Influence of Voluntary Contraction Level, Test Stimulus Intensity and Normalization Procedures on the Evaluation of Short-Interval Intracortical Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
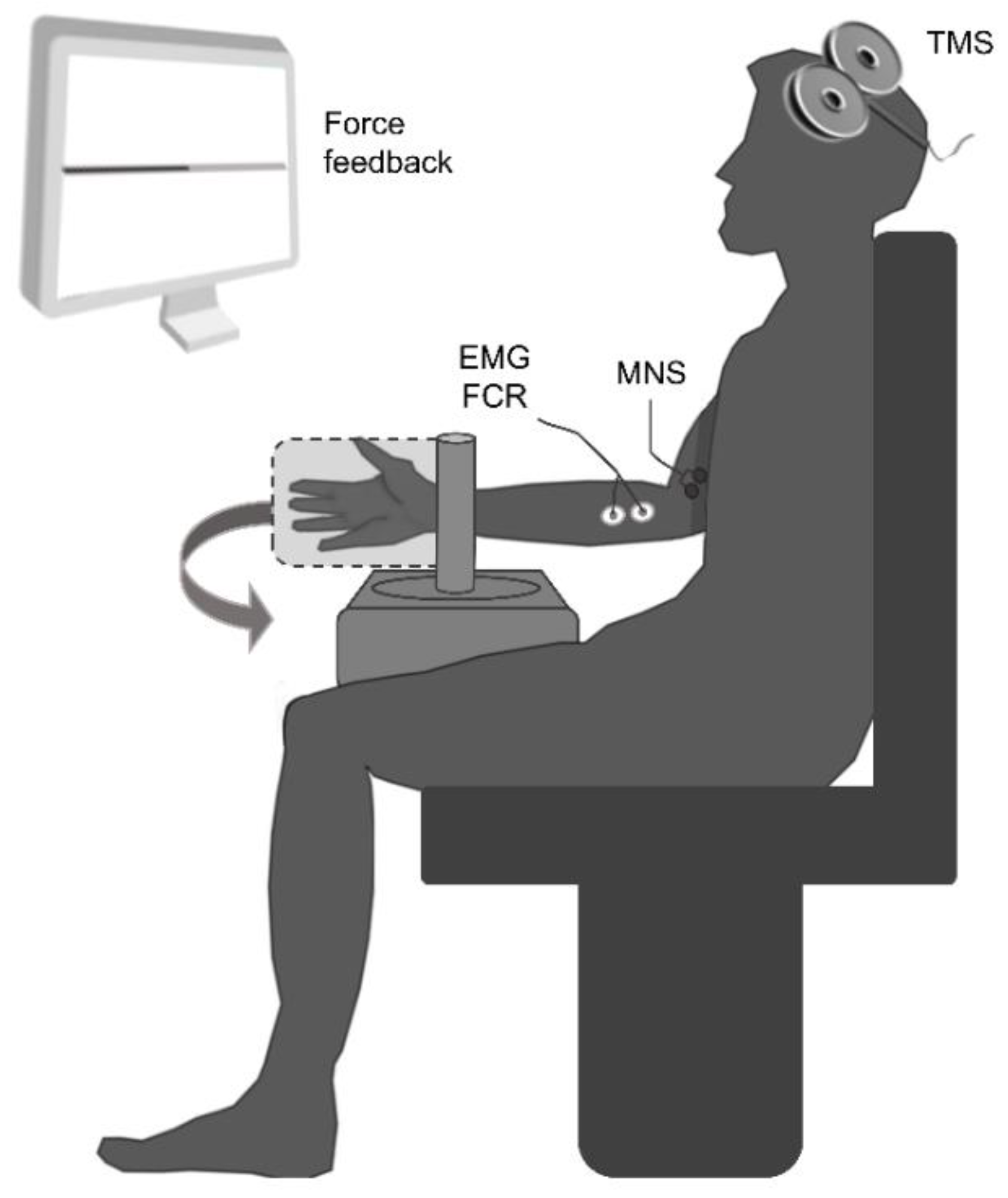
2.2. General Experimental Procedure
2.3. EMG Recordings
2.4. Electrical Stimulation Protocol
2.5. Transcranial Magnetic Stimulation Protocol
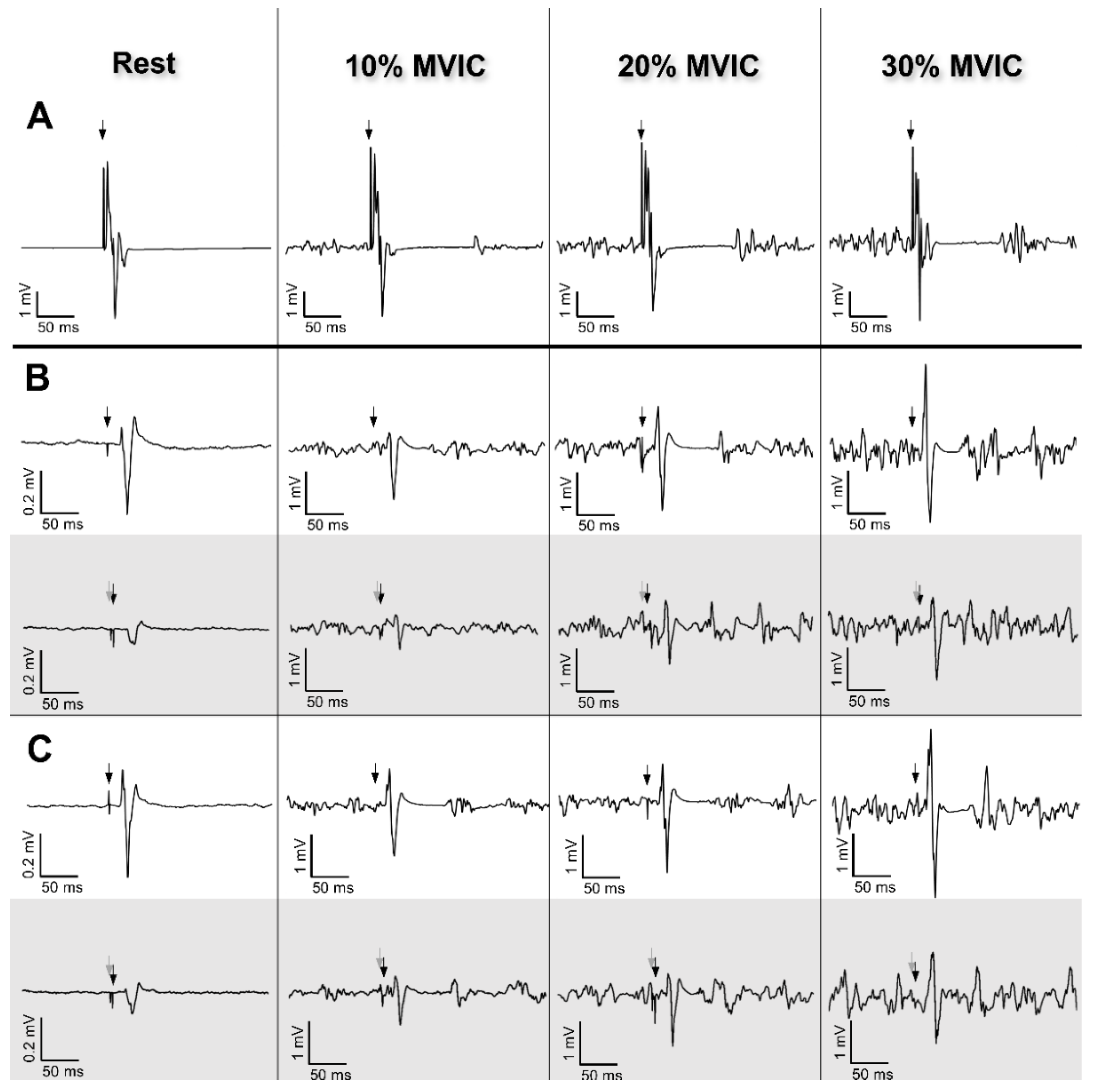
2.6. Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Methodological Considerations
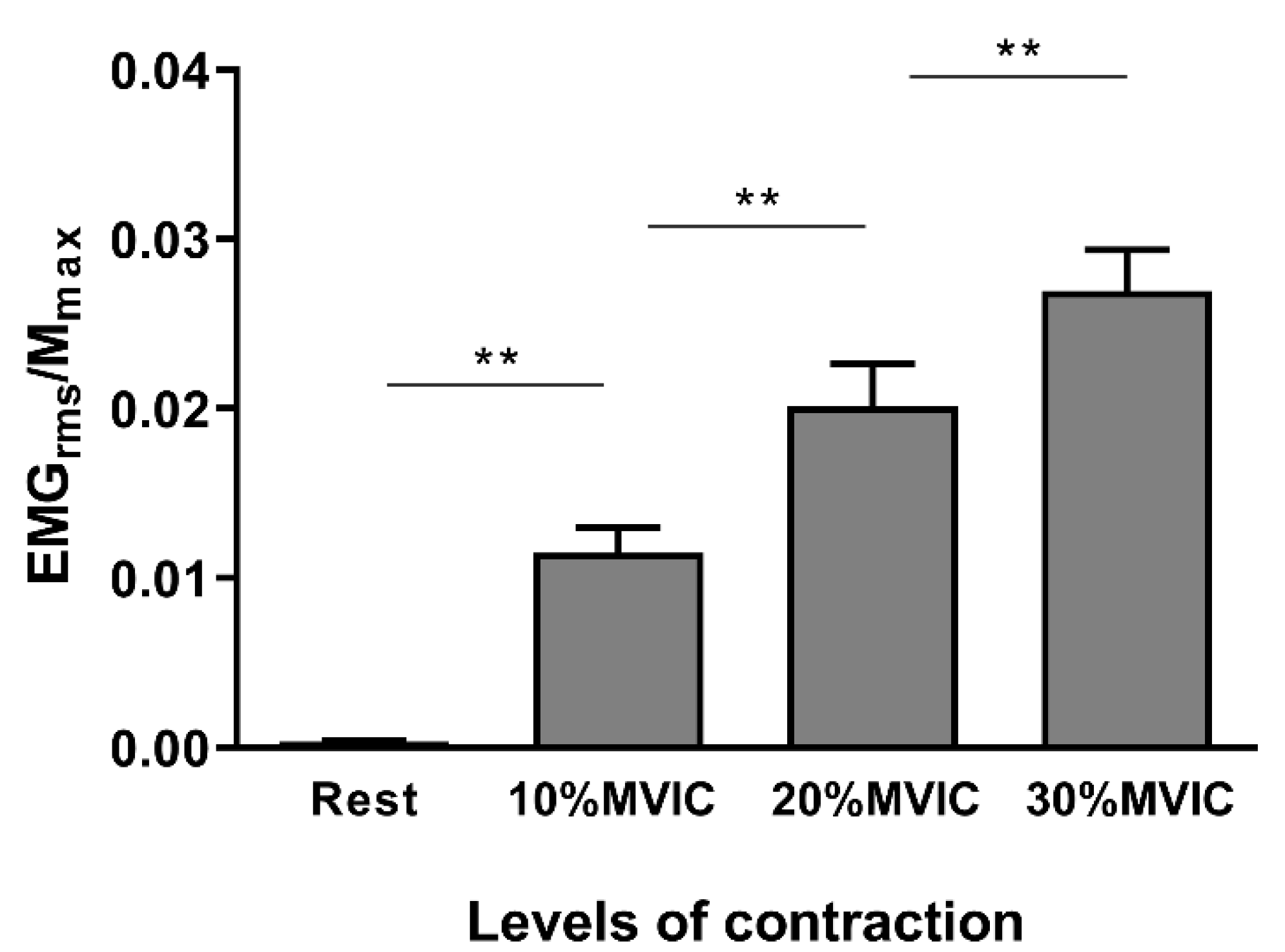
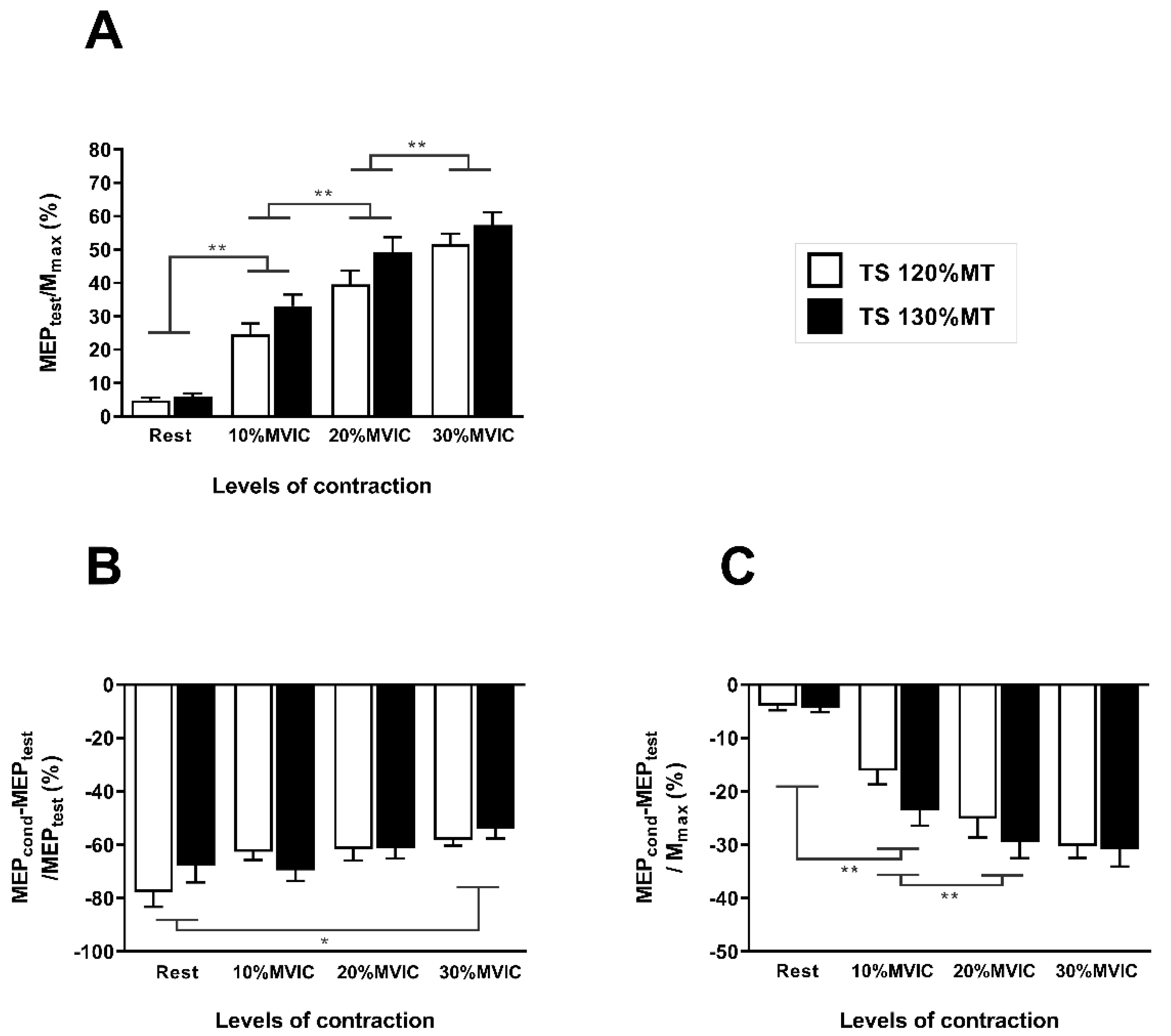
3.2. Neurophysiological Data
4. Discussion
4.1. Modulation of Corticospinal Excitability and SICIMEPtest
4.2. Modulation of SICIMmax and Its Relationship with MEPtest/Mmax Ratio
4.3. Methodological Recommendations
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Available
References
- Reis, J.; Swayne, O.B.; Vandermeeren, Y.; Camus, M.; Dimyan, M.A.; Harris-Love, M.; Perez, M.A.; Ragert, P.; Rothwell, J.C.; Cohen, L.G. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol. 2008, 586, 325–351. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, D.J.; Bonanno, D.R.; Frazer, A.K.; Howatson, G.; Pearce, A.J. Corticospinal responses following strength training: A systematic review and meta-analysis. Eur. J. Neurosci. 2017, 46, 2648–2661. [Google Scholar] [CrossRef] [PubMed]
- Weier, A.T.; Pearce, A.J.; Kidgell, D.J. Strength training reduces intracortical inhibition. Acta Physiol. 2012, 206, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Siddique, U.; Rahman, S.; Frazer, A.; Leung, M.; Pearce, A.J.; Kidgell, D.J. Task-dependent modulation of corticospinal excitability and inhibition following strength training. J. Electromyogr. Kinesiol. 2020, 52, 102411. [Google Scholar] [CrossRef]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical inhibition in human motor cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- Fisher, R.J.; Nakamura, Y.; Bestmann, S.; Rothwell, J.C.; Bostock, H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp. Brain Res. 2002, 143, 240–248. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Restuccia, D.; Oliviero, A.; Profice, P.; Ferrara, L.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp. Brain Res. 1998, 119, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Rothwell, J.; Capogna, M. Noninvasive stimulation of the human brain: Activation of multiple cortical circuits. Neuroscientist 2018, 24, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Lackmy, A.; Marchand-Pauvert, V. The estimation of short intra-cortical inhibition depends on the proportion of spinal motoneurones activated by corticospinal inputs. Clin. Neurophysiol. 2010, 121, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Garry, M.I.; Thomson, R.H.S. The effect of test TMS intensity on short-interval intracortical inhibition in different excitability states. Exp. Brain Res. 2009, 193, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sanger, T.D.; Garg, R.R.; Chen, R. Interactions between two different inhibitory systems in the human motor cortex. J. Physiol. 2001, 530, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Roshan, L.; Paradiso, G.O.; Chen, R. Two phases of short-interval intracortical inhibition. Exp. Brain Res. 2003, 151, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Grosprêtre, S.; Papaxanthis, C.; Martin, A. Modulation of spinal excitability by a sub-threshold stimulation of M1 area during muscle lengthening. Neuroscience 2014, 263, 60–71. [Google Scholar] [CrossRef]
- Opie, G.M.; Semmler, J.G. Modulation of short- and long-interval intracortical inhibition with increasing motor evoked potential amplitude in a human hand muscle. Clin. Neurophysiol. 2014, 125, 1440–1450. [Google Scholar] [CrossRef]
