Recent Advances in Antigen-Specific Immunotherapies for the Treatment of Multiple Sclerosis
Abstract
1. Introduction
2. Immunopathogenesis of MS
3. MS Therapies
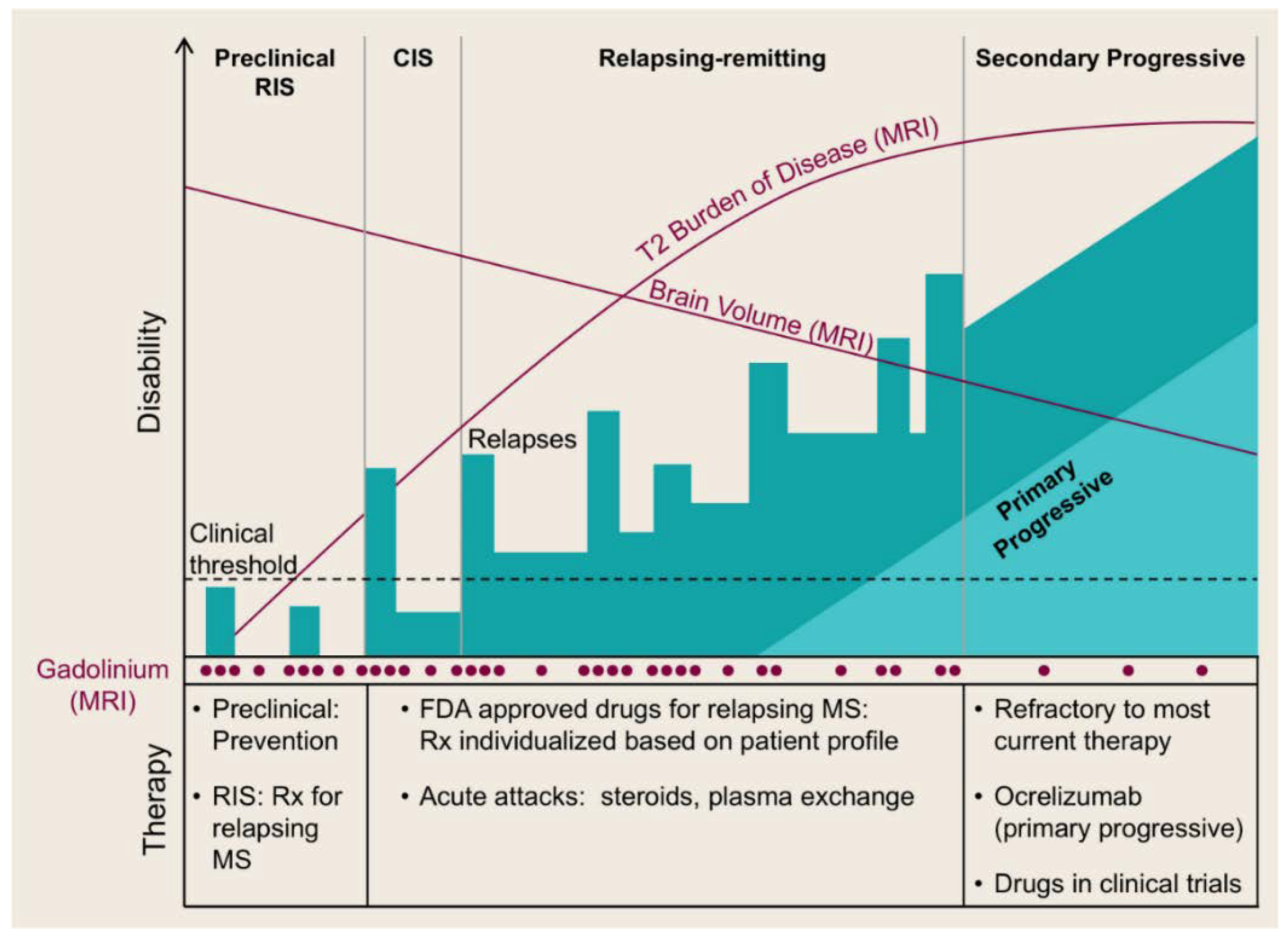
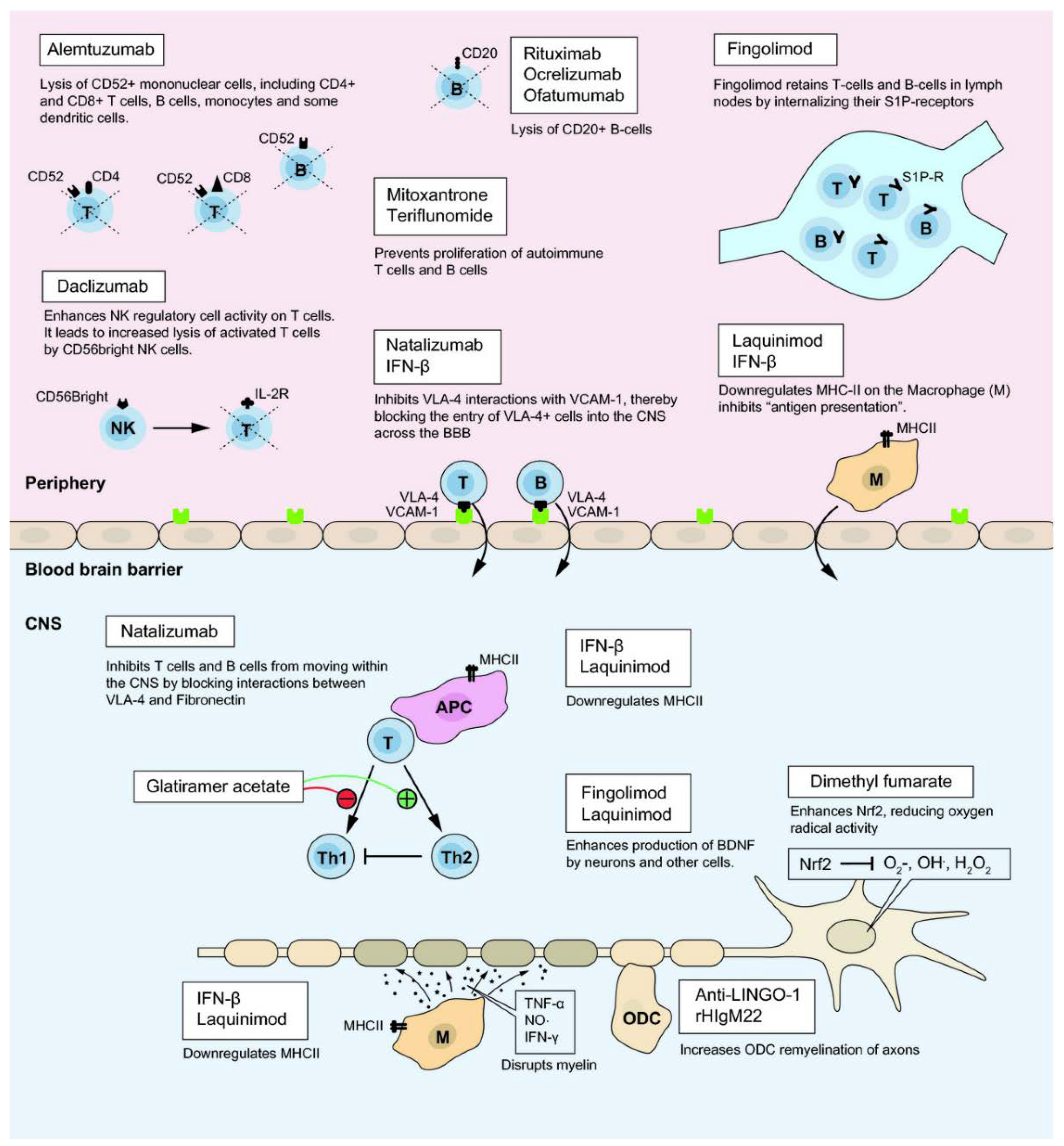
3.1. Disease-Modifying Therapies
3.2. Antigen-Specific Immunotherapies
4. In Vivo Assessment of Tolerance-Inducing Vaccination in MS
4.1. Animal Model of MS
4.2. Myelin Peptide-Based Vaccination
4.2.1. Immunodominant Myelin Petides
4.2.2. Altered Peptide Ligands (APLs)
4.2.3. Y-MSPc
4.2.4. Cytokine-Neuroantigen (NAg) Fusion Proteins
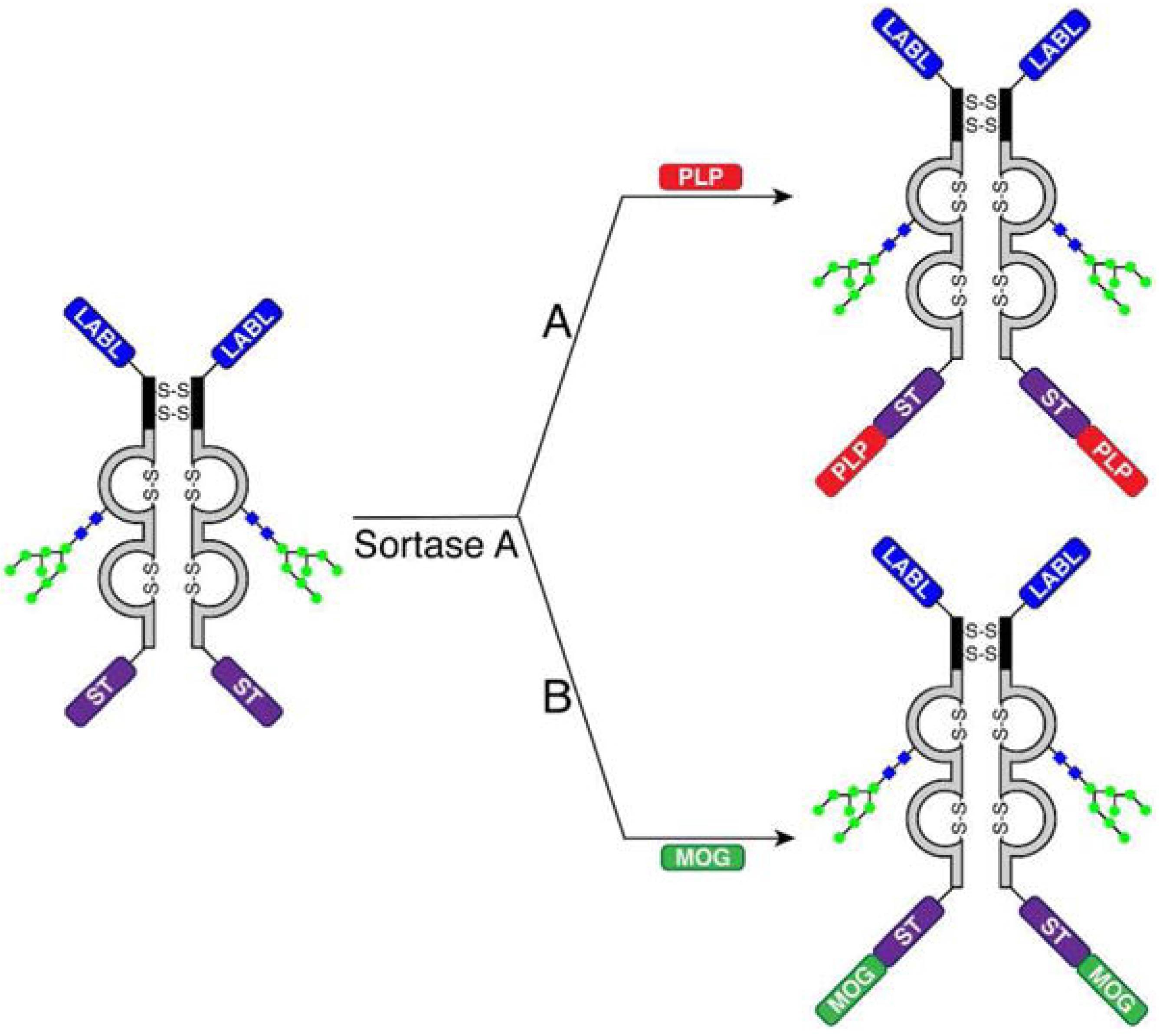
4.2.5. Antibodies Coupled with Myelin Peptides
4.2.6. Recombinant T-cell Receptor Ligands (RTLs)
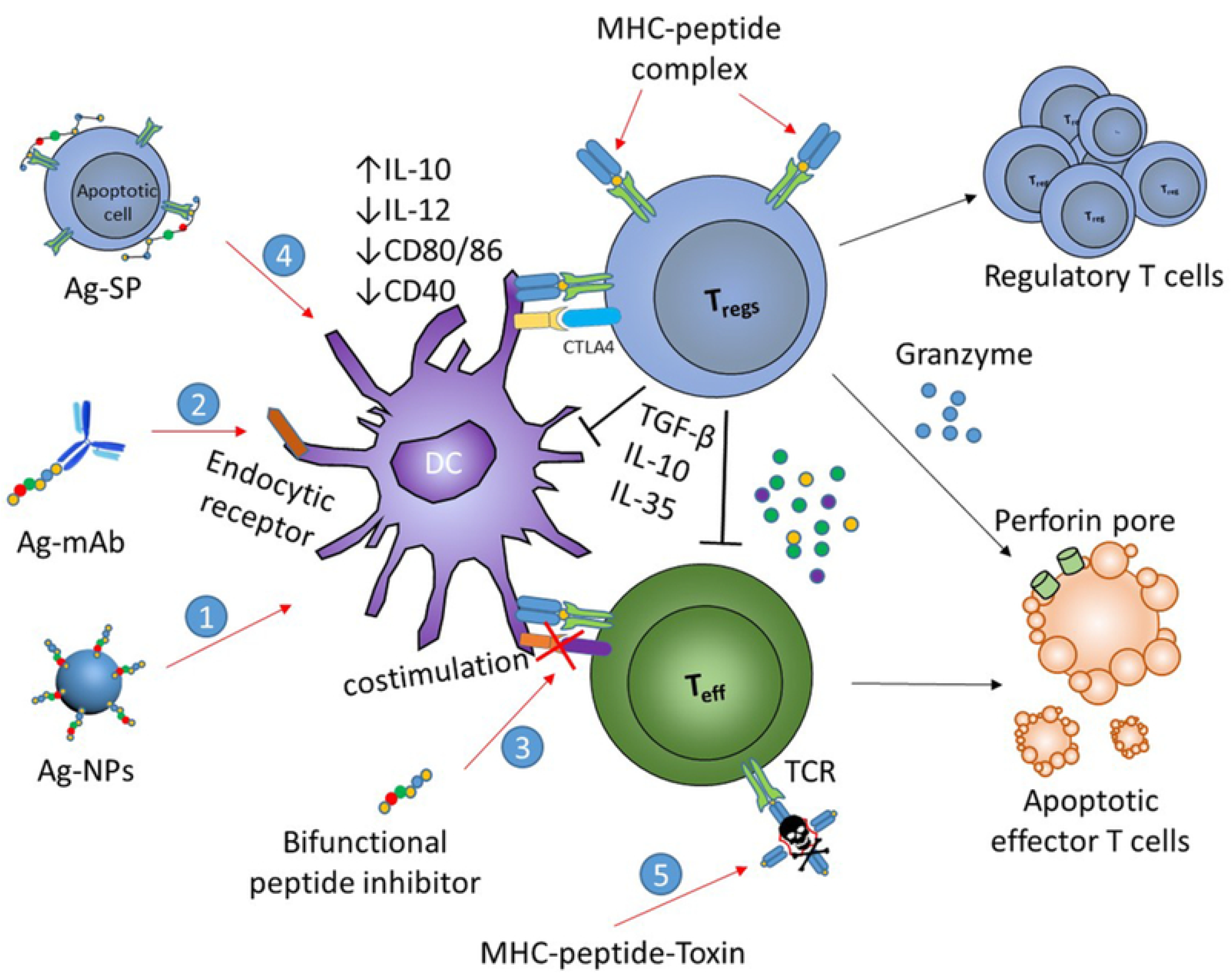
4.2.7. Bifunctional Peptide Inhibitors (BPIs)
4.2.8. Antigen-Drug Conjugates
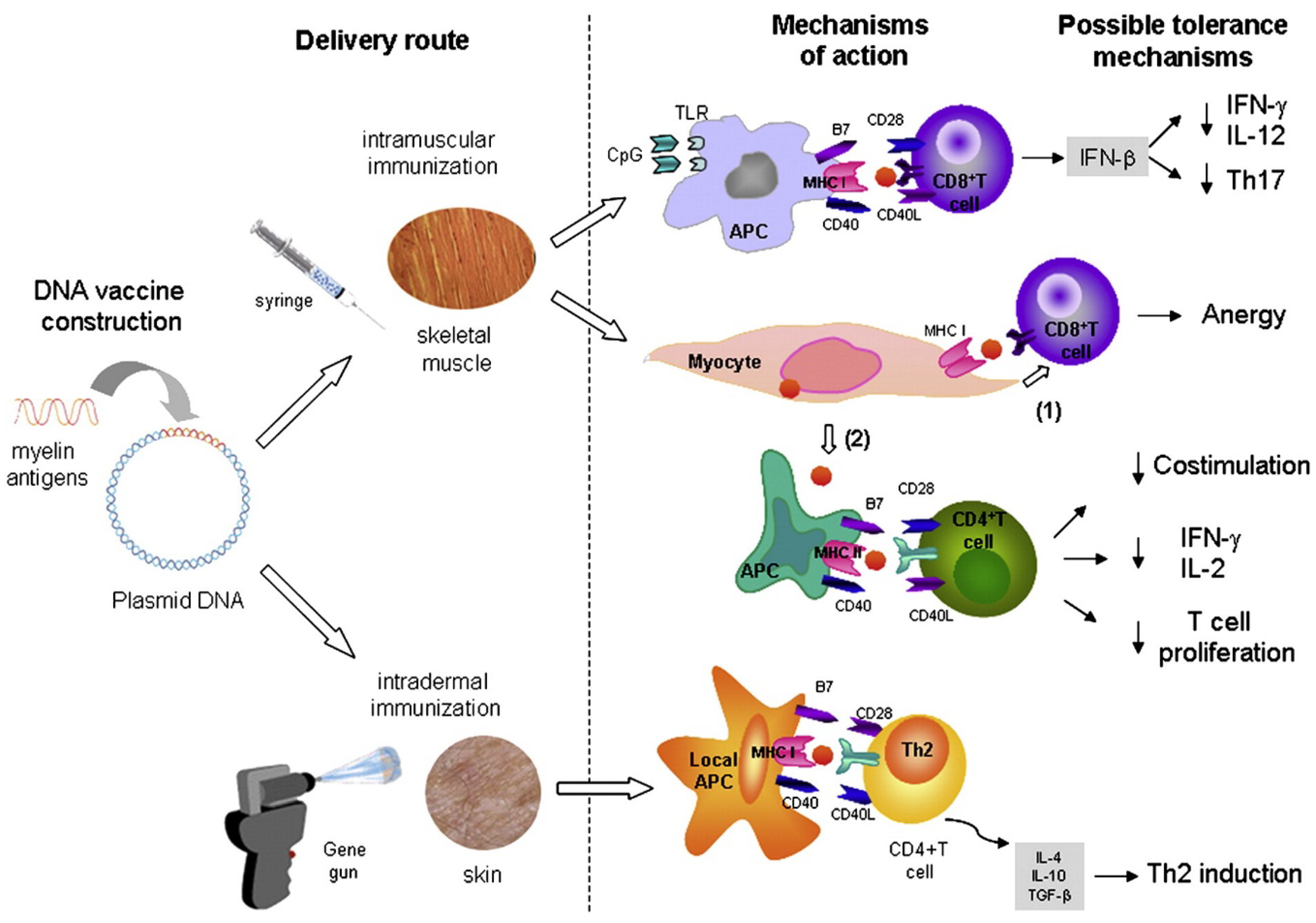
4.3. DNA Vaccination
4.4. Cell-Based Vaccination
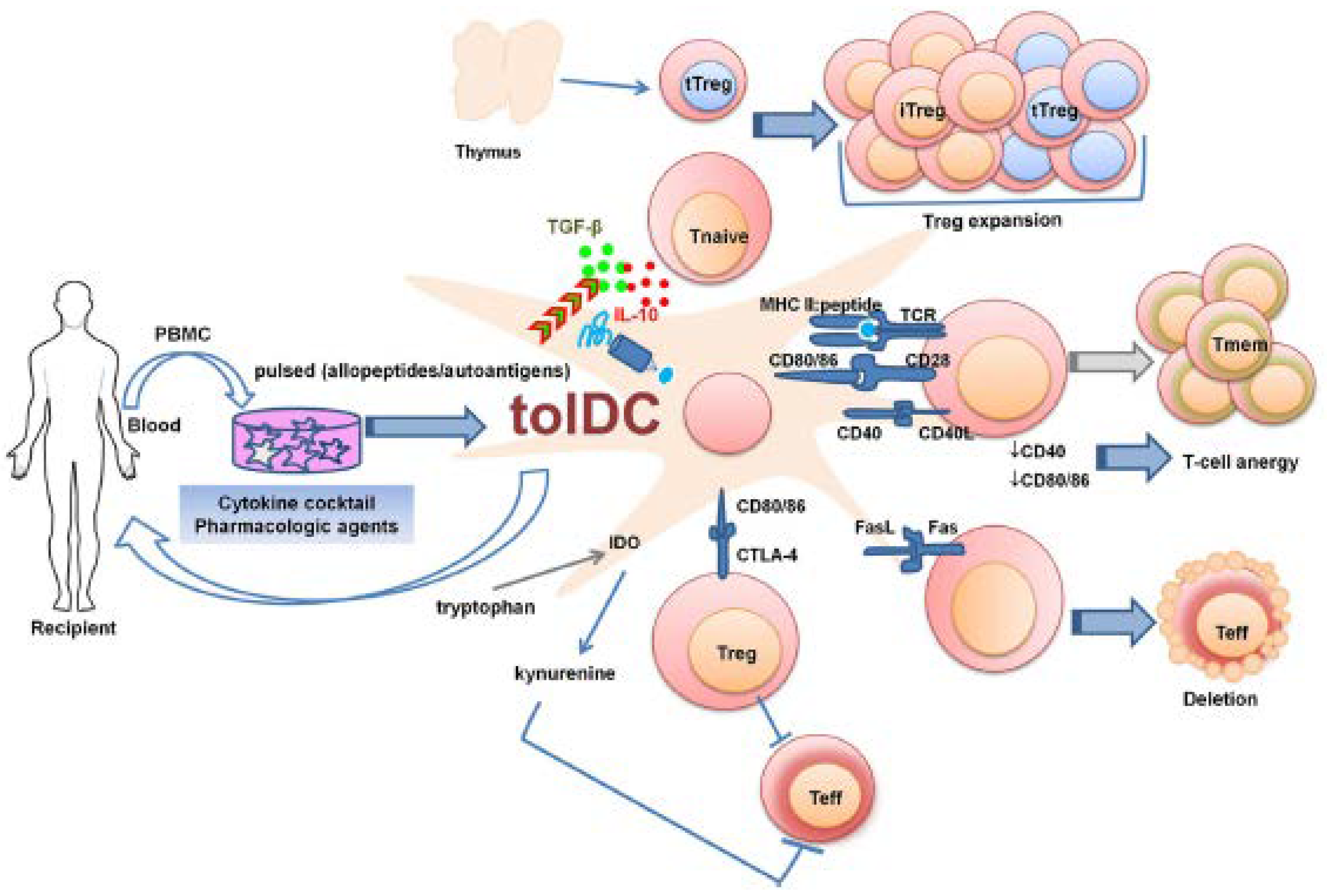
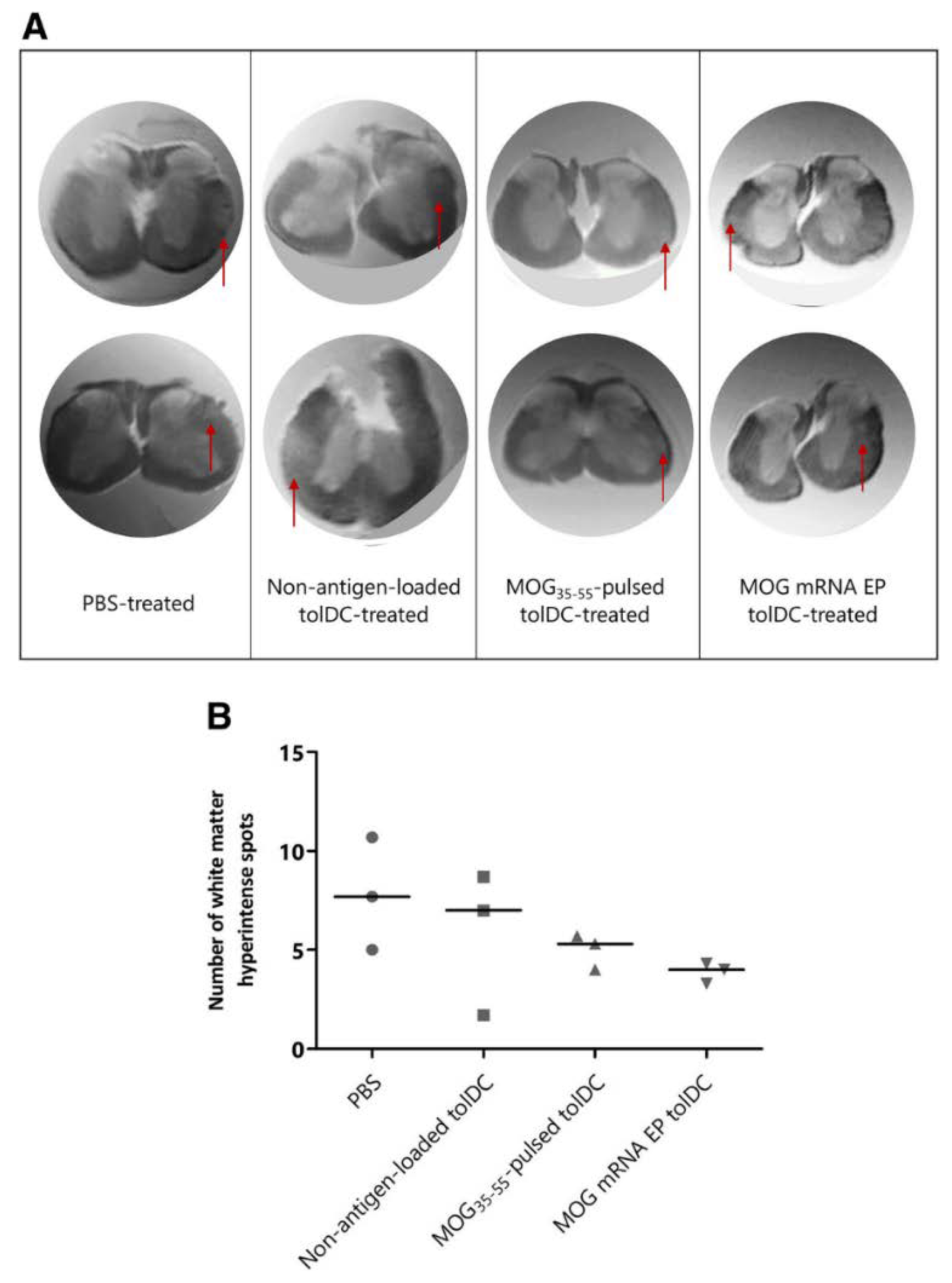
4.4.1. Antigen-Specific Tolerogenic Dendritic Cells (tolDCs)
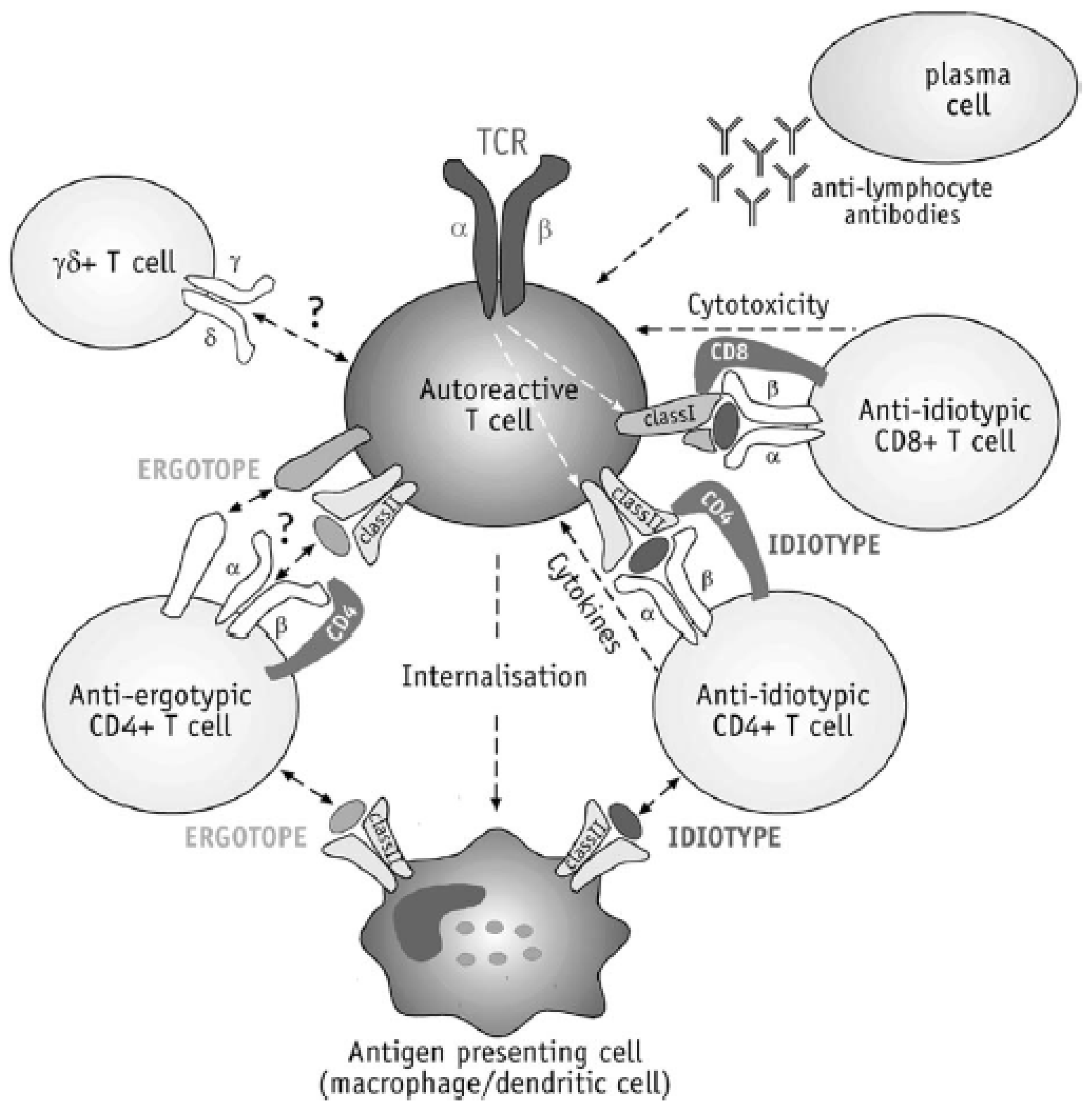
4.4.2. T Cell Vaccination (TCV)
4.4.3. Antigen-Coupled Cells
4.5. Carrier-Aided Vaccination
4.5.1. Polymer Particles
4.5.2. Soluble Antigen Arrays
4.5.3. Immune Polyelectrolyte Multilayers (iPEMs)
4.5.4. pMHC-Nanoparticles (pMHC-NPs)
4.5.5. Mannan-Peptide Conjugates
4.5.6. Liposomes
4.5.7. Microneedle Patches
5. Clinical Trials
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harrington, E.P.; Bergles, D.E.; Calabresi, P.A. Immune cell modulation of oligodendrocyte lineage cells. Neurosci. Lett. 2020, 715, 134601. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple sclerosis: Mechanisms and immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nature Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Afshar, B.; Khalifehzadeh-Esfahani, Z.; Seyfizadeh, N.; Danbaran, G.R.; Hemmatzadeh, M.; Mohammadi, H. The role of immune regulatory molecules in multiple sclerosis. J. Neuroimmunol. 2019, 337, 577061. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.M.; Pender, M.P. Myelin proteolipid protein: An effective autoantigen and target of autoimmunity in multiple sclerosis. J. Autoimmun. 2008, 31, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Iwanowski, P.; Losy, J. Immunological differences between classical phenothypes of multiple sclerosis. J. Neurol. Sci. 2015, 349, 10–14. [Google Scholar] [CrossRef]
- Lee, D.-H.; Linker, R.A. The role of myelin oligodendrocyte glycoprotein in autoimmune demyelination: A target for multiple sclerosis therapy? Expert Opin. Ther. Targets 2012, 16, 451–462. [Google Scholar] [CrossRef]
- Rangachari, M.; Kuchroo, V.K. Using EAE to better understand principles of immune function and autoimmune pathology. J. Autoimmun. 2013, 45, 31–39. [Google Scholar] [CrossRef]
- Lüssi, F.; Zipp, F.; Witsch, E. Dendritic cells as therapeutic targets in neuroinflammation. Cell. Mol. Life Sci. 2016, 73, 2425–2450. [Google Scholar] [CrossRef]
- Ho, P.P.; Fontoura, P.; Platten, M.; Sobel, R.A.; DeVoss, J.J.; Lee, L.Y.; Kidd, B.A.; Tomooka, B.H.; Capers, J.; Agrawal, A.; et al. A Suppressive oligodeoxynucleotide enhances the efficacy of myelin cocktail/IL-4-tolerizing DNA vaccination and treats autoimmune disease. J. Immunol. 2005, 175, 6226–6234. [Google Scholar] [CrossRef]
- Hemmer, B.; Nessler, S.; Zhou, D.; Kieseier, B.; Hartung, H.-P. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat. Clin. Prac. Neurol. 2006, 2, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Hellings, N.; Raus, J.; Stinissen, P. T-cell based immunotherapy in multiple sclerosis: Induction of regulatory immune networks by T-cell vaccination. Expert Rev. Clin. Immunol. 2006, 2, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, L.; Peng, L.; Qiu, W. TLR9 and its signaling pathway in multiple sclerosis. J. Neurol. Sci. 2017, 373, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Willekens, B.; Cools, N. Beyond the magic bullet: Current progress of therapeutic vaccination in multiple sclerosis. CNS Drugs 2018, 32, 401–410. [Google Scholar] [CrossRef]
- Skaper, S.D. Chapter 4—Oligodendrocyte precursor cells as a therapeutic target for demyelinating diseases, Prog. Brain Res. 2019, 245, 119–144. [Google Scholar] [CrossRef]
- Gholamzad, M.; Ebtekar, M.; Ardestani, M.S.; Azimi, M.; Mahmodi, Z.; Mousavi, M.J.; Aslani, S. A comprehensive review on the treatment approaches of multiple sclerosis: Currently and in the future. Inflamm. Res. 2019, 68, 25–38. [Google Scholar] [CrossRef]
- Derfuss, T. Personalized medicine in multiple sclerosis: Hope or reality? BMC Medicine. 2012, 10, 116. [Google Scholar] [CrossRef]
- Lassmann, H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front. Immunol. 2019, 9, 3116. [Google Scholar] [CrossRef]
- Xie, Z.-X.; Zhang, H.-L.; Wu, X.-J.; Zhu, J.; Ma, D.-H.; Jin, T. Role of the immunogenic and tolerogenic subsets of dendritic cells in multiple sclerosis. Mediat. Inflamm. 2015, 20, 513295. [Google Scholar] [CrossRef]
- Rostami, A.; Ciric, B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J. Neurol. Sci. 2013, 333, 76–87. [Google Scholar] [CrossRef]
- Baldassari, L.E.; Fox, R.J. Therapeutic advances and challenges in the treatment of progressive multiple sclerosis. Drugs 2018, 78, 1549–1566. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, R.; Howell, O.; Vora, A.; Serafini, B.; Nicholas, R.; Puopolo, M.; Reynolds, R.; Aloisi, F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007, 130, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Babaloo, Z.; Jadidi-Niaragh, F.; Ayromlou, H.; Sadreddini, S.; Yousefi, M. Multiple sclerosis: Therapeutic applications of advancing drug delivery systems. Biomed. Pharmacother. 2017, 86, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, K.W.; Strominger, J.L. Molecular mimicry in T cell-mediated autoimmunity: Viral peptides activate human T cell clones specific for myelin basic protein. Cell 1995, 80, 695–705. [Google Scholar] [CrossRef]
- Fujinami, R.S.; von Herrath, M.G.; Christen, U.; Whitton, J.L. Molecular mimicry, bystander activation, or viral persistence: Infections and autoimmune disease. Clin. Microbiol. Rev. 2006, 19, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-S.; Shin, E.-C. The activation of bystander CD8+ T cells and their roles in viral infection. Exp. Mol. Med. 2019, 51, 154. [Google Scholar] [CrossRef]
- Giacomini, P.S.; Bar-Or, A. Antigen-specific therapies in multiple sclerosis. Expert Opin. Emerg. Drugs 2009, 14, 551–560. [Google Scholar] [CrossRef]
- Szczepanik, M. Mechanisms of immunological tolerance to the antigens of the central nervous system. Skin-induced tolerance as a new therapeutic concept. J. Physiol. Pharmacol. 2011, 62, 159–165. [Google Scholar]
- Hellings, N.; Raus, J.; Stinissen, P. T-cell vaccination in multiple sclerosis: Update on clinical application and mode of action. Autoimmun. Rev. 2004, 3, 267–275. [Google Scholar] [CrossRef]
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokatlian, T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef]
- Selter, R.C.; Hemmer, B. Update on immunopathogenesis and immunotherapy in multiple sclerosis. Immunotargets Ther. 2013, 2, 21–30. [Google Scholar] [CrossRef]
