Neuronal-Specific Inhibition of Endoplasmic Reticulum Mg2+/Ca2+ ATPase Ca2+ Uptake in a Mixed Primary Hippocampal Culture Model of Status Epilepticus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Primary Hippocampal Cell Cultures
2.3. SE in the Culture Dish
2.4. Electrophysiological Monitoring of SE in Hippocampal Neurons
2.5. Determination of [Ca2+]i Levels in Hippocampal Culture Neurons Using Fluorescent Ca2+ Imaging
2.6. ER-Mediated Ca2+ Uptake Measured in the Unfractionated Homogenates
2.7. Data and Statistical Analyses
3. Results
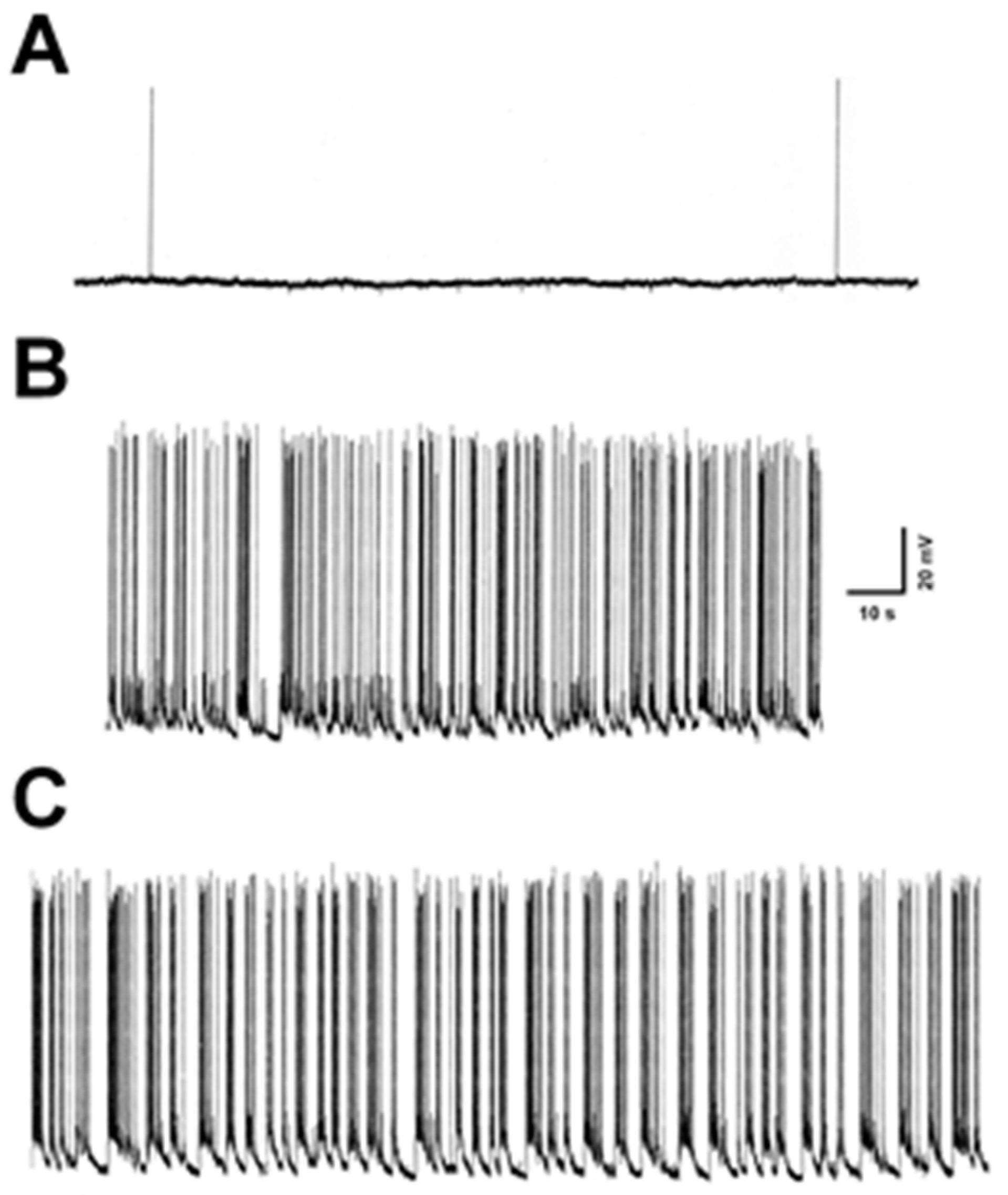
3.1. Electrophysiological Monitoring of SE in Hippocampal Neurons
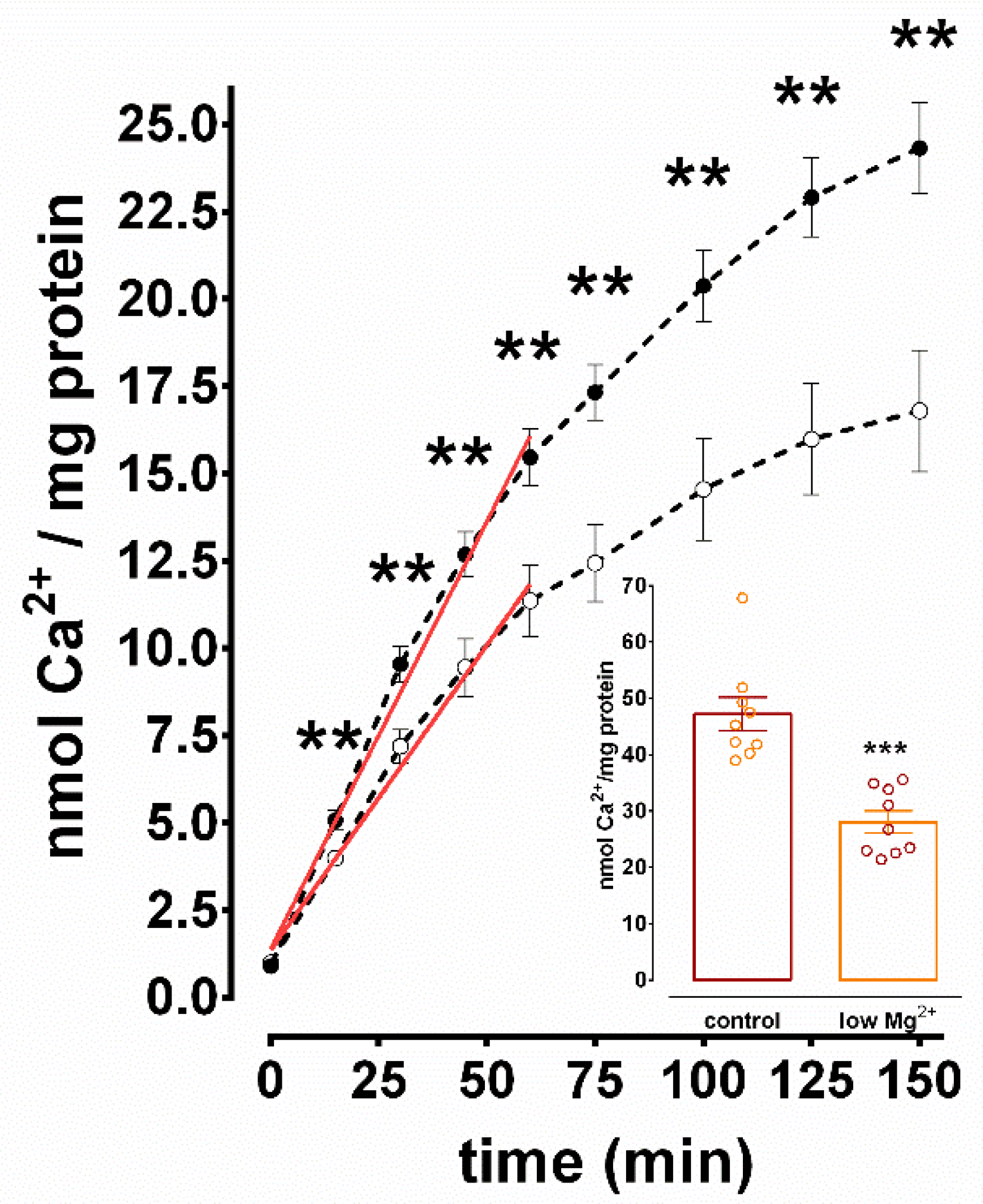
3.2. Inhibition of SERCA-Mediated Ca2+ Uptake in N+G Homogenates Exposed to Low Mg2+ Media
3.3. Inhibition of Ca2+ Uptake in Homogenates of Glia Incubated in Low Mg2+
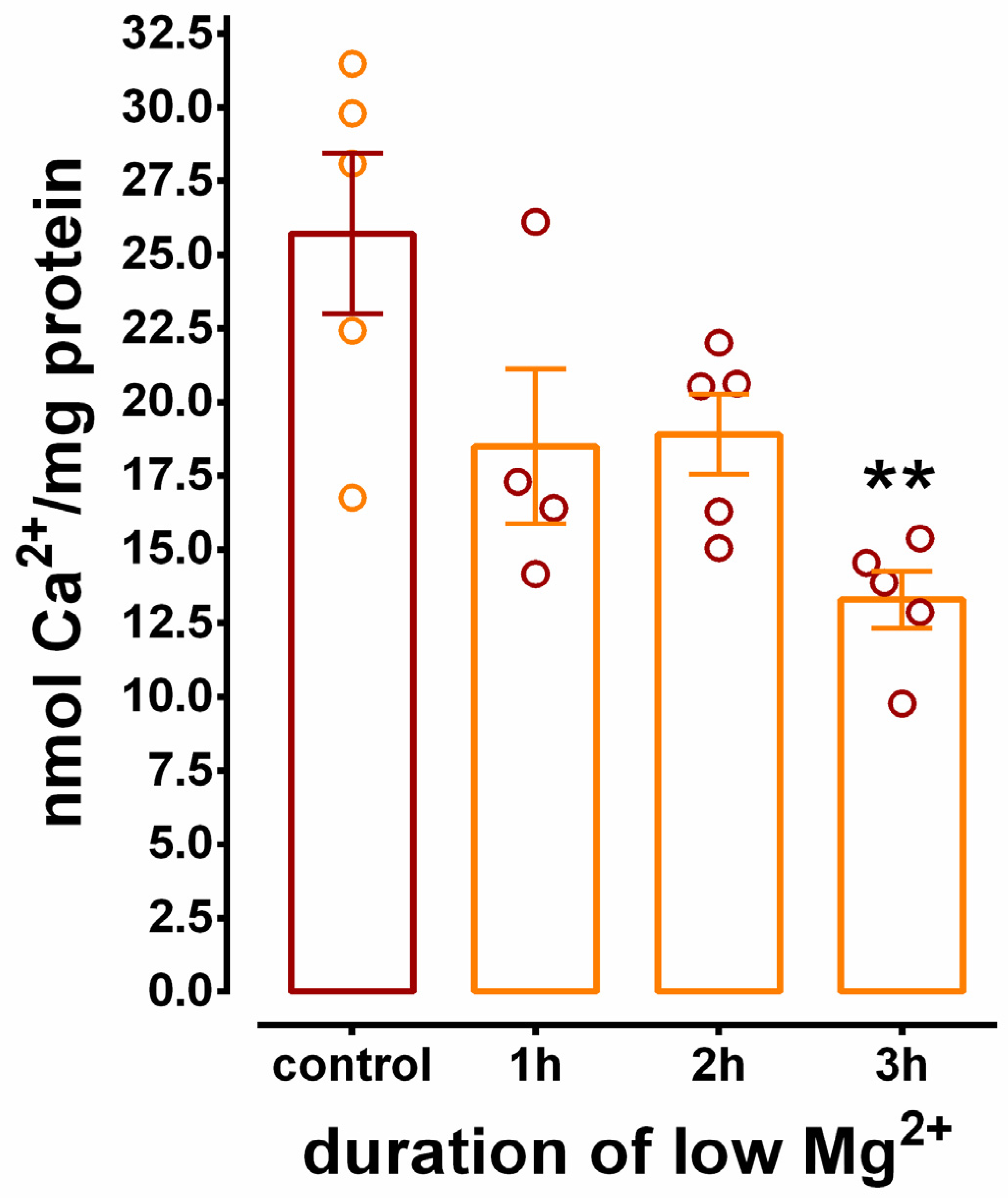
3.4. Inhibition of SERCA-Mediated Ca2+ Uptake in Hippocampal N+G Homogenates Is Duration Dependent
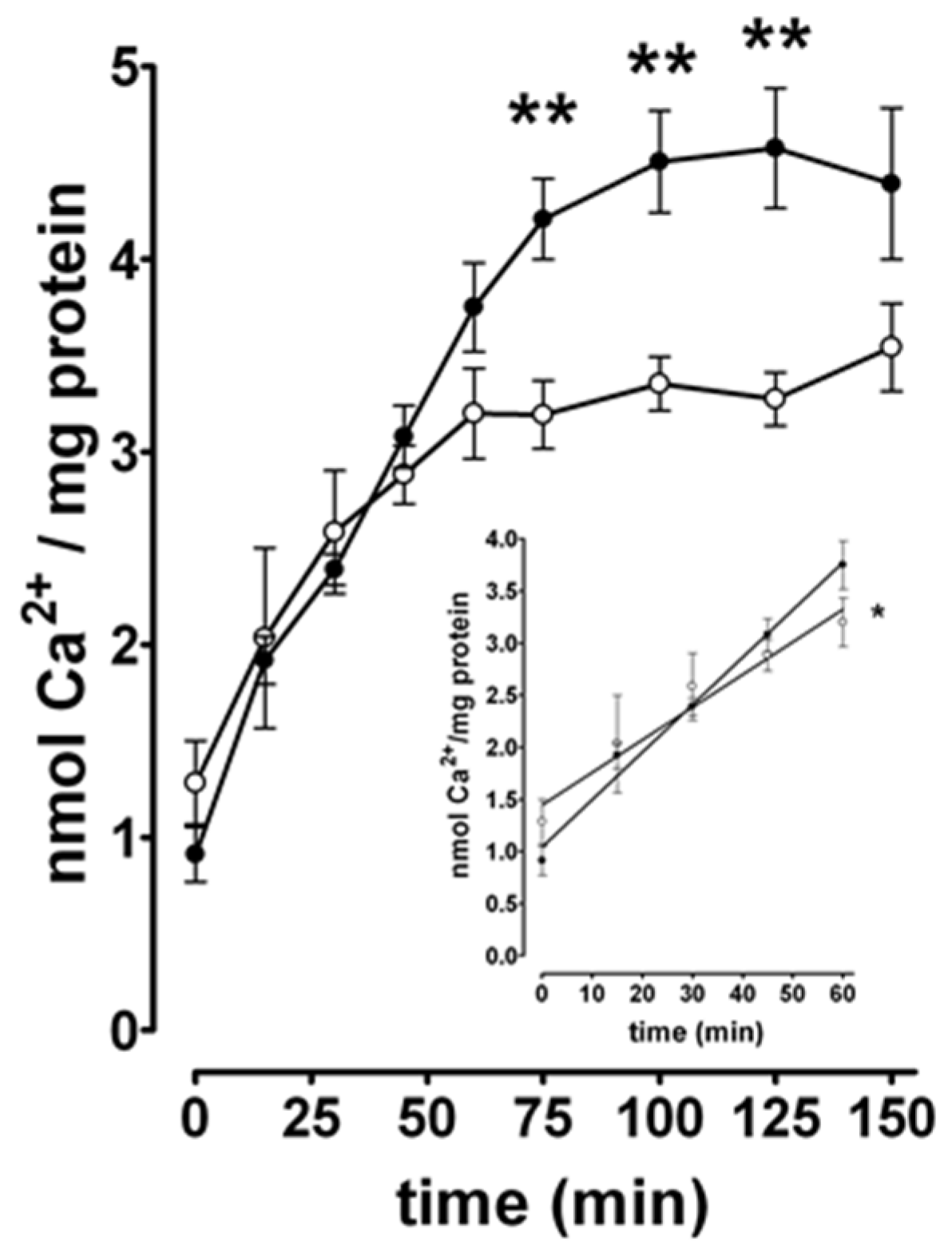
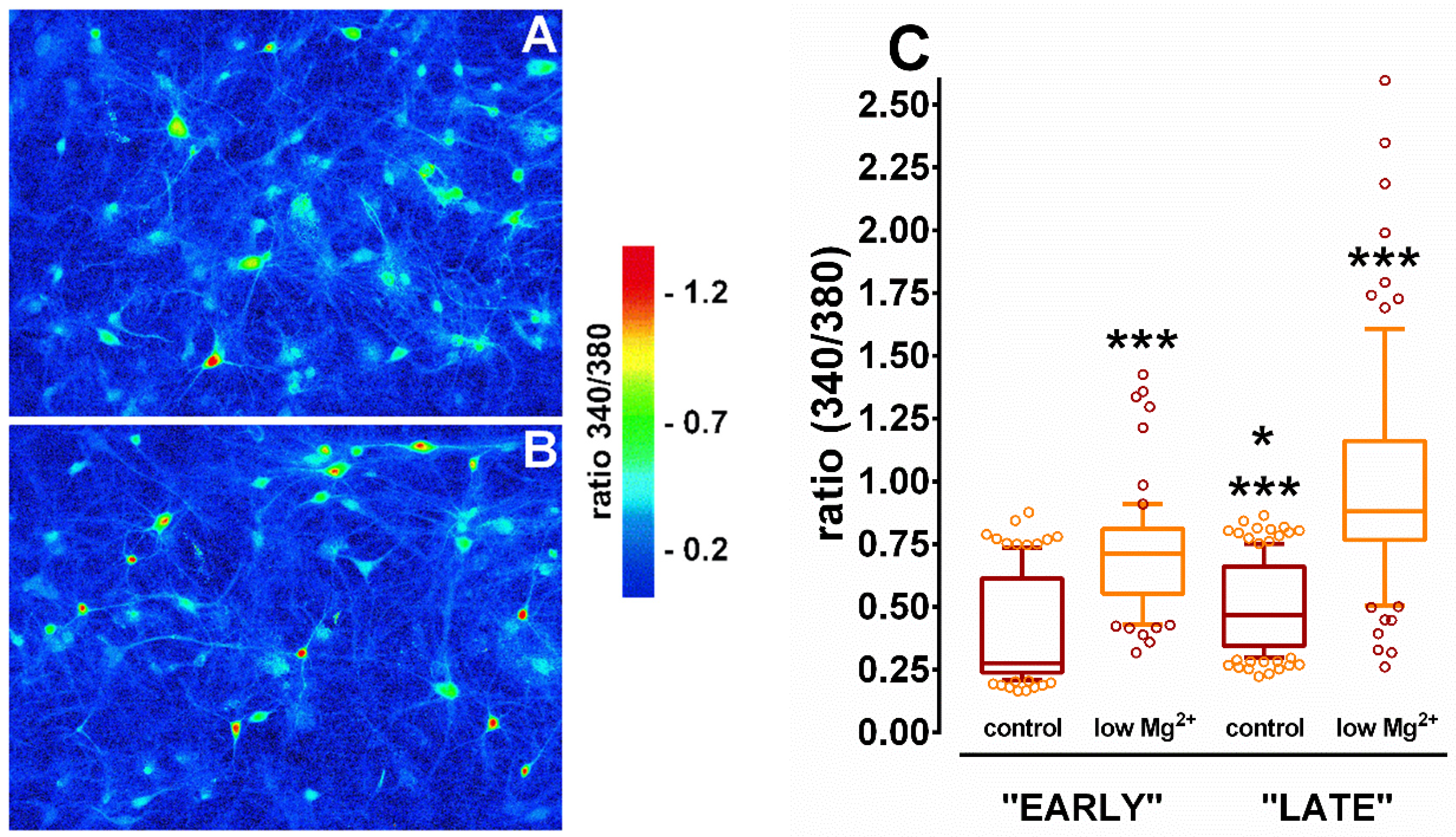
3.5. Changes in [Ca2+]i in Hippocampal Neurons During SE
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gurcharran, K.; Grinspan, Z.M. The burden of pediatric status epilepticus: Epidemiology, morbidity, mortality, and costs. Seizure 2019, 68, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.E.; Varelas, P.N. Status Epilepticus, refractory status epilepticus, and super-refractory status epilepticus. Contin. Lifelong Learn. Neurol. 2018, 24, 1683–1707. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Ribeiro, L.; Inacio, A.R.; Wieloch, T.; Duarte, C.B. GABA(A) receptor dephosphorylation followed by internalization is coupled to neuronal death in in vitro ischemia. Neurobiol. Dis. 2014, 65, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Calcium signaling during brain aging and its influence on the hippocampal synaptic plasticity. Adv. Exp. Med. Biol. 2020, 1131, 985–1012. [Google Scholar] [CrossRef]
- Huang, Y.Z.; He, X.P.; Krishnamurthy, K.; McNamara, J.O. TrkB-Shc signaling protects against hippocampal injury following status epilepticus. J. Neurosci. 2019, 39, 4624–4630. [Google Scholar] [CrossRef]
- Griffiths, T.; Evans, M.C.; Meldrum, B.S. Intracellular sites of early calcium accumulation in the rat hippocampus during status epilepticus. Neurosci. Lett. 1982, 30, 329–334. [Google Scholar] [CrossRef]
- Deshpande, L.S.; DeLorenzo, R.J. Novel therapeutics for treating organophosphate-induced status epilepticus co-morbidities, based on changes in calcium homeostasis. Neurobiol. Dis. 2020, 133, 104418. [Google Scholar] [CrossRef]
- Choi, H.S.; Lee, C.H. Time-course changes of hippocalcin expression in the mouse hippocampus following pilocarpine-induced status epilepticus. J. Vet. Sci. 2016, 17, 137–144. [Google Scholar] [CrossRef]
- Delorenzo, R.J.; Sun, D.A.; Deshpande, L.S. Cellular mechanisms underlying acquired epilepsy: The calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol. Ther. 2005, 105, 229–266. [Google Scholar] [CrossRef]
- Phillips, K.F.; Deshpande, L.S.; DeLorenzo, R.J. Hypothermia reduces mortality, prevents the calcium plateau, and is neuroprotective following status epilepticus in rats. Front. Neurol. 2018, 9, 438. [Google Scholar] [CrossRef]
