, 609–612. [Google Scholar] [CrossRef]
- Ludyga, S.; Mucke, M.; Colledge, F.; Puhse, U.; Gerber, M. A Combined EEG-fNIRS Study Investigating Mechanisms Underlying the Association between Aerobic Fitness and Inhibitory Control in Young Adults. Neuroscience 2019. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, J.; Sun, B.; Luo, Q.; Gong, H. Studying hemispheric lateralization during a Stroop task through near-infrared spectroscopy-based connectivity. J. Biomed. Opt. 2014, 19, 57012. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.S.; Erickson, K.I.; Colcombe, S.J.; Kim, J.S.; Voss, M.W.; Kramer, A.F. Age-related differences in the involvement of the prefrontal cortex in attentional control. Brain Cogn. 2009, 71, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyodo, K.; Dan, I.; Kyutoku, Y.; Suwabe, K.; Byun, K.; Ochi, G.; Kato, M.; Soya, H. The association between aerobic fitness and cognitive function in older men mediated by frontal lateralization. Neuroimage 2016, 125, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.; Hilsendegen, P.; Thomas, M.; Haeussinger, F.B.; Metzger, F.G.; Nuerk, H.-C.; Fallgatter, A.J.; Nieratschker, V.; Ehlis, A.-C. Cortical hemodynamic changes during the Trier Social Stress Test: An fNIRS study. Neuroimage 2018, 171, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.; Thomas, M.; Hilsendegen, P.; Metzger, F.G.; Haeussinger, F.B.; Nuerk, H.-C.; Fallgatter, A.J.; Nieratschker, V.; Ehlis, A.-C. Stress-related dysfunction of the right inferior frontal cortex in high ruminators: An fNIRS study. Neuroimage Clin. 2018, 18, 510–517. [Google Scholar] [CrossRef]
- Schaal, N.K.; Hepp, P.; Schweda, A.; Wolf, O.T.; Krampe, C. A Functional Near-Infrared Spectroscopy Study on the Cortical Haemodynamic Responses During the Maastricht Acute Stress Test. Sci. Rep. 2019, 9, 13459. [Google Scholar] [CrossRef] [Green Version]
- Herman, J.P.; Figueiredo, H.; Mueller, N.K.; Ulrich-Lai, Y.; Ostrander, M.M.; Choi, D.C.; Cullinan, W.E. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front. Neuroendocrinol. 2003, 24, 151–180. [Google Scholar] [CrossRef]
- Pechtel, P.; Pizzagalli, D.A. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology 2011, 214, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- Klaperski, S.; von Dawans, B.; Heinrichs, M.; Fuchs, R. Effects of a 12-week endurance training program on the physiological response to psychosocial stress in men: A randomized controlled trial. J. Behav. Med. 2014, 37, 1118–1133. [Google Scholar] [CrossRef]
- Mücke, M.; Ludyga, S.; Colledge, F.; Gerber, M. Influence of Regular Physical Activity and Fitness on Stress Reactivity as Measured with the Trier Social Stress Test Protocol: A Systematic Review. Sports Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kudielka, B.M.; Kirschbaum, C. Sex differences in HPA axis responses to stress: A review. Biol. Psychol. 2005, 69, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Rosselli, M.; Ardila, A. The impact of culture and education on non-verbal neuropsychological measurements: A critical review. Brain Cogn. 2003, 52, 326–333. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.M.; Brähler, E.; Dreier, M.; Reinecke, L.; Müller, K.W.; Schmutzer, G.; Wölfling, K.; Beutel, M.E. The German version of the Perceived Stress Scale - psychometric characteristics in a representative German community sample. BMC Psychiatry 2016, 16, 159. [Google Scholar] [CrossRef] [Green Version]
- Gerber, M.; Lang, C.; Lemola, S.; Colledge, F.; Kalak, N.; Holsboer-Trachsler, E.; Pühse, U.; Brand, S. Validation of the German version of the insomnia severity index in adolescents, young adults and adult workers: Results from three cross-sectional studies. BMC Psychiatry 2016, 16, 174. [Google Scholar] [CrossRef] [Green Version]
- Goodman, R. Psychometric properties of the strengths and difficulties questionnaire. J. Am. Acad. Child. Adolesc. Psychiatry 2001, 40, 1337–1345. [Google Scholar] [CrossRef]
- Taber, K.S. The Use of Cronbach’s Alpha When Developing and Reporting Research Instruments in Science Education. Res. Sci. Educ. 2018, 48, 1273–1296. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Schommer, N.C.; Hellhammer, D.H.; Kirschbaum, C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology 2004, 29, 983–992. [Google Scholar] [CrossRef]