- Lim, E.T.; Giovannoni, G. Immunopathogenesis and immunotherapeutic approaches in multiple sclerosis. Expert Rev. Neurother. 2005, 5, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadis, N.; van Pesch, V. A basic overview of multiple sclerosis immunopathology. Eur. J. Neurol. 2015, 22, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Sie, C.; Korn, T.; Mitsdoerffer, M. Th17 cells in central nervous system autoimmunity. Exp. Neurol. 2014, 262, 18–27. [Google Scholar] [CrossRef] [PubMed]
- García-González, P.; Ubilla-Olguín, G.; Catalán, D.; Schinnerling, K.; Aguillón, J.C. Tolerogenic dendritic cells for reprogramming of lymphocyte responses in autoimmune diseases. Autoimmun. Rev. 2016, 15, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- AGreenfield, L.; Hauser, S.L. B Cell therapy for multiple sclerosis: Entering an era. Ann. Neurol. 2018, 83, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-F.; Rudensky, A. Molecular orchestration of differentiation and function of regulatory T cells. Genes Dev. 2009, 23, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Gregori, S.; Goudy, K.S.; Roncarolo, M.G. The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front. Immunol. 2012, 3, 30. [Google Scholar] [CrossRef]
- Lu, W.; Chen, S.; Lai, C.; Lai, M.; Fang, H.; Dao, H.; Kang, J.; Fan, J.; Guo, W.; Fu, L.; et al. Suppression of HIV replication by CD8(+) regulatory T cells in elite controllers. Front. Immunol. 2016, 7, 134. [Google Scholar] [CrossRef]
- Vuddamalay, Y.; van Meerwijk, J.P.M. CD28- and CD28lowCD8+ regulatory T cells: Of mice and men. Front. Immunol. 2017, 8, 31. [Google Scholar] [CrossRef]
- Milo, R. Therapeutic strategies targeting B-cells in multiple sclerosis. Autoimmun. Rev. 2016, 15, 714–718. [Google Scholar] [CrossRef]
- Zhang, Y.; Salter, A.; Wallström, E.; Cutter, G.; Stüve, O. Evolution of clinical trials in multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Dargahi, N.; Katsara, M.; Tselios, T.; Androutsou, M.-E.; de Courten, M.; Matsoukas, J.; Apostolopoulos, V. Multiple sclerosis: Immunopathology and treatment update. Brain Sci. 2017, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Gentile, A.; Musella, A.; de Vito, F.; Rizzo, F.R.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Bassi, M.S.; Buttari, F.; et al. Immunomodulatory effects of exercise in experimental multiple sclerosis. Front. Immunol. 2019, 10, 2197. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, R.A.D.; Pinschewer, D.D.; Merkler, D. Immunological mechanism of action and clinical profile of disease-modifying treatments in multiple sclerosis. CNS Drugs 2014, 28, 535–558. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Cross, A.H.; Naismith, R.T. Established and novel disease-modifying treatments in multiple sclerosis. J. Intern. Med. 2014, 275, 350–363. [Google Scholar] [CrossRef]
- Piehl, F. A changing treatment landscape for multiple sclerosis: Challenges and opportunities. J. Intern. Med. 2014, 275, 364–381. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Carter, J.L. Multiple Sclerosis: Current and Emerging Disease-Modifying Therapies and Treatment Strategies. Mayo Clin. Proc. 2014, 89, 225–240. [Google Scholar] [CrossRef]
- Tramacere, I.; del Giovane, C.; Salanti, G.; D’Amico, R.; Pacchetti, I.; Filippini, G. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: A network meta-analysis. Cochrane Database Syst. Rev. 2015, 9, CD011381. [Google Scholar] [CrossRef]
- Wraith, D.C. The future of immunotherapy: A 20-year perspective. Front. Immunol. 2017, 8, 1668. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, E.; Patti, F.; Zanghì, A.; Zappia, M. A personalized approach in progressive multiple sclerosis: The current status of disease modifying therapies (DMTs) and future perspectives. Int. J. Mol. Sci. 2016, 17, 1725. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, J.R.; Cross, A.H. Disease-Modifying Treatment in Progressive Multiple Sclerosis. Curr. Treat. Options Neurol. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Targets of therapy in progressive MS. Mult. Scler. 2017, 23, 1593–1599. [Google Scholar] [CrossRef]
- Novartis Receives FDA Approval for Mayzent® (Siponimod), the First Oral Drug to Treat Secondary Progressive MS with Active Disease. Available online: https://novartis.gcs-web.com/Novartis-receives-FDA-approval-for-Mayzent-siponimod-the-first-oral-drug-to-treat-secondary-progressive-MS-with-active-disease?_ga=2.241998658.1110943223.1587297344-1758107691.1587297344 (accessed on 14 April 2020).
- Zeposia (Ozanimod). Available online: https://multiplesclerosisnewstoday.com/zeposia-ozanimod-rpc1063-rrms/ (accessed on 14 April 2020).
- Cladribine. Available online: https://en.wikipedia.org/wiki/Cladribine (accessed on 14 April 2020).
- Wildner, P.; Selmaj, K.W. Multiple sclerosis: Skin-induced antigen-specific immune tolerance. J. Neuroimmunol. 2017, 311, 49–58. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Antigen-Specific Therapies in Multiple Sclerosis. Int. Rev. Immunol. 2005, 24, 393–413. [Google Scholar] [CrossRef]
- Blanchfield, J.L. Antigen-specific tolerogenic vaccines inhibit autoimmune disease in a rodent model of multiple sclerosis. Ph.D. Thesis, The Faculty of the Department of Microbiology and Immunology Brody School of Medicine at East Carolina University, Greenville, USA, 2010. [Google Scholar]
- Pickens, C.J.; Christopher, M.A.; Leon, M.A.; Pressnall, M.M.; Johnson, S.N.; Thati, S.; Sullivan, B.P.; Berkland, C. Antigen-drug conjugates as a novel therapeutic class for the treatment of antigen-specific autoimmune disorders. Mol. Pharm. 2019, 16, 2452–2461. [Google Scholar] [CrossRef]
- Chunsong, Y.; Jingchao, X.; Meng, L.; Myunggi, A.; Haipeng, L. Bioconjugate strategies for the induction of antigen-specific tolerance in autoimmune diseases. Bioconjug. Chem. 2018, 29, 29719–29732. [Google Scholar] [CrossRef]
- Mannie, M.D.; Curtis, A.D. II Tolerogenic vaccines for multiple sclerosis. Hum. Vac. Immunother. 2013, 9, 1032–1038. [Google Scholar] [CrossRef][Green Version]
- Yannakakis, M.P.; Tzoupis, H.; Michailidou, E.; Mantzourani, E.; Simal, C.; Tselios, T. Molecular dynamics at the receptor level of immunodominant myelin oligodendrocyte glycoprotein 35–55 epitope implicated in multiple sclerosis. J. Mol. Graph. Model. 2016, 68, 78–86. [Google Scholar] [CrossRef]
- Lutterotti, A.; Martin, R. Antigen-specific tolerization approaches in multiple sclerosis. Expert Opin. Investig. Drugs 2014, 23, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Wraith, D. Antigen-specific immunotherapy. Nature 2016, 530, 422–423. [Google Scholar] [CrossRef] [PubMed]
- Spence, A.; Klementowicz, J.E.; Bluestone, J.A.; Tang, Q. Targeting Treg signaling for the treatment of autoimmune diseases. Curr. Opin. Immunol. 2015, 37, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sabatos-Peyton, C.A.; Verhagen, J.; Wraith, D.C. Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr. Opin. Immunol. 2010, 22, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. The re-emergence of antigen-specific tolerance as a potential therapy for MS. Mult. Scler. J. 2015, 21, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Comi, C.; Chiocchetti, A.; Dianzani, U. Exploiting PLGA-based biocompatible nanoparticles for next-generation tolerogenic vaccines against autoimmune disease. Int. J. Mol. Sci. 2019, 20, 204. [Google Scholar] [CrossRef]
- Vanderlugt, C.L.; Miller, S.D. Epitope spreading inimmune-mediated diseases: Implications for immunotherapy. Nat. Rev. Immunol. 2002, 2, 85–95. [Google Scholar] [CrossRef]