- Ridding, M.C.; Taylor, J.L.; Rothwell, J.C. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J. Physiol. 1995, 487, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ortu, E.; Deriu, F.; Suppa, A.; Tolu, E.; Rothwell, J.C. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J. Physiol. 2008, 586, 5147–5159. [Google Scholar] [CrossRef]
- Hendy, A.M.; Ekblom, M.M.; Latella, C.; Teo, W.P. Investigating the effects of muscle contraction and conditioning stimulus intensity on short-interval intracortical inhibition. Eur. J. Neurosci. 2019, 50, 3133–3140. [Google Scholar] [CrossRef]
- Zoghi, M.; Nordstrom, M.A. Progressive suppression of intracortical inhibition during graded isometric contraction of a hand muscle is not influenced by hand preference. Exp. Brain Res. 2007, 177, 266–274. [Google Scholar] [CrossRef]
- Rantalainen, T.; Weier, A.; Leung, M.; Brandner, C.; Spittle, M.; Kidgell, D. Short-interval intracortical inhibition is not affected by varying visual feedback in an isometric task in biceps brachii muscle. Front. Hum. Neurosci. 2013, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Rothwell, J.C.; Hallett, M.; Berardelli, A.; Eisen, A.; Rossini, P.; Paulus, W. Magnetic stimulation: Motor evoked potentials. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 97–103. [Google Scholar] [PubMed]
- Rossini, P.; Barker, A.; Berardelli, A. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef]
- Pfister, R.; Schwarz, K.; Carson, R.; Jancyzk, M. Easy methods for extracting individual regression slopes: Comparing SPSS, R, and Excel. Tutor. Quant. Methods Psychol. 2013, 9, 72–78. [Google Scholar] [CrossRef]
- Valls-Solé, J.; Pascual-Leone, A.; Brasil-Neto, J.P.; Cammarota, A.; McShane, A.; Hallett, M. Abnormal facilitation of the response to transcranial magnetic stimulation in patients with parkinson’s disease. Neurology 1994, 44, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Van den Bos, M.A.J.; Geevasinga, N.; Menon, P.; Burke, D.; Kiernan, M.C.; Vucic, S. Physiological processes influencing motor-evoked potential duration with voluntary contraction. J. Neurophysiol. 2017, 117, 1156–1162. [Google Scholar] [CrossRef]