- Xiang, L.; Ren, Y.; Cai, H.; Zhao, W.; Song, Y. MicroRNA-132 aggravates epileptiform discharges via suppression of BDNF/TrkB signaling in cultured hippocampal neurons. Brain Res. 2015, 1622, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Sombati, S.; Delorenzo, R.J. Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J. Neurophysiol. 1995, 73, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.E.; Sombati, S.; Lawrence, D.C.; McCay, B.D.; DeLorenzo, R.J. Epileptogenesis causes acute and chronic increases in GABAA receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of acquired epilepsy. J. Pharmacol. Exp. Ther. 2004, 310, 871–880. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzo, R.J.; Pal, S.; Sombati, S. Prolonged activation of the N-methyl-D-aspartate receptor-Ca2+ transduction pathway causes spontaneous recurrent epileptiform discharges in hippocampal neurons in culture. Proc. Natl. Acad. Sci. USA 1998, 95, 14482–14487. [Google Scholar] [CrossRef]
- Pal, S.; Sombati, S.; Limbrick, D.D., Jr.; DeLorenzo, R.J. In vitro status epilepticus causes sustained elevation of intracellular calcium levels in hippocampal neurons. Brain Res. 1999, 851, 20–31. [Google Scholar] [CrossRef]
- Handforth, A.; Treiman, D.M. Functional mapping of the early stages of status epilepticus: A 14C-2-deoxyglucose study in the lithium-pilocarpine model in rat. Neuroscience 1995, 64, 1057–1073. [Google Scholar] [CrossRef]
- Smith, Z.Z.; Benison, A.M.; Bercum, F.M.; Dudek, F.E.; Barth, D.S. Progression of convulsive and nonconvulsive seizures during epileptogenesis after pilocarpine-induced status epilepticus. J. Neurophysiol. 2018, 119, 1818–1835. [Google Scholar] [CrossRef]