- Miller, S.D.; Turley, D.M.; Podojil, J.R. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol. 2007, 7, 665–677. [Google Scholar] [CrossRef]
- Lutterotti, A.; Sospedra, M.; Martin, R. Antigen-specific therapies in MS—Current concepts and novel approaches. J. Neurol. Sci. 2008, 274, 18–22. [Google Scholar] [CrossRef]
- Bielekova, B.; Goodwin, B.; Richert, N.; Cortese, I.; Kondo, T.; Afshar, G.; Grani, B.; Eaton, J.; Antel, J.; Frank, J.A.; et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 2000, 6, 1167–1175. [Google Scholar] [CrossRef]
- Turley, D.M.; Miller, S.D. Prospects for antigen-specific tolerance based therapies for the treatment of multiple sclerosis, Results Probl. Cell Differ. 2010, 51, 217–235. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.M.; Lalor, S.J.; Sweeney, C.M.; Tubridy, N.; Mills, K.H. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Libbey, J.E.; Fujinami, R.S. Experimental autoimmune encephalomyelitis as a testing paradigm for adjuvants and vaccines. Vaccine 2011, 29, 3356–3362. [Google Scholar] [CrossRef] [PubMed]
- Tabansky, I.; Keskin, D.B.; Watts, D.; Petzold, C.; Funaro, M.; Sands, W.; Wright, P.; Yunis, E.J.; Najjar, S.; Diamond, B.; et al. Targeting DEC-205−DCIR2+ dendritic cells promotes immunological tolerance in proteolipid protein-induced experimental autoimmune encephalomyelitis. Mol. Med. 2018, 24, 17. [Google Scholar] [CrossRef]
- Huang, X.; Lu, H.W.Q. The mechanisms and applications of T cell vaccination for autoimmune diseases: A comprehensive review. Clinic. Rev. Allerg. Immunol. 2014, 47, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Grau-López, L.; Raïch, D.; Ramo-Tello, C.; Naranjo-Gómez, M.; Dàvalos, A.; Pujol-Borrell, R.; Borràs, F.E.; Martínez-Cáceres, E. Myelin peptides in multiple sclerosis. Autoimmun. Rev. 2009, 8, 650–653. [Google Scholar] [CrossRef]
- Kuchroo, V.K.; Anderson, A.C.; Waldner, H.; Munder, M.; Bettelli, E.; Nicholson, L.B. T cell response in experimental autoimmune encephalomyelitis (EAE): Role of Self and Cross-Reactive Antigens in Shaping, Tuning, and Regulating the Autopathogenic T Cell Repertoire. Annu. Rev. Immunol. 2002, 20, 101–123. [Google Scholar] [CrossRef]
- Matsoukas, J.; Apostolopoulos, V.; Kalbacher, H.; Papini, A.-M.; Tselios, T.; Chatzantoni, K.; Biagioli, T.; Lolli, F.; Deraos, S.; Papathanassopoulos, P.; et al. Design and synthesis of a novel potent myelin basic protein epitope 87-99 cyclic analogue: Enhanced stability and biological properties of mimics render them a potentially new class of immunomodulators. J. Med. Chem. 2005, 48, 1470–1480. [Google Scholar] [CrossRef]
- Reindl, M.; Waters, P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat. Rev. Neurol. 2019, 15, 89–102. [Google Scholar] [CrossRef]
- Kaushansky, N.; Eisenstein, M.; Zilkha-Falb, R.; Ben-Nun, A. The myelin-associated oligodendrocytic basic protein (MOBP) as a relevant primary target autoantigen in multiple sclerosis. Autoimmun. Rev. 2010, 9, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Androutsou, M.E.; Tapeinou, A.; Vlamis-Gardikas, A.; Tselios, T. Myelin oligodendrocyte glycoprotein and multiple sclerosis. Med. Chem. 2018, 14, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Tselios, T.; Aggelidakis, M.; Tapeinou, A.; Tseveleki, V.; Kanistras, I.; Gatos, D.; Matsoukas, J. Rational design and synthesis of altered peptide ligands based on human myelin oligodendrocyte glycoprotein 35–55 epitope: Inhibition of chronic experimental autoimmune encephalomyelitis in mice. Molecules 2014, 19, 17968–17984. [Google Scholar] [CrossRef] [PubMed]
- Deraos, G.; Kritsi, E.; Matsoukas, M.-T.; Christopoulou, K.; Kalbacher, H.; Zoumpoulakis, P.; Apostolopoulos, V.; Matsoukas, J. Design of linear and cyclic mutant analogues of dirucotide peptide (MBP82–98) against multiple sclerosis: Conformational and binding studies to MHC Class II. Brain Sci. 2018, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Tapeinou, A.; Giannopoulou, E.; Hansen, C.S.B.E.; Kalofonos, H.; Apostolopoulosd, V.; Vlamis-Gardikas, A.; Tselios, T. Design, synthesis and evaluation of an anthraquinone derivative conjugated to myelin basic protein immunodominant (MBP85-99) epitope: Towards selective immunosuppression. Eur. J. Med. Chem. 2018, 143, 621–631. [Google Scholar] [CrossRef]
- Yannakakis, M.-P.; Simal, C.; Tzoupis, H.; Rodi, M.; Dargahi, N.; Prakash, M.; Mouzaki, A.; Platts, J.A.; Apostolopoulos, V.; Tselios, T.V. Design and synthesis of non-peptide mimetics mapping the immunodominant myelin basic protein (MBP83–96) epitope to function as T-cell receptor antagonists. Int. J. Mol. Sci. 2017, 18, 1215. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M.; Gilmore, W. Vaccines for multiple sclerosis: Progress to date. CNS Drugs 2008, 22, 175–198. [Google Scholar] [CrossRef]
- Mantzourani, E.D.; Platts, J.A.; Brancale, A.; Mavromoustakos, T.M.; Tselios, T.V. Molecular dynamics at the receptor level of immunodominant myelin basic protein epitope 87–99 implicated in multiple sclerosis and its antagonists altered peptide ligands: Triggering of immune response. J. Mol. Graph. Model. 2007, 26, 471–481. [Google Scholar] [CrossRef]
- Kaushansky, N.; de Rosbo, N.K.; Zilkha-Falb, R.; Yosef-Hemo, R.; Cohen, L.; Ben-Nun, A. ‘Multi-epitope-targeted’ immune-specific therapy for a multiple sclerosis-like disease via engineered multi-epitope protein is superior to peptides. PLoS ONE 2011, 6, e27860. [Google Scholar] [CrossRef]
- Kaushansky, N.; Kaminitz, A.; Allouche-Arnon, H.; Ben-Nun, A. Modulation of MS-like disease by a multi epitope protein is mediated by induction of CD11c+ CD11b+ Gr1+ myeloid-derived dendritic cells. J. Neuroimmunol. 2019, 333, 476953. [Google Scholar] [CrossRef]
- Moorman, C.D.; Curtis, A.D., 2nd; Bastian, A.G.; Elliott, S.E.; Mannie, M.D. A GMCSF-Neuroantigen Tolerogenic Vaccine Elicits Systemic Lymphocytosis of CD4+ CD25high FOXP3+ Regulatory T Cells in Myelin-Specific TCR Transgenic Mice Contingent Upon Low-Efficiency T Cell Antigen Receptor Recognition. Front. Immunol. 2019, 9, 3119. [Google Scholar] [CrossRef] [PubMed]
- Mannie, M.D.; Blanchfield, J.L.; Islam, S.M.T.; Abbott, D.J. Cytokine-neuroantigen fusion proteins as a new class of tolerogenic, therapeutic vaccines for treatment of inflammatory demyelinating disease in rodent models of multiple sclerosis. Front. Immunol. 2012, 3, 255. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.J.; Blanchfield, J.L.; Martinson, D.A.; Russell, S.C.; Taslim, N.; Curtis, A.D.; Mannie, M.D. Neuroantigen-specific, tolerogenic vaccines: GMCSF is a fusion partner that facilitates tolerance rather than immunity to dominant self-epitopes of myelin in murine models of experimental autoimmune encephalomyelitis (EAE). BMC Immunol. 2011, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Blanchfield, J.L.; Mannie, M.D. A GMCSF-neuroantigen fusion protein is a potent tolerogen in experimental autoimmune encephalomyelitis (EAE) that is associated with efficient targeting of neuroantigen to APC. J. Leukoc. Biol. 2010, 87, 509–521. [Google Scholar] [CrossRef][Green Version]
- Petzold, C.; Schallenberg, S.; Stern, J.N.H.; Kretschmer, K. Targeted antigen delivery to DEC-205+ dendritic cells for tolerogenic vaccination. Rev. Diabet. Stud. 2012, 9, 305–318. [Google Scholar] [CrossRef][Green Version]
- Idoyaga, J.; Fiorese, C.; Zbytnuik, L.; Lubkin, A.; Miller, J.; Malissen, B.; Mucida, D.; Merad, M.; Steinman, R.M. Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Investig. 2013, 123, 844–854. [Google Scholar] [CrossRef]