- Zoghi, M.; Pearce, S.L.; Nordstrom, M.A. Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J. Physiol. 2003, 550, 933–946. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Restuccia, D.; Oliviero, A.; Profice, P.; Ferrara, L.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J. Physiol. 1998, 508, 625–633. [Google Scholar] [CrossRef]
- Terao, Y.; Ugawa, Y. Basic mechanisms of TMS. J. Clin. Neurophysiol. 2002, 19, 322–343. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Profice, P.; Ranieri, F.; Capone, F.; Dileone, M.; Oliviero, A.; Pilato, F. I-wave origin and modulation. Brain Stimul. 2012, 5, 512–525. [Google Scholar] [CrossRef]
- Hanajima, R.; Ugawa, Y.; Terao, Y.; Sakai, K.; Furubayashi, T.; Machii, K.; Kanazawa, I. Paired-pulse magnetic stimulation of the human motor cortex: Differences among I waves. J. Physiol. 1998, 509, 607–618. [Google Scholar] [CrossRef]
- Wagle-Shukla, A.; Ni, Z.; Gunraj, C.A.; Bahl, N.; Chen, R. Effects of short interval intracortical inhibition and intracortical facilitation on short interval intracortical facilitation in human primary motor cortex. J. Physiol. 2009, 587, 5665–5678. [Google Scholar] [CrossRef] [PubMed]
- Lackmy-Vallee, A.; Giboin, L.S.; Marchand-Pauvert, V. Non-linear input-output properties of the cortical networks mediating TMS-induced short-interval intracortical inhibition in humans. Eur. J. Neurosci. 2012, 35, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Classen, J.; Cohen, L.G.; Hallett, M. Task-dependent changes of intracortical inhibition. Exp. Brain Res. 1998, 118, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Lungholt, B.K.S.; Nyborg, K.; Nielsen, J.B. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp. Brain Res. 2004, 159, 197–205. [Google Scholar] [CrossRef]