- Blair, R.E.; Churn, S.B.; Sombati, S.; Lou, J.K.; DeLorenzo, R.J. Long-lasting decrease in neuronal Ca2+/calmodulin-dependent protein kinase II activity in a hippocampal neuronal culture model of spontaneous recurrent seizures. Brain Res. 1999, 851, 54–65. [Google Scholar] [CrossRef]
- Pedersen, P.L.; Carafoli, E. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem. Sci. 1987, 12, 146–150. [Google Scholar] [CrossRef]
- Parsons, J.T.; Churn, S.B.; DeLorenzo, R.J. Chronic inhibition of cortex microsomal Mg(2+)/Ca(2+) ATPase-mediated Ca(2+) uptake in the rat pilocarpine model following epileptogenesis. J. Neurochem. 2001, 79, 319–327. [Google Scholar] [CrossRef]
- Parsons, J.T.; Churn, S.B.; Kochan, L.D.; DeLorenzo, R.J. Pilocarpine-induced status epilepticus causes N-methyl-D-aspartate receptor-dependent inhibition of microsomal Mg(2+)/Ca(2+) ATPase-mediated Ca(2+) uptake. J. Neurochem. 2000, 75, 1209–1218. [Google Scholar] [CrossRef]
- Parsons, J.T.; Churn, S.B.; DeLorenzo, R.J. Ischemia-induced inhibition of calcium uptake into rat brain microsomes mediated by Mg2+/Ca2+ ATPase. J. Neurochem. 1997, 68, 1124–1134. [Google Scholar] [CrossRef]
- Parsons, J.T.; Sun, D.A.; DeLorenzo, R.J.; Churn, S.B. Neuronal-specific endoplasmic reticulum Mg(2+)/Ca(2+) ATPase Ca(2+) sequestration in mixed primary hippocampal culture homogenates. Anal. Biochem. 2004, 330, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.A.; Sombati, S.; Blair, R.E.; DeLorenzo, R.J. Calcium-dependent epileptogenesis in an in vitro model of stroke-induced “epilepsy”. Epilepsia 2002, 43, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Limbrick, D.D., Jr.; Churn, S.B.; Sombati, S.; DeLorenzo, R.J. Inability to restore resting intracellular calcium levels as an early indicator of delayed neuronal cell death. Brain Res. 1995, 690, 145–156. [Google Scholar] [CrossRef]