- Stern, J.N.H.; Keskin, D.B.; Kato, Z.; Waldner, H.; Schallenberg, S.; Anderson, A.; von Boehmer, H.; Kretschmer, K.; Strominger, J.L. Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proc. Natl. Acad. Sci. USA 2010, 107, 17280–17285. [Google Scholar] [CrossRef]
- Ring, S.; Maas, M.; Nettelbeck, D.M.; Enk, A.H.; Mahnke, K. Targeting of autoantigens to DEC205+ dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J. Immunol. 2013, 191, 2938–2947. [Google Scholar] [CrossRef]
- Kasagi, S.; Wang, D.; Zhang, P.; Zanvit, P.; Chen, H.; Zhang, D.; Li, J.; Che, L.; Maruyama, T.; Nakatsukasa, H.; et al. Combination of apoptotic T cell induction and self-peptide administration for therapy of experimental autoimmune encephalomyelitis. EBioMedicine 2019, 44, 50–59. [Google Scholar] [CrossRef]
- Clemente-Casares, X.; Tsai, S.; Yang, Y.; Santamaria, P. Peptide-MHC-based nanovaccines for the treatment of autoimmunity: A “one size fits all” approach? J. Mol. Med. 2011, 89, 733–742. [Google Scholar] [CrossRef]
- Offner, H.; Sinha, S.; Wang, C.; Burrows, G.G.; Vandenbark, A.A. Recombinant T-cell receptor ligands: Immunomodulatory, neuroprotective and neuroregenerative effects suggest application as therapy for multiple sclerosis. Rev. Neurosci. 2008, 19, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Subramanian, S.; Emerson-Webber, A.; Lindner, M.; Burrows, G.G.; Grafe, M.; Linington, C.; Vandenbark, A.A.; Bernard, C.C.A.; Offner, H. Recombinant TCR ligand reverses clinical signs and CNS damage of EAE induced by recombinant human MOG. J. Neuroimmune Pharmacol. 2010, 5, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, Z.; Liang, Z.; Duan, H.; Ouyang, L.; Yu, Q.; Xu, Z.; Shen, G.; Weng, X.; Wu, X. Soluble MOG35-55/I-Ab Dimers Ameliorate Experimental Autoimmune Encephalomyelitis by Reducing Encephalitogenic T Cells. PLoS ONE 2012, 7, e47435. [Google Scholar] [CrossRef] [PubMed]
- Vandenbark, A.A.; Rich, C.; Mooney, J.; Zamora, A.; Wang, C.; Huan, J.; Fugger, L.; Offner, H.; Jones, R.; Burrows, G.G. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35-55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J. Immunol. 2003, 171, 127–133. [Google Scholar] [CrossRef]
- White, D.R.; Khedri, Z.; Kiptoo, P.; Siahaan, T.J.; Tolbert, T.J. Synthesis of a bifunctional peptide inhibitor–IgG1 Fc fusion that suppresses experimental autoimmune encephalomyelitis. Bioconjug. Chem. 2017, 28, 1867–1877. [Google Scholar] [CrossRef]
- Ridwan, R.; Kiptoo, P.; Kobayashi, N.; Weir, S.; Hughes, M.; Williams, T.; Soegianto, R.; Siahaan, T.J. Antigen-specific suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor: Structure optimization and pharmacokinetics. JPET 2010, 332, 1136–1145. [Google Scholar] [CrossRef]
- Badawi, A.H.; Siahaan, T.J. Suppression of MOG- and PLP-induced experimental autoimmune encephalomyelitis using a novel multivalent bifunctional peptide inhibitor. J. Neuroimmunol. 2013, 263, 20–27. [Google Scholar] [CrossRef]
- Majewska, M.; Zając, K.; Srebro, Z.; Sura, P.; Książek, L.; Zemelka, M.; Szczepanik, M. Epicutaneous immunization with myelin basic protein protects from the experimental autoimmune encephalomyelitis. Pharmacol. Rep. 2007, 59, 74–79. [Google Scholar]
- Szczepanik, M.; Tutaj, M.; Bryniarski, K.; Dittel, B.N. Epicutaneously induced TGF-h-dependent tolerance inhibits experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005, 164, 105–114. [Google Scholar] [CrossRef]
- Tutaj, M.; Szczepanik, M. Epicutaneous (EC) immunization with myelin basic protein (MBP) induces TCRabþ CD4þ CD8þ double positive suppressor cells that protect from experimental autoimmune encephalomyelitis (EAE). J. Autoimmun. 2007, 28, 208–215. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.-X.; Chen, Y.; Xu, H.; Fitzgerald, D.C.; Zhao, Z.; Rostami, A. CD11c+CD11b+ Dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J. Immunol. 2008, 181, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Lourbopoulos, A.; Deraos, G.; Matsoukas, M.-T.; Touloumi, O.; Giannakopoulou, A.; Kalbacher, H.; Grigoriadis, N.; Apostolopoulos, V.; Matsoukas, J. Cyclic MOG35–55 ameliorates clinical and neuropathological features of experimental autoimmune encephalomyelitis, Bioorgan. Med. Chem. 2017, 25, 4163–4174. [Google Scholar] [CrossRef] [PubMed]
- Streeter, H.B.; Rigden, R.; Martin, K.F.; Scolding, N.J.; Wraith, D.C. Preclinical development and first-in-human study of ATX-MS-1467 for immunotherapy of MS. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e93. [Google Scholar] [CrossRef] [PubMed]
- Billetta, R.; Ghahramani, N.; Morrow, O.; Prakken, B.; de Jong, H.; Meschter, C.; Lanza, P.; Albani, S. Epitope-specific immune tolerization ameliorates experimental autoimmune encephalomyelitis. Clin. Immunol. 2012, 145, 94–101. [Google Scholar] [CrossRef]
- Peron, J.P.S.; Yang, K.; Chen, M.-L.; Brandao, W.N.; Basso, A.S.; Commodaro, A.G.; Weiner, H.L.; Rizzo, L.V. Oral tolerance reduces Th17 cells as well as the overall inflammation in the central nervous system of EAE mice. J. Neuroimmunol. 2010, 227, 10–17. [Google Scholar] [CrossRef]
- Song, F.; Guan, Z.; Gienapp, I.E.; Shawler, T.; Benson, J.; Whitacre, C.C. The thymus plays a role in oral tolerance in experimental autoimmune encephalomyelitis. J. Immunol. 2006, 177, 1500–1509. [Google Scholar] [CrossRef]
- Deraos, G.; Rodi, M.; Kalbacher, H.; Chatzantoni, K.; Karagiannis, F.; Synodinos, L.; Plotas, P.; Papalois, A.; Dimisianos, N.; Papathanasopoulos, P.; et al. Properties of myelin altered peptide ligand cyclo(8–99)(Ala91,Ala96) MBP87-99 render it a promising drug lead for immunotherapy of multiple sclerosis. Eur. J. Med.Chem. 2015, 101, 13–23. [Google Scholar] [CrossRef]
- Islam, S.M.T.; Curtis, A.D., 2nd; Taslim, N.; Wilkinson, D.S.; Mannie, M.D. GM-CSF-neuroantigen fusion proteins reverse experimental autoimmune encephalomyelitis and mediate tolerogenic activity in adjuvant-primed environments: Association with inflammation-dependent, inhibitory antigen presentation. J. Immunol. 2014, 193, 2317–2329. [Google Scholar] [CrossRef]
- Mannie, M.D.; Clayson, B.A.; Buskirk, E.J.; DeVine, J.L.; Hernandez, J.J.; Abbott, D.J. IL-2/Neuroantigen fusion proteins as antigen-specific tolerogens in experimental autoimmune encephalomyelitis (EAE): Correlation of T cell-mediated antigen presentation and tolerance induction. J. Immunol. 2007, 178, 2835–2843. [Google Scholar] [CrossRef]
- Link, J.M.; Rich, C.M.; Korat, M.; Burrows, G.G.; Offner, H.; Vandenbark, A.A. Monomeric DR2/MOG-35--55 recombinant TCR ligand treats relapses of experimental encephalomyelitis in DR2 transgenic mice. Clin. Immunol. 2007, 123, 95–104. [Google Scholar] [CrossRef]
- Huan, J.; Subramanian, S.; Jones, R.; Rich, C.; Link, J.; Mooney, J.; Bourdette, D.N.; Vandenbark, A.A.; Burrows, G.G.; Offner, H. Monomeric recombinant TCR ligand reduces relapse rate and severity of experimental autoimmune encephalomyelitis in SJL/J mice through cytokine switch. J. Immunol. 2004, 172, 4556–4566. [Google Scholar] [CrossRef] [PubMed]