- Goodwill, A.M.; Pearce, A.J.; Kidgell, D.J. Corticomotor plasticity following unilateral strength training. Muscle Nerve 2012, 46, 384–393. [Google Scholar] [CrossRef]
- Hendy, A.M.; Kidgell, D.J. Anodal tDCS applied during strength training enhances motor cortical plasticity. Med. Sci. Sports Exerc. 2013, 45, 1721–1729. [Google Scholar] [CrossRef]
- Mason, J.; Frazer, A.K.; Pearce, A.J.; Goodwill, A.M.; Howatson, G.; Jaberzadeh, S.; Kidgell, D.J. Determining the early corticospinal-motoneuronal responses to strength training: A systematic review and meta-analysis. Rev. Neurosci. 2019, 30, 463–476. [Google Scholar] [CrossRef]
- Berghuis, K.M.M.; Veldman, M.P.; Solnik, S.; Koch, G.; Zijdewind, I.; Hortobágyi, T. Neuronal mechanisms of motor learning and motor memory consolidation in healthy old adults. Age 2015, 37, 53. [Google Scholar] [CrossRef]
- Gruet, M.; Temesi, J.; Rupp, T.; Levy, P.; Millet, G.Y.; Verges, S. Stimulation of the motor cortex and corticospinal tract to assess human muscle fatigue. Neuroscience 2013, 231, 384–399. [Google Scholar] [CrossRef]
- Latella, C.; Hendy, A.; Vanderwesthuizen, D.; Teo, W.-P. The modulation of corticospinal excitability and inhibition following acute resistance exercise in males and females. Eur. J. Sport Sci. 2018, 18, 984–993. [Google Scholar] [CrossRef]
- Latella, C.; Teo, W.-P.; Harris, D.; Major, B.; VanderWesthuizen, D.; Hendy, A.M. Effects of acute resistance training modality on corticospinal excitability, intra-cortical and neuromuscular responses. Eur. J. Appl. Physiol. 2017, 117, 2211–2224. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Ziemann, U.; Lemon, R.N. State of the art: Physiology of transcranial motor cortex stimulation. Brain Stimul. 2008, 1, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.; Howatson, G.; Frazer, A.K.; Pearce, A.J.; Jaberzadeh, S.; Avela, J.; Kidgell, D.J. Modulation of intracortical inhibition and excitation in agonist and antagonist muscles following acute strength training. Eur. J. Appl. Physiol. 2019, 119, 2185–2199. [Google Scholar] [CrossRef]