- Gibbs, J.W., 3rd; Sombati, S.; DeLorenzo, R.J.; Coulter, D.A. Physiological and pharmacological alterations in postsynaptic GABA(A) receptor function in a hippocampal culture model of chronic spontaneous seizures. J. Neurophysiol. 1997, 77, 2139–2152. [Google Scholar] [CrossRef][Green Version]
- Coulter, D.A.; Sombati, S.; DeLorenzo, R.J. Electrophysiology of glutamate neurotoxicity in vitro: Induction of a calcium-dependent extended neuronal depolarization. J. Neurophysiol. 1992, 68, 362–373. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflug. Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Limbrick, D.D., Jr.; Pal, S.; DeLorenzo, R.J. Hippocampal neurons exhibit both persistent Ca2+ influx and impairment of Ca2+ sequestration/extrusion mechanisms following excitotoxic glutamate exposure. Brain Res. 2001, 894, 56–67. [Google Scholar] [CrossRef]
- Pal, S.; Sun, D.; Limbrick, D.; Rafiq, A.; DeLorenzo, R.J. Epileptogenesis induces long-term alterations in intracellular calcium release and sequestration mechanisms in the hippocampal neuronal culture model of epilepsy. Cell Calcium 2001, 30, 285–296. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Morris, G.; Leite, M.; Kullmann, D.M.; Pavlov, I.; Schorge, S.; Lignani, G. Activity clamp provides insights into paradoxical effects of the anti-seizure drug carbamazepine. J. Neurosci. 2017, 37, 5484–5495. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, L.S.; DeLorenzo, R.J. Acetaminophen inhibits status epilepticus in cultured hippocampal neurons. Neuroreport 2011, 22, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Churn, S.B.; DeLorenzo, R.J. Global ischemia-induced inhibition of the coupling ratio of calcium uptake and ATP hydrolysis by rat whole brain microsomal Mg(2+)/Ca(2+) ATPase. Brain Res. 1999, 834, 32–41. [Google Scholar] [CrossRef]
- Miller, R.J. The control of neuronal Ca2+ homeostasis. Prog. Neurobiol. 1991, 37, 255–285. [Google Scholar] [CrossRef]
- Thastrup, O.; Cullen, P.J.; Drobak, B.K.; Hanley, M.R.; Dawson, A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. USA 1990, 87, 2466–2470. [Google Scholar] [CrossRef]
- Trotta, E.E.; de Meis, L. ATP-dependent calcium accumulation in brain microsomes. Enhancement by phosphate and oxalate. Biochim. Biophys. Acta 1975, 394, 239–247. [Google Scholar] [CrossRef]
- Xu, A.; Hawkins, C.; Narayanan, N. Phosphorylation and activation of the Ca(2+)-pumping ATPase of cardiac sarcoplasmic reticulum by Ca2+/calmodulin-dependent protein kinase. J. Biol. Chem. 1993, 268, 8394–8397. [Google Scholar]
- Lytton, J.; Westlin, M.; Burk, S.E.; Shull, G.E.; MacLennan, D.H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 1992, 267, 14483–14489. [Google Scholar]
- Bronstein, J.M.; Farber, D.B.; Wasterlain, C.G. Regulation of type-II calmodulin kinase: Functional implications. Brain Res. Rev. 1993, 18, 135–147. [Google Scholar] [CrossRef]