- Offner, H.; Subramanian, S.; Wang, C.; Afentoulis, M.; Vandenbark, A.A.; Huan, J.; Burrows, G.G. Treatment of passive experimental autoimmune encephalomyelitis in SJL mice with a recombinant TCR ligand induces IL-13 and prevents axonal injury. J. Immunol. 2005, 175, 4103–4111. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Subramanian, S.; Proctor, T.M.; Kaler, L.J.; Grafe, M.; Dahan, R.; Huan, J.; Vandenbark, A.A.; Burrows, G.G.; Offner, H. A promising therapeutic approach for multiple sclerosis: Recombinant T-cell receptor ligands modulate experimental autoimmune encephalomyelitis by reducing interleukin-17 production and inhibiting migration of encephalitogenic cells into the CNS. J. Neurosci. 2007, 27, 12531–12539. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Subramanian, S.; Miller, L.; Proctor, T.M.; Roberts, C.; Burrows, G.G.; Vandenbark, A.A.; Offner, H. Cytokine switch and bystander suppression of autoimmune responses to multiple antigens in experimental autoimmune encephalomyelitis by a single recombinant T-Cell receptor ligand. J. Neurosci. 2009, 29, 3816–3823. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Miller, L.; Subramanian, S.; McCarty, O.; Proctor, T.; Meza-Romero, R.; Burrows, G.G.; Vandenbark, A.A.; Offner, H. Binding of recombinant T cell receptor ligands (RTL) to antigen presenting cells prevents upregulation of CD11b and inhibits T cell activation and transfer of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2010, 225, 52–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.; Gold, B.G.; Kaler, L.J.; Yu, X.; Afentoulis, M.E.; Burrows, G.G.; Vandenbark, A.A.; Bourdette, D.N.; Offner, H. Antigen-specific therapy promotes repair of myelin and axonal damage in established EAE. J. Neurochem. 2006, 98, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.H.; Kiptoo, P.; Siahaan, T.J. Immune tolerance induction against experimental Autoimmune Encephalomyelitis (EAE) Using A New PLP-B7AP Conjugate that simultaneously targets B7/CD28 costimulatory signal and TCR/MHC-II signal. J. Mult. Scler. (Foster City) 2015, 2, 1000131. [Google Scholar]
- Badawi, A.H.; Kiptoo, P.; Wang, W.-T.; Choi, I.-Y.; Lee, P.; Vines, C.M.; Siahaan, T.J. Suppression of EAE and prevention of blood-brain barrier breakdown after vaccination with novel bifunctional peptide inhibitor. Neuropharmacology 2012, 62, 1874–1881. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, N.; Kobayashi, H.; Gu, L.; Malefyt, T.; Siahaan, T.J. Antigen-specific suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor. JPET 2007, 322, 879–886. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kiptoo, P.; Kobayashi, H.; Ridwan, R.; Brocke, S.; Siahaan, T.J. Prophylactic and therapeutic suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor. Clin. Immunol. 2008, 129, 69–79. [Google Scholar] [CrossRef]
- Kiptoo, P.; Büyüktimkin, B.; Badawi, A.H.; Stewart, J.; Ridwan, R.; Siahaan, T.J. Controlling immune response and demyelination using highly potent bifunctional peptide inhibitors in the suppression of experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2012, 172, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Fissolo, N.; Montalban, X.; Comabella, M. DNA vaccination techniques. Methods Mol. Biol. 2016, 1304, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Garren, H. DNA vaccines for autoimmune diseases. Expert Rev. Vaccines 2009, 8, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, P.; Garren, H.; Steinman, L. Antigen-specific therapies in multiple sclerosis: Going beyond proteins and peptides. Int. Rev. Immunol. 2005, 24, 415–446. [Google Scholar] [CrossRef] [PubMed]
- Fissolo, N.; Montalban, X.; Comabella, M. DNA-based vaccines for multiple sclerosis: Current status and future directions. Clin. Immunol. 2012, 142, 76–83. [Google Scholar] [CrossRef]
- Stuve, O.; Cravens, P.; Eagar, T.N. DNA-based vaccines: The future of multiple sclerosis therapy? Expert Rev. Neurother. 2008, 8, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Jakimovski, D.; Weinstock-Guttman, B.; Ramanathan, M.; Dwyer, M.G.; Zivadinov, R. Infections, vaccines and autoimmunity: A multiple sclerosis perspective. Vaccines 2020, 8, 50. [Google Scholar] [CrossRef]
- Garren, H.; Ruiz, P.J.; Watkins, T.A.; Fontoura, P.; Nguyen, L.-V.T.; Estline, E.R.; Hirschberg, D.L.; Steinman, L. Combination of gene delivery and DNA vaccination to protect from and reverse Th1 autoimmune disease via deviation to the Th2 pathway. Immunity 2001, 15, 15–22. [Google Scholar] [CrossRef]
- Wefer, J.; Harris, R.A.; Lobell, A. Protective DNA vaccination against experimental autoimmune encephalomyelitis is associated with induction of IFNh. J. Neuroimmunol. 2004, 149, 66–76. [Google Scholar] [CrossRef]
- Schif-Zuck, S.; Wildbaum, G.; Karin, N. Coadministration of plasmid DNA constructs encoding an encephalitogenic determinant and IL-10 elicits regulatory T cell-mediated protective immunity in the central nervous system. J. Immunol. 2006, 177, 8241–8247. [Google Scholar] [CrossRef]
- Lobell, A.; Weissert, R.; Eltayeb, S.; de Graaf, K.L.; Wefer, J.; Storch, M.K.; Lassmann, H.; Wigzell, H.; Olsson, T. Suppressive DNA vaccination in myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis involves a T1-biased immune response. J. Immunol. 2003, 170, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Isaksson, M.; Wefer, J.; Norling, A.; Flores-Morales, A.; Rorsman, F.; Kämpe, O.; Harris, R.A.; Lobell, A. Impaired autoimmune T helper 17 cell responses following DNA vaccination against rat experimental autoimmune encephalomyelitis. PLoS ONE 2008, 3, e3682. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Sun, Y.; Zhang, J.; Gao, W.; Kang, J.; Wang, Y.; Wang, B.; Xia, G. Treg cell resistance to apoptosis in DNA vaccination for experimental autoimmune encephalomyelitis treatment. PLoS ONE 2012, 7, e49994. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.; Szymanska, B.; Selmaj, K. Differential prevention of experimental autoimmune encephalomyelitis with antigen-specific DNA vaccination. Clin. Neurol. Neurosurg. 2004, 106, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, X. Regulatory dendritic cells in autoimmunity: A comprehensive review. J. Autoimmun. 2015, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Domogalla, M.P.; Rostan, P.V.; Raker, V.K.; Steinbrink, K. Tolerance through education: How tolerogenic dendritic cells shape immunity. Front. Immunol. 2017, 8, 1764. [Google Scholar] [CrossRef]
- Van Brussel, I.; Lee, W.P.; Rombouts, M.; Nuyts, A.H.; Heylen, M.; DeWinter, B.Y.; Cools, N.; Schrijvers, D.M. Tolerogenic dendritic cell vaccines to treat autoimmune diseases: Can the unattainable dream turn into reality? Autoimmun. Rev. 2014, 13, 138–150. [Google Scholar] [CrossRef]
- Flórez-Grau, G.; Zubizarreta, I.; Cabezón, R.; Villoslada, P.; Benitez-Ribas, D. Tolerogenic dendritic cells as a promising antigen-specific therapy in the treatment of multiple sclerosis and neuromyelitis optica from preclinical to clinical trials. Front. Immunol. 2018, 9, 1169. [Google Scholar] [CrossRef]
- Obregon, C.; Kumar, R.; Pascual, M.A.; Vassalli, G.; Golshayan, D. Update on dendritic cell-induced immunological and clinical tolerance. Front. Immunol. 2017, 8, 1514. [Google Scholar] [CrossRef]
- Derdelinckx, J.; Mansilla, M.J.; de Laere, M.; Lee, W.-P.; Navarro-Barriuso, J.; Wens, I.; Nkansah, I.; Daans, J.; de Reu, H.; Keliris, A.J.; et al. Clinical and immunological control of experimental autoimmune encephalomyelitis by tolerogenic dendritic cells loaded with MOG-encoding RNA. J. Neuroinflam. 2019, 16, 167. [Google Scholar] [CrossRef]