- Nuzzo, J.L.; Barry, B.K.; Jones, M.D.; Gandevia, S.C.; Taylor, J.L. Effects of four weeks of strength training on the corticomotoneuronal pathway. Med. Sci. Sports Exerc. 2017, 49, 2286–2296. [Google Scholar] [CrossRef]
- Mason, J.; Frazer, A.; Horvath, D.M.; Pearce, A.J.; Avela, J.; Howatson, G.; Kidgell, D. Adaptations in corticospinal excitability and inhibition are not spatially confined to the agonist muscle following strength training. Eur. J. Appl. Physiol. 2017, 117, 1359–1371. [Google Scholar] [CrossRef]
- Wu, L.; Goto, Y.; Taniwaki, T.; Kinukawa, N.; Tobimatsu, S. Different patterns of excitation and inhibition of the small hand and forearm muscles from magnetic brain stimulation in humans. Clin. Neurophysiol. 2002, 113, 1286–1294. [Google Scholar] [CrossRef]
- Crone, C.; Johnsen, L.L.; Hultborn, H.; Orsnes, G.B. Amplitude of the maximum motor response (Mmax) in human muscles typically decreases during the course of an experiment. Exp. Brain Res. 1999, 124, 265–270. [Google Scholar] [CrossRef]
- Nuzzo, J.L.; Barry, B.K.; Gandevia, S.C.; Taylor, J.L. Acute strength training increases responses to stimulation of corticospinal axons. Med. Sci. Sports Exerc. 2016, 48, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Biabani, M.; Farrell, M.; Zoghi, M.; Egan, G.; Jaberzadeh, S. The minimal number of TMS trials required for the reliable assessment of corticospinal excitability, short interval intracortical inhibition, and intracortical facilitation. Neurosci. Lett. 2018, 674, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, J.; Spampinato, D.A.; Paraneetharan, V.; Rothwell, J.C. SICI during changing brain states: Differences in methodology can lead to different conclusions. Brain Stimul. 