- Murray, K.D.; Gall, C.M.; Benson, D.L.; Jones, E.G.; Isackson, P.J. Decreased expression of the alpha subunit of Ca2+/ calmodulin-dependent protein kinase type II mRNA in the adult rat CNS following recurrent limbic seizures. Brain Res. Mol. Brain Res. 1995, 32, 221–232. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Yao, L.; Zhang, X.; Gan, D.; Jiang, M.; Wang, N.; Chen, G.; Wang, X. Altered norbin expression in patients with epilepsy and a rat model. Sci. Rep. 2017, 7, 13970. [Google Scholar] [CrossRef] [PubMed]
- Churn, S.B.; Limbrick, D.; Sombati, S.; DeLorenzo, R.J. Excitotoxic activation of the NMDA receptor results in inhibition of calcium/calmodulin kinase II activity in cultured hippocampal neurons. J. Neurosci. 1995, 15, 3200–3214. [Google Scholar] [CrossRef] [PubMed]
- Winder, D.G.; Sweatt, J.D. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat. Rev. Neurosci. 2001, 2, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Kurz, J.E.; Sheets, D.; Parsons, J.T.; Rana, A.; Delorenzo, R.J.; Churn, S.B. A significant increase in both basal and maximal calcineurin activity in the rat pilocarpine model of status epilepticus. J. Neurochem. 2001, 78, 304–315. [Google Scholar] [CrossRef]
- Kurz, J.E.; Rana, A.; Parsons, J.T.; Churn, S.B. Status epilepticus-induced changes in the subcellular distribution and activity of calcineurin in rat forebrain. Neurobiol. Dis. 2003, 14, 483–493. [Google Scholar] [CrossRef]
- Wang, B.; Stanford, K.R.; Kundu, M. ER-to-golgi trafficking and its implication in neurological diseases. Cells 2020, 9, 408. [Google Scholar] [CrossRef]
- Saito, A.; Imaizumi, K. The broad spectrum of signaling pathways regulated by unfolded protein response in neuronal homeostasis. Neurochem. Int. 2018, 119, 26–34. [Google Scholar] [CrossRef]