- Iberg, C.A.; Hawiger, D. Natural and induced tolerogenic dendritic cells. J. Immunol. 2020, 204, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Vandenbark, A.A.; Abulafia-Lapid, R. Autologous T-cell vaccination for multiple sclerosis: A perspective on progress. BioDrugs 2008, 22, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Volovitz, I.; Marmora, Y.; Mor, F.; Flügel, A.; Odoardi, F.; Eisenbach, L.; Cohen, I.R. T cell vaccination induces the elimination of EAE effector T cells: Analysis using GFP-transduced, encephalitogenic T cells. J. Autoimmun. 2010, 35, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Turley, D.M.; Miller, S.D. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J. Immunol. 2007, 178, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Turley, D.M.; Smith, C.E.; Harp, C.T.; McCarthy, D.; Feeney, E.M.; Getts, M.T.; Martin, A.J.; Luo, X.; Terry, R.R.; et al. Tolerance Induced by Apoptotic Antigen-Coupled Leukocytes is Induced by PD-L1+, IL-10-Producing Splenic Macrophages and Maintained by Tregs. J. Immunol. 2011, 187, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Pishesha, N.; Bilate, A.M.; Wibowo, M.C.; Huang, N.-J.; Lia, Z.; Deshycka, R.; Bousbaine, D.; Lia, H.; Pattersona, H.C.; Dougana, S.K.; et al. Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc. Natl. Acad. Sci. USA 2017, 114, 3157–3162. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, D.; Peng, X.; Lin, J.; Suc, Z.; Lia, J.; Zhang, X.; Weng, Y. Beneficial effect of atorvastatin-modified dendritic cells pulsed with myelin oligodendrocyte glycoprotein autoantigen on experimental autoimmune encephalomyelitis. Neuroreport 2018, 29, 317–327. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Baylink, D.J.; Li1, C.-H.; Watts, D.M.; Xu, Y.; Qin, X.; Walter, M.H.; Tang, X. Targeting non-classical myelin epitopes to treat experimental autoimmune encephalomyelitis. Sci. Rep. 2016, 6, 36064. [Google Scholar] [CrossRef]
- Kalantari, T.; Karimi, M.H.; Ciric, B.; Yan, Y.; Rostami, A.; Kamali-Sarvestani, E. Tolerogenic dendritic cells produced by lentiviral-mediated CD40- and interleukin-23p19-specific shRNA can ameliorate experimental autoimmune encephalomyelitis by suppressing T helper type 17 cells. Clin. Exp. Immunol. 2014, 176, 180–189. [Google Scholar] [CrossRef]
- Mansilla, M.J.; Sellès-Moreno, C.; Fàbregas-Puig, S.; Amoedo, J.; Navarro-Barriuso, J.; Teniente-Serra, A.; Grau-López, L.; Ramo-Tello, C.; Martínez-Cáceres, E.M. Beneficial effect of tolerogenic dendritic cells pulsed with MOG autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci. Ther. 2015, 21, 222–230. [Google Scholar] [CrossRef]
- Mansilla, M.J.; Contreras-Cardone, R.; Navarro-Barriuso, J.; Cools, N.; Berneman, Z.; Ramo-Tello, C.; Martínez-Cáceres, E.M. Cryopreserved vitamin D3-tolerogenic dendritic cells pulsed with autoantigens as a potential therapy for multiple sclerosis patients. J. Neuroinflamm. 2016, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Leng, X.; Luo, S.; Su, Z.; Luo, X.; Guo, H.; Mo, C.; Zou, Q.; Liu, Y.; Wang, Y. Tolerogenic dendritic cells generated with tofacitinib ameliorate experimental autoimmune encephalomyelitis through modulation of Th17/Treg balance. J. Immunol. Res. 2016, 2016, 5021537. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, J.; Zheng, C.; Wu, J.; Cheng, Y.; Zhu, S.; Lin, C.; Cao, Q.; Zhu, J.; Jin, T. 1,25-dihydroxyvitamin D3-induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells. Immunology 2017, 152, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Papenfuss, T.L.; Powell, N.D.; McClain, M.A.; Bedarf, A.; Singh, A.; Gienapp, I.E.; Shawler, T.; Whitacre, C.C. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J. Immunol. 2011, 15, 186–3346. [Google Scholar] [CrossRef] [PubMed]
- Menges, M.; Rößner, S.; Voigtländer, C.; Schindler, H.; Kukutsch, N.A.; Bogdan, C.; Erb, K.; Schuler, G.; Lutz, M.B. Repetitive injections of dendritic cells matured with tumor necrosis factor α induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 2002, 195, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.-G.; Huang, Y.-M.; Yang, J.-S.; Xu, L.-Y.; Link, H. Bone marrow-derived dendritic cells from experimental allergic encephalomyelitis induce immune tolerance to EAE in Lewis rats. Clin. Exp. Immunol. 2001, 125, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Zhang, A.-H.; Yoon, J.; Culp, W.E.; Leesa, J.R.; Wucherpfennig, K.W.; Scott, D.W. Engineered MBP-specific human Tregs ameliorate MOG-induced EAE through IL-2-triggered inhibition of effector T cells. J. Autoimmun. 2018, 92, 77–86. [Google Scholar] [CrossRef]
- Pereira, B.d.; Fraefel, C.; Hilbe, M.; Ackermann, M.; Dresch, C. Transcriptional targeting of DCs with lentiviral vectors induces antigen-specific tolerance in a mouse model of multiple sclerosis. Gene Ther. 2013, 20, 556–566. [Google Scholar] [CrossRef]
- Eixarch, H.; Espejo, C.; Gómez, A.; Mansilla, M.J.; Castillo, M.; Mildner, A.; Vidal, F.; Gimeno, R.; Prinz, M.; Montalban, X.; et al. Tolerance induction in experimental autoimmune encephalomyelitis using non-myeloablative hematopoietic gene therapy with autoantigen. Mol. Ther. 2009, 17, 897–905. [Google Scholar] [CrossRef]
- Casacuberta-Serra, S.; Costa, C.; Eixarch, H.; Mansilla, M.J.; López-Estévez, S.; Martorell, L.; Parés, M.; Montalban, X.; Espejo, C.; Barquinero, J. Myeloid-derived suppressor cells expressing a self-antigen ameliorate experimental autoimmune encephalomyelitis. Exp. Neurol. 2016, 286, 50–60. [Google Scholar] [CrossRef]
- Tabansky, I.; Messina, M.D.; Bangeranye, C.; Goldstein, J.; Blitz-Shabbir, K.M.; Machado, S.; Jeganathan, V.; Wright, P.; Najjar, S.; Cao, Y.; et al. Advancing drug delivery systems for the treatment of multiple sclerosis. Immunol. Res. 2015, 63, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Ballerini, C.; Baldi, G.; Aldinucci, A.; Maggi, P. Nanomaterial applications in multiple sclerosis inflamed Brain. J. Neuroimmune Pharmacol. 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, M.; Majewski, S.; Foldvari, M. Therapeutic applications of nanomedicine in autoimmune diseases: From immunosuppression to tolerance induction. Nanomed. Nanotechnol. 2015, 11, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.R.; Marques, F.; Sousa, J.C.; Cerqueira, J.; Pinto, I.M. Nano- and micro-based systems for immunotolerance induction in multiple sclerosis. Hum. Vaccines Immunother. 2016, 12, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Veld, R.H.I.; da Silva, C.G.; Kaijzel, E.L.; Chan, A.B.; Cruz, L.J. The Potential of nano-vehicle mediated therapy in vasculitis and multiple sclerosis. Curr. Pharm. Des. 2017, 23, 1985–1992. [Google Scholar] [CrossRef]
- Gammon, J.M.; Jewell, C.M. Engineering immune tolerance with biomaterials. Adv. Healthc. Mater. 2019, 8, e1801419. [Google Scholar] [CrossRef]
- Pei, W.; Wan, X.; Shahzad, K.A.; Zhang, L.; Song, S.; Jin, X.; Wang, L.; Zhao, C.; Shen, C. Direct modulation of myelin-autoreactive CD4+ and CD8+ T cells in EAE mice by a tolerogenic nanoparticle co-carrying myelin peptide-loaded major histocompatibility complexes, CD47 and multiple regulatory molecules. Int. J. Nanomed. 2018, 13, 3731–3750. [Google Scholar] [CrossRef]
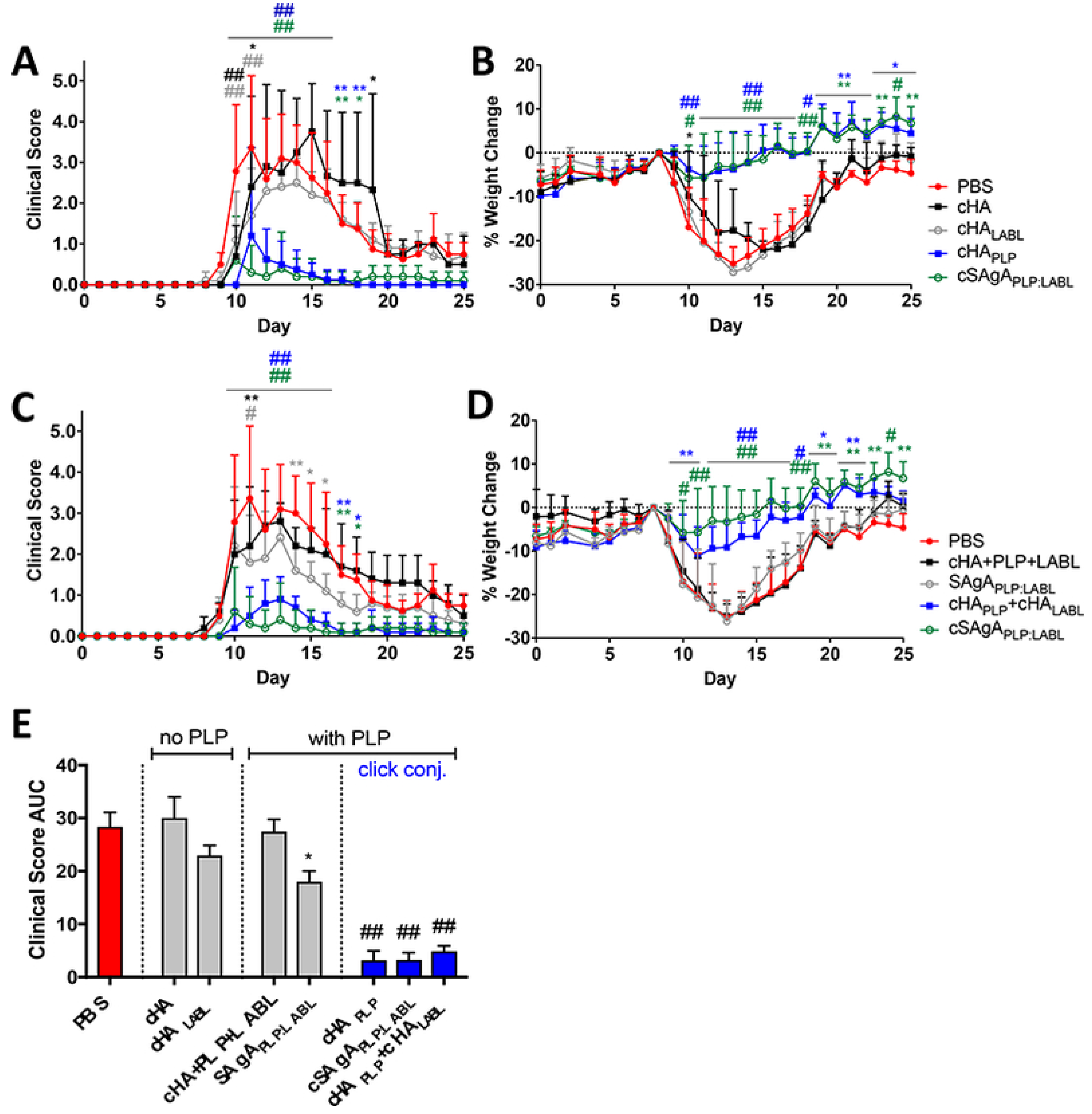
- Sestak, J.O.; Sullivan, B.P.; Thati, S.; Northrup, L.; Hartwell, B.; Antunez, L.; Forrest, M.L.; Vines, C.M.; Siahaan, T.J.; Berkland, C. Codelivery of antigen and an immune cell adhesion inhibitor is necessary for efficacy of soluble antigen arrays in experimental autoimmune encephalomyelitis. Mol. Ther. Methods Clin. Dev. 2014, 1, 14008. [Google Scholar] [CrossRef]