2020, 13, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Neige, C.; Rannaud Monany, D.; Stinear, C.M.; Byblow, W.D.; Papaxanthis, C.; Lebon, F. Unravelling the modulation of intracortical inhibition during motor imagery: An adaptive threshold-hunting study. Neuroscience 2020, 434, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Cheah, B.C.; Krishnan, A.V.; Burke, D.; Kiernan, M.C. The effects of alterations in conditioning stimulus intensity on short interval intracortical inhibition. Brain Res. 2009, 1273, 39–47. [Google Scholar] [CrossRef]
- Stagg, C.J.; Bestmann, S.; Constantinescu, A.O.; Moreno Moreno, L.; Allman, C.; Mekle, R.; Woolrich, M.; Near, J.; Johansen-Berg, H.; Rothwell, J.C. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol. 2011, 589, 5845–5855. [Google Scholar] [CrossRef] [PubMed]
- Peurala, S.H.; Müller-Dahlhaus, J.F.M.; Arai, N.; Ziemann, U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF). Clin. Neurophysiol. 2008, 119, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, J.; Byblow, W.D. Threshold tracking primary motor cortex inhibition: The influence of current direction. Eur. J. Neurosci. 2016, 44, 2614–2621. [Google Scholar] [CrossRef]

| Variables | Levels of Muscle Contraction (%MVIC) | |||
|---|---|---|---|---|
| Rest | 10% | 20% | 30% | |
| MT (%MSO) | 40.87 ± 7.3 ** | 31.73 ± 8.1 | 31.53 ± 7.2 | 31.27 ± 7 |
| EMGrms MEP (μV) | 1.97 ± 0.8 | 67.18 ± 28.2 | 113.58 ± 46.5 | 160.32 ± 64.5 |
| EMGrms (%EMGrms-MVIC) | 0.31 ± 0.2 | 9.65 ± 5.7 | 16.06 ± 8.7 | 21.96 ± 10.5 |
| Mmax (mV) | 6.97 ± 3.7 | 6.82 ± 3.8 | 6.60 ± 3.7 | 6.70 ± 3.9 |
| EMGrms Mmax (μV) | 1.75 ± 0.6 | 65.04 ± 27.7 | 114.83 ± 61 | 160.73 ± 78.8 |
| Subject | Slope | Intercept | R2 | p Value | Range of the MEPtest/Mmax Ratios (%) |
|---|---|---|---|---|---|
| 1 | −0.553 | 0.118 | 0.911 | <0.001 | 1.19–46.9 |
| 2 | −0.798 | 0.508 | 0.944 | <0.001 | 2.97–32.91 |
| 3 | −0.593 | −0.320 | 0.879 | =0.001 | 7.54–46.85 |
| 4 | −0.546 | −1.474 | 0.895 | <0.001 | 1.23–46.74 |