- Ren, Z.; Riley, N.J.; Needleman, L.A.; Sanders, J.M.; Swanson, G.T.; Marshall, J. Cell surface expression of GluR5 kainate receptors is regulated by an endoplasmic reticulum retention signal. J. Biol. Chem. 2003, 278, 52700–52709. [Google Scholar] [CrossRef]
- Kang, J.Q.; Macdonald, R.L. The GABAA receptor gamma2 subunit R43Q mutation linked to childhood absence epilepsy and febrile seizures causes retention of alpha1beta2gamma2S receptors in the endoplasmic reticulum. J. Neurosci. 2004, 24, 8672–8677. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshpande, L.S.; DeLorenzo, R.J.; Churn, S.B.; Parsons, J.T. Neuronal-Specific Inhibition of Endoplasmic Reticulum Mg2+/Ca2+ ATPase Ca2+ Uptake in a Mixed Primary Hippocampal Culture Model of Status Epilepticus. Brain Sci. 2020, 10, 438. https://doi.org/10.3390/brainsci10070438
Deshpande LS, DeLorenzo RJ, Churn SB, Parsons JT. Neuronal-Specific Inhibition of Endoplasmic Reticulum Mg2+/Ca2+ ATPase Ca2+ Uptake in a Mixed Primary Hippocampal Culture Model of Status Epilepticus. Brain Sciences. 2020; 10(7):438. https://doi.org/10.3390/brainsci10070438
Chicago/Turabian StyleDeshpande, Laxmikant S., Robert J. DeLorenzo, Severn B. Churn, and J. Travis Parsons. 2020. "Neuronal-Specific Inhibition of Endoplasmic Reticulum Mg2+/Ca2+ ATPase Ca2+ Uptake in a Mixed Primary Hippocampal Culture Model of Status Epilepticus" Brain Sciences 10, no. 7: 438. https://doi.org/10.3390/brainsci10070438
APA StyleDeshpande, L. S., DeLorenzo, R. J., Churn, S. B., & Parsons, J. T. (2020). Neuronal-Specific Inhibition of Endoplasmic Reticulum Mg2+/Ca2+ ATPase Ca2+ Uptake in a Mixed Primary Hippocampal Culture Model of Status Epilepticus. Brain Sciences, 10(7), 438. https://doi.org/10.3390/brainsci10070438