- Thati, S.; Kuehl, C.; Hartwell, B.; Sestak, J.; Siahaan, T.; Forrest, M.L.; Berkland, C. Routes of Administration and dose optimization of soluble antigen arrays in mice with experimental autoimmune encephalomyelitis. J. Pharm. Sci. 2015, 104, 714–721. [Google Scholar] [CrossRef][Green Version]
- Hartwell, B.L.; Pickens, C.J.; Leon, M.; Berkland, C. Multivalent antigen arrays exhibit high avidity binding and modulation of B cell receptor-mediated signaling to drive efficacy against experimental autoimmune encephalomyelitis. Biomacromolecules 2017, 18, 1893–1907. [Google Scholar] [CrossRef]
- Hartwell, B.L.; Pickens, C.J.; Leon, M.; Northrup, L.; Christopher, M.; Griffin, J.D.; Martinez-Becerra, F.; Berkland, C. Soluble antigen arrays disarm antigen-specific B cells to promote lasting immune tolerance in experimental autoimmune encephalomyelitis. J. Autoimmun. 2018, 93, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Tostanoski, L.H.; Chiu, Y.-C.; Andorko, J.I.; Guo, M.; Zeng, X.; Zhang, P.; Royal, W., III; Jewell, C.M. Design of polyelectrolyte multilayers to promote immunological tolerance. ACS Nano 2016, 10, 9334–9345. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.L.; Andorko, J.I.; Tostanoski, L.H.; Jewell, C.M. Polyplexes assembled from self-peptides and regulatory nucleic acids blunt toll-like receptor signaling to combat autoimmunity. Biomaterials 2017, 118, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Tseveleki, V.; Tselios, T.; Kanistras, I.; Koutsoni, O.; Karamita, M.; Vamvakas, S.-S.; Apostolopoulos, V.; Dotsika, E.; Matsoukas, J.; Lassmann, H.; et al. Mannan-conjugated myelin peptides prime non-pathogenic Th1 and Th17 cells and ameliorate experimental autoimmune encephalomyelitis. Exp. Neurol. 2015, 267, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Belogurov, A.A., Jr.; Stepanov, A.V.; Smirnov, I.V.; Melamed, D.; Bacon, A.; Mamedov, A.E.; Boitsov, V.M.; Sashchenko, L.P.; Ponomarenko, N.A.; Sharanova, S.N.; et al. Liposome-encapsulated peptides protect against experimental allergic encephalitis. FASEB J. 2013, 27, 222–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pujol-Autonell, I.; Mansilla, M.-J.; Rodriguez-Fernandez, S.; Cano-Sarabia, M.; Navarro-Barriuso1, J.; Ampudia, R.-M.; Rius, A.; Garcia-Jimeno, S.; Perna-Barrull, D.; Martinez-Caceres, E.; et al. Liposome-based immunotherapy against autoimmune diseases: Therapeutic effect on multiple sclerosis. Nanomedicine 2017, 12, 1231–1242. [Google Scholar] [CrossRef]
- Belogurov, A.; Zakharov, K.; Lomakin, Y.; Surkov, K.; Avtushenko, S.; Kruglyakov, P.; Smirnov, I.; Makshakov, G.; Lockshin, C.; Gregoriadis, G.; et al. CD206-targeted liposomal myelin basic protein peptides in patients with multiple sclerosis resistant to first-line disease-modifying therapies: A first-in-human, proof-of-concept dose-escalation study. Neurotherapeutics 2016, 13, 895–904. [Google Scholar] [CrossRef]
- Lomakin, Y.; Belogurov, A., Jr.; Glagoleva, I.; Stepanov, A.; Zakharov, K.; Okunola, J.; Smirnov, I.; Genkin, D.; Gabibov, A. Administration of myelin basic protein peptides encapsulated in mannosylated liposomes normalizes level of serum TNF-α and IL-2 and chemoattractants CCL2 and CCL4 in multiple sclerosis patients. Mediat. Inflamm. 2016, 2016, 2847232. [Google Scholar] [CrossRef]
- Cappellano, G.; Woldetsadik, A.D.; Orilieri, E.; Shivakumar, Y.; Rizzi, M.; Carniato, F.; Gigliotti, C.L.; Boggio, E.; Clemente, N.; Comi, C.; et al. Subcutaneous inverse vaccination with PLGA particles loaded with aMOG peptide and IL-10 decreases the severity of experimental autoimmune encephalomyelitis. Vaccine 2014, 32, 5681–5689. [Google Scholar] [CrossRef]
- Casey, L.M.; Pearson, R.M.; Hughes, K.R.; Liu, J.M.H.; Rose, J.A.; North, M.G.; Wang, L.Z.; Lei, M.; Miller, S.D.; Shea, L.D. Conjugation of transforming growth factor Beta to antigen-loaded Poly(lactide-co-glycolide) nanoparticles enhances efficiency of antigen-specific tolerance. Bioconjug. Chem. 2018, 29, 813–823. [Google Scholar] [CrossRef]
- Cho, J.J.; Stewart, J.M.; Drashansky, T.T.; Brusko, M.A.; Zuniga, A.N.; Lorentsen, K.J.; Keselowsky, B.G.; Avram, D. An antigen-specific semi-therapeutic treatment with local delivery of tolerogenic factors through a dual-sized microparticle system blocks experimental autoimmune encephalomyelitis. Biomaterials 2017, 143, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Gholamzad, M.; Ebtekar, M.; Ardestani, M.S. Intravenous injection of myelin oligodendrocyte glycoprotein-coated PLGA microparticles have tolerogenic effects in experimental autoimmune encephalomyelitis. Iran J. Allergy Asthma Immunol. 2017, 16, 27–281. [Google Scholar]
- Hunter, Z.; McCarthy, D.P.; Yap, W.T.; Harp, C.T.; Getts, D.R.; Shea, L.D.; Miller, S.D. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano 2014, 8, 2148–2160. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.A.; LaMothe, R.A.; Ferrari, J.D.; Zhang, A.-H.; Rossi, R.J.; Kolte, P.N.; Griset, A.P.; O’Neil, C.; Altreuter, D.H.; Browning, E.; et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl. Acad. Sci. USA 2014, 112, E156–E165. [Google Scholar] [CrossRef]
- McCarthy, D.P.; Yap, J.W.-T.; Harp, C.T.; Song, W.K.; Chen, J.; Pearson, R.M.; Miller, S.D.; Shea, L.D. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomedicine 2017, 13, 191–200. [Google Scholar] [CrossRef]
- Saito, E.; Kuo, R.; Kramer, K.R.; Gohel, N.; Giles, D.A.; Moore, B.B.; Miller, S.D.; Shea, L.D. Design of biodegradable nanoparticles to modulate phenotypes of antigen presenting cells for antigen-specific treatment of autoimmune disease. Biomaterials 2019, 222, 119432. [Google Scholar] [CrossRef]
- Wan, X.; Pei, W.; Shahzad, K.A.; Zhang, L.; Song, S.; Jin, X.; Wang, L.; Zhao, C.; Shen, C. A Tolerogenic artificial APC durably ameliorates experimental autoimmune encephalomyelitis by directly and selectively modulating myelin peptide–autoreactive CD4+ and CD8+ T cell. J. Immunol. 2018, 201, 1194–1210. [Google Scholar] [CrossRef]
- Zhao, H.; Kiptoo, P.; Williams, T.D.; Siahaan, T.J.; Topp, E.M. Immune response to controlled release of immunomodulating peptides in a murine experimental autoimmune encephalomyelitis (EAE) model. J. Control. Release 2010, 141, 145–152. [Google Scholar] [CrossRef]
- Pearson, R.M.; Casey, L.M.; Hughes, K.R.; Wang, L.Z.; North, M.G.; Getts, D.R.; Miller, S.D.; Shea, L.D. Controlled delivery of single or multiple antigens in tolerogenic nanoparticles using peptide-polymer bioconjugates. Mol. Ther. 2017, 25, 1655–1664. [Google Scholar] [CrossRef]
- Sestak, J.O.; Fakhari, A.; Badawi, A.H.; Siahaan, T.J.; Berkland, C. Structure, size, and solubility of antigen arrays determines efficacy in experimental autoimmune encephalomyelitis. AAPS J. 2014, 16, 1185–1193. [Google Scholar] [CrossRef]
- Kuo, R.; Saito, E.; Miller, S.D.; Shea, L.D. Peptide-conjugated nanoparticles reduce positive co-stimulatory expression and T cell activity to induce tolerance. Mol. Ther. 2017, 25, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Martin, A.J.; McCarthy, D.P.; Terry, R.L.; Hunter, Z.N.; Yap, W.T.; Getts, M.T.; Pleiss, M.; Luo, X.; King, N.J.C.; et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 2012, 30, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Tostanoski, L.H.; Chiu, Y.-C.; Gammon, J.M.; Simon, T.; Andorko, J.I.; Bromberg, J.S.; Jewell, C.M. Reprogramming the local lymph node microenvironment promotes tolerance that is systemic and antigen-specific. Cell Rep. 2016, 16, 2940–2952. [Google Scholar] [CrossRef] [PubMed]