| 5 | −0.518 | −1.143 | 0.806 | =0.002 | 6.40–45.38 |
| 6 | −0.693 | 0.728 | 0.941 | <0.001 | 1.11–47.68 |
| 7 | −0.725 | −1.884 | 0.943 | <0.001 | 6.07–72.37 |
| 8 | −0.455 | −6.172 | 0.848 | =0.001 | 10.66–74.25 |
| 9 | −0.626 | −1.282 | 0.898 | <0.001 | 2.44–69.65 |
| 10 | −0.486 | −3.472 | 0.947 | <0.001 | 8.28–62.33 |
| 11 | −0.610 | 0.231 | 0.961 | <0.001 | 1.52–58.23 |
| 12 | −0.466 | −2.872 | 0.856 | =0.001 | 5.38–49.67 |
| 13 | −0.405 | −5.942 | 0.791 | =0.003 | 7.29–66.39 |
| 14 | −0.385 | −1.949 | 0.921 | <0.001 | 1.75–79.66 |
| 15 | −0.671 | −1.563 | 0.937 | <0.001 | 5.84–89.71 |
| Mean | −0.569 | −1.706 | 0.898 | 4.65–59.25 | |
| SD | 0.120 | 2 | 0.053 | 3.09–15.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neige, C.; Grosprêtre, S.; Martin, A.; Lebon, F. Influence of Voluntary Contraction Level, Test Stimulus Intensity and Normalization Procedures on the Evaluation of Short-Interval Intracortical Inhibition. Brain Sci. 2020, 10, 433. https://doi.org/10.3390/brainsci10070433
Neige C, Grosprêtre S, Martin A, Lebon F. Influence of Voluntary Contraction Level, Test Stimulus Intensity and Normalization Procedures on the Evaluation of Short-Interval Intracortical Inhibition. Brain Sciences. 2020; 10(7):433. https://doi.org/10.3390/brainsci10070433
Chicago/Turabian StyleNeige, Cécilia, Sidney Grosprêtre, Alain Martin, and Florent Lebon. 2020. "Influence of Voluntary Contraction Level, Test Stimulus Intensity and Normalization Procedures on the Evaluation of Short-Interval Intracortical Inhibition" Brain Sciences 10, no. 7: 433. https://doi.org/10.3390/brainsci10070433
APA StyleNeige, C., Grosprêtre, S., Martin, A., & Lebon, F. (2020). Influence of Voluntary Contraction Level, Test Stimulus Intensity and Normalization Procedures on the Evaluation of Short-Interval Intracortical Inhibition. Brain Sciences, 10(7), 433. https://doi.org/10.3390/brainsci10070433