- Klaperski, S.; von Dawans, B.; Heinrichs, M.; Fuchs, R. Does the level of physical exercise affect physiological and psychological responses to psychosocial stress in women? Psychol. Sport Exerc. 2013, 14, 266–274. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Taylor, S.; Iusitini, L.; Stewart, T.; Savila, F.; Tautolo, E.-S.; Plank, L.; Jalili-Moghaddam, S.; Paterson, J.; Rush, E. Accelerometer data treatment for adolescents: Fitting a piece of the puzzle. Prev. Med. Rep. 2017, 5, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Homack, S.; Riccio, C.A. A meta-analysis of the sensitivity and specificity of the Stroop Color and Word Test with children. Arch. Clin. Neuropsychol. 2004, 19, 725–743. [Google Scholar] [CrossRef]
- Penner, I.-K.; Kobel, M.; Stöcklin, M.; Weber, P.; Opwis, K.; Calabrese, P. The Stroop task: Comparison between the original paradigm and computerized versions in children and adults. Clin. Neuropsychol. 2012, 26, 1142–1153. [Google Scholar] [CrossRef]
- Din, N.C.; Tat Meng, E.C. Computerized Stroop Tests: A Review. J. Psychol. Psychother. 2019, 9, 1–5. [Google Scholar]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Tak, S.; Ye, J.C. Statistical analysis of fNIRS data: A comprehensive review. Neuroimage 2014, 85 Pt. 1, 72–91. [Google Scholar] [CrossRef]
- Orihuela-Espina, F.; Leff, D.R.; James, D.R.C.; Darzi, A.W.; Yang, G.Z. Quality control and assurance in functional near infrared spectroscopy (fNIRS) experimentation. Phys. Med. Biol. 2010, 55, 3701–3724. [Google Scholar] [CrossRef]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Müller, N.G. Applications of Functional Near-Infrared Spectroscopy (fNIRS) Neuroimaging in Exercise—Cognition Science: A Systematic, Methodology-Focused Review. J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigadoi, S.; Ceccherini, L.; Cutini, S.; Scarpa, F.; Scatturin, P.; Selb, J.; Gagnon, L.; Boas, D.A.; Cooper, R.J. Motion artifacts in functional near-infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. Neuroimage 2014, 85 Pt. 1, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Cooper, R.J.; Selb, J.; Gagnon, L.; Phillip, D.; Schytz, H.W.; Iversen, H.K.; Ashina, M.; Boas, D.A. A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Front. Neurosci. 2012, 6, 147. [Google Scholar] [CrossRef] [Green Version]
- Scholkmann, F.; Spichtig, S.; Muehlemann, T.; Wolf, M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol. Meas. 2010, 31, 649–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, K.; Hyodo, K.; Suwabe, K.; Ochi, G.; Sakairi, Y.; Kato, M.; Dan, I.; Soya, H. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: An fNIRS study. Neuroimage 2014, 98, 336–345. [Google Scholar] [CrossRef]
- Schroeter, M.L.; Zysset, S.; Kupka, T.; Kruggel, F.; Yves von Cramon, D. Near-infrared spectroscopy can detect brain activity during a color-word matching Stroop task in an event-related design. Hum. Brain Mapp. 2002, 17, 61–71. [Google Scholar] [CrossRef]
- Yennu, A.; Tian, F.; Smith-Osborne, A.; J Gatchel, R.; Woon, F.L.; Liu, H. Prefrontal responses to Stroop tasks in subjects with post-traumatic stress disorder assessed by functional near infrared spectroscopy. Sci. Rep. 2016, 6, 30157. [Google Scholar] [CrossRef] [Green Version]
- Schecklmann, M.; Ehlis, A.-C.; Plichta, M.M.; Fallgatter, A.J. Functional near-infrared spectroscopy: A long-term reliable tool for measuring brain activity during verbal fluency. Neuroimage 2008, 43, 147–155. [Google Scholar] [CrossRef]
- Allen, A.P.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014, 38, 94–124. [Google Scholar] [CrossRef]
- Belanger, H.G.; Cimino, C.R. The lateralized Stroop: A meta-analysis and its implications for models of semantic processing. Brain Lang. 2002, 83, 384–402. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tomporowski, P.D.; Pesce, C. Exercise, sports, and performance arts benefit cognition via a common process. Psychol. Bull. 2019, 145, 929–951. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, R.; Forte, R.; Borzacchini, M.; Vazou, S.; Tomporowski, P.D.; Pesce, C. Physical and Motor Fitness, Sport Skills and Executive Function in Adolescents: A Moderated Prediction Model. PSYCH 2015, 06, 1915–1929. [Google Scholar] [CrossRef] [Green Version]
- Gerber, M.; Ludyga, S.; Mücke, M.; Colledge, F.; Brand, S.; Pühse, U. Low vigorous physical activity is associated with increased adrenocortical reactivity to psychosocial stress in students with high stress perceptions. Psychoneuroendocrinology 2017, 80, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Plieger, T.; Felten, A.; Diks, E.; Tepel, J.; Mies, M.; Reuter, M. The impact of acute stress on cognitive functioning: A matter of cognitive demands? Cogn. Neuropsychiatry 2017, 22, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Bunge, S.A.; Gross, J.J.; Gabrieli, J.D.E. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002, 14, 1215–1229. [Google Scholar] [CrossRef] [Green Version]
- Delgado, M.R.; Nearing, K.I.; Ledoux, J.E.; Phelps, E.A. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 2008, 59, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Golkar, A.; Lonsdorf, T.B.; Olsson, A.; Lindstrom, K.M.; Berrebi, J.; Fransson, P.; Schalling, M.; Ingvar, M.; Öhman, A. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS ONE 2012, 7, e48107. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Hamann, S. Neural correlates of positive and negative emotion regulation. J. Cogn. Neurosci. 2007, 19, 776–798. [Google Scholar] [CrossRef]
- Ishizuka, K.; Hillier, A.; Beversdorf, D.Q. Effect of the cold pressor test on memory and cognitive flexibility. Neurocase 2007, 13, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Chajut, E.; Algom, D. Selective attention improves under stress: Implications for theories of social cognition. J. Personal. Soc. Psychol. 2003, 85, 231–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwabe, L.; Hoffken, O.; Tegenthoff, M.; Wolf, O.T. Stress-induced enhancement of response inhibition depends on mineralocorticoid receptor activation. Psychoneuroendocrinology 2013, 38, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Roos, L.E.; Knight, E.L.; Beauchamp, K.G.; Berkman, E.T.; Faraday, K.; Hyslop, K.; Fisher, P.A. Acute stress impairs inhibitory control based on individual differences in parasympathetic nervous system activity. Biol. Psychol. 2017, 125, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanger, J.; Bechtold, L.; Schoofs, D.; Blaszkewicz, M.; Wascher, E. The influence of acute stress on attention mechanisms and its electrophysiological correlates. Front. Behav. Neurosci. 2014, 8, 353. [Google Scholar] [CrossRef] [Green Version]
- Vinski, M.T.; Watter, S. Being a grump only makes things worse: A transactional account of acute stress on mind wandering. Front. Psychol. 2013, 4, 730. [Google Scholar] [CrossRef] [Green Version]
- Mendl, M. Performing under pressure: Stress and cognitive function. Appl. Anim. Behav. Sci. 1999, 65, 221–244. [Google Scholar] [CrossRef]
| Low Exercise Group (n = 21) | High Exercise Group (n = 21) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | T | |
| Age (years) | 17.2 | 1.1 | 17.1 | 1.2 | 0.14 |
| BMI (kg/m2) | 22.9 | 5.1 | 22.0 | 2.1 | 0.81 |
| Socioeconomic status | 3.1 | 0.6 | 3.5 | 0.8 | −2.01 |
| Sleep complaints (ISI) | 6.3 | 3.7 | 5.9 | 4.1 | 0.28 |
| Chronic stress (PSS) | 14.3 | 5.4 | 13.3 | 3.6 | 0.67 |
| Psychopathology (SDQ) | 14.8 | 9.5 | 13.5 | 4.5 | 0.58 |
| VPA (min/day) | 6.4 | 8.6 | 15.0 | 7.3 | −3.38 * |
| MVPA (min/day) | 60.6 | 22.4 | 83.3 | 20.0 | −3.39 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mücke, M.; Ludyga, S.; Colledge, F.; Pühse, U.; Gerber, M. Association of Exercise with Inhibitory Control and Prefrontal Brain Activity Under Acute Psychosocial Stress. Brain Sci. 2020, 10, 439. https://doi.org/10.3390/brainsci10070439
Mücke M, Ludyga S, Colledge F, Pühse U, Gerber M. Association of Exercise with Inhibitory Control and Prefrontal Brain Activity Under Acute Psychosocial Stress. Brain Sciences. 2020; 10(7):439. https://doi.org/10.3390/brainsci10070439
Chicago/Turabian StyleMücke, Manuel, Sebastian Ludyga, Flora Colledge, Uwe Pühse, and Markus Gerber. 2020. "Association of Exercise with Inhibitory Control and Prefrontal Brain Activity Under Acute Psychosocial Stress" Brain Sciences 10, no. 7: 439. https://doi.org/10.3390/brainsci10070439