- Büyüktimkin, B.; Wang, Q.; Kiptoo, P.; Stewart, J.M.; Berkland, C.; Siahaan, T.J. Vaccine-like controlled-release delivery of an immunomodulating peptide to treat experimental autoimmune encephalomyelitis. Mol. Pharm. 2012, 9, 979–985. [Google Scholar] [CrossRef]
- Northrup, L.; Sestak, J.O.; Sullivan, B.P.; Thati, S.; Hartwell, B.L.; Siahaan, T.J.; Vines, C.M.; Berkland, C. Co-delivery of autoantigen and B7 pathway modulators suppresses experimental autoimmune encephalomyelitis. AAPS J. 2014, 16, 1204–1213. [Google Scholar] [CrossRef]
- Kuehl, C.; Thati, S.; Sullivan, B.; Sestak, J.; Thompson, M.; Siahaan, T.; Berkland, C. Pulmonary administration of soluble antigen arrays is superior to antigen in treatment of experimental autoimmune encephalomyelitis. J. Pharm. Sci. 2017, 106, 3293–3302. [Google Scholar] [CrossRef]
- Hess, K.L.; Oh, E.; Tostanoski, L.H.; Andorko, J.I.; Susumu, K.; Deschamps, J.R.; Medintz, I.L.; Jewell, C.M. Engineering immunological tolerance using quantum dots to tune the density of self-antigen display. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Clemente-Casares, X.; Blanco, J.; Ambalavanan, P.; Yamanouchi, J.; Singha, S.; Fandos, C.; Tsai, S.; Wang, J.; Garabatos, N.; Izquierdo, C.; et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 2016, 530, 434–440. [Google Scholar] [CrossRef]
- Yeste, A.; Nadeau, M.; Burns, E.J.; Weiner, H.L.; Qu, F.J. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2012, 109, 11270–11275. [Google Scholar] [CrossRef]
- Katsara, M.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. Design of novel cyclic altered peptide ligands of myelin basic protein MBP83–99 that modulate immune responses in SJL/J mice. J. Med. Chem. 2008, 51, 3971–3978. [Google Scholar] [CrossRef]
- Yu, L.; Yang, F.; Jiang, L.; Chen, Y.; Wang, K.; Xu, F.; Wei, Y.; Cao, X.; Wang, J.; Cai, Z. Exosomes with membrane-associated TGF-β1 from gene-modified dendritic cells inhibit murine EAE independently of MHC restriction. Eur. J. Immunol. 2013, 43, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Buerth, C.; Mausberg, A.K.; Heininger, M.K.; Hartung, H.-P.; Kieseier, B.C.; Ernst, J.F. Oral tolerance induction in experimental autoimmune encephalomyelitis with Candida utilis expressing the immunogenic MOG35-55 peptide. PLoS ONE 2016, 11, e0155082. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Comi, G.; Panitch, H.; Oger, J.; Antel, J.; Conlon, P.; Steinman, L. The Altered peptide ligand in relapsing MS study group, Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat. Med. 2000, 6, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Goodkin, D.E.; Shulman, M.; Winkelhake, J.; Waubant, E.; Andersson, P.; Stewart, T.; Nelson, S.; Fischbein, N.; Coyle, P.K.; Frohman, E.; et al. A phase I trial of solubilized DR2:MBP84-102 (AG284) in multiple sclerosis. Neurology 2000, 54, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.G.; Catz, I.; Ferenczi, L.Z.; Krantz, M.J. Intravenous synthetic peptide MBP8298 delayed disease progression in an HLA Class II-defined cohort of patients with progressive multiple sclerosis: Results of a 24-month double-blind placebo-controlled clinical trial and 5 years of follow-up treatment. Eur. J. Neurol. 2006, 13, 887–895. [Google Scholar] [CrossRef]
- Freedman, M.S.; Bar-Or, A.; Oger, J.; Traboulsee, A.; Patry, D.; Young, C.; Olsson, T.; Li, D.; Hartung, H.-P.; Krantz, M.; et al. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology 2011, 77, 1551–1560. [Google Scholar] [CrossRef]
- Offner, H.; Burrows, G.G.; Ferro, A.J.; Vandenbark, A.A. RTL therapy for multiple sclerosis: A Phase I clinical study. J. Neuroimmunol. 2011, 231, 7–14. [Google Scholar] [CrossRef][Green Version]
- Yadav, V.; Bourdette, D.N.; Bowen, J.D.; Lynch, S.G.; Mattson, D.; Preiningerova, J.; Bever, C.T.; Simon, J.; Goldstein, A.; Burrows, G.G.; et al. Recombinant T-cell receptor ligand (RTL) for treatment of multiple sclerosis: A double-blind, placebo-controlled, Phase 1, dose-escalation study. Autoimmune Dis. 2012, 2012, 954739. [Google Scholar] [CrossRef]
- Loo, E.W.; Krantz, M.J.; Agrawal, B. High dose antigen treatment with a peptide epitope of myelin basic protein modulates T cells in multiple sclerosis patients. Cell. Immunol. 2012, 280, 10–15. [Google Scholar] [CrossRef]
- Lutterotti, A.; Yousef, S.; Sputtek, A.; Stürner, K.H.; Stellmann, J.-P.; Breiden, P.; Reinhardt, S.; Schulze, C.; Bester, M.; Heesen, C.; et al. Antigen-specific tolerance by autologous myelin peptide–coupled cells: A Phase 1 trial in multiple sclerosis. Sci. Transl. Med. 2013, 5, 188ra75. [Google Scholar] [CrossRef]
- Bar-Or, A.; Vollmer, T.; Antel, J.; Arnold, D.L.; Bodner, C.A.; Campagnolo, D.; Gianettoni, J.; Jalili, F.; Kachuck, N.; Lapierre, Y.; et al. Induction of antigen-specific tolerance in multiple sclerosis after immunization with a DNA encoding myelin basic protein in a randomized, placebo-controlled Phase I/II trial. Arch. Neurol. 2007, 64, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Juryńczyk, M.; Walczak, A.; Jurewicz, A.; Jesionek-Kupnicka, D.; Szczepanik, M.; Selmaj, K. Immune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann. Neurol. 2010, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.; Siger, M.; Ciach, A.; Szczepanik, M.; Selmaj, K. Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA Neurol. 2013, 70, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Garren, H.; Robinson, W.H.; Krasulova´, E.; Havrdova´, E.; Nadj, C.; Selmaj, K.; Losy, J.; Nadj, I.; Radue, E.-W.; Kidd, B.A.; et al. Steinman, and the BHT-3009 Study Group, Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann. Neurol. 2008, 63, 611–620. [Google Scholar] [CrossRef]
- Papadopoulou, A.; von Felten, S.; Traud, S.; Rahman, A.; Quan, J.; King, R.; Garren, H.; Steinman, L.; Cutter, G.; Kappos, L.; et al. Evolution of MS lesions to black holes under DNA vaccine treatment. J. Neurol. 2012, 259, 1375–1382. [Google Scholar] [CrossRef]
- Chataway, J.; Martin, K.; Barrell, K.; Sharrack, B.; Stolt, P.; Wraith, D.C. Effects of ATX-MS-1467 immunotherapy over 16 weeks in relapsing multiple sclerosis. Neurology 2018, 90, e955–e962. [Google Scholar] [CrossRef]
- Zubizarreta, I.; Flórez-Graub, G.; Vila, G.; Cabezón, R.; España, C.; Andorra, M.; Saiza, A.; Llufriu, S.; Sepulveda, M.; Sola-Valls, N.; et al. Immune tolerance in multiple sclerosis and neuromyelitis optica with peptide-loaded tolerogenic dendritic cells in a phase 1b trial. Proc. Natl. Acad. Sci. USA 2019, 116, 8463–8470. [Google Scholar] [CrossRef]
- Willekens, B.; Presas-Rodríguez, S.; Mansilla, M.J.; Derdelinckx, J.; Lee, W.-P.; Nijs, G.; de Laere, M.; Wens, I.; Cras, P.; Parizel, P.; et al. On behalf of the RESTORE consortium, Tolerogenic dendritic cell-based treatment for multiple sclerosis (MS): A harmonized study protocol for two phase I clinical trials comparing intradermal and intranodal cell administration. BMJ Open 2019, 9, e030309. [Google Scholar] [CrossRef]
- Seledtsova, G.V.; Ivanova, I.P.; Shishkov, A.A.; Seledtsov, V.I. Immune responses to polyclonal T-cell vaccination in patients with progressive multiple sclerosis. J. Immunotoxicol. 2016, 13, 879–884. [Google Scholar] [CrossRef]
- Loftus, B.; Newsom, B.; Montgomery, M.; von Gynz-Rekowski, K.; Riser, M.; Inman, S.; Garces, P.; Rill, D.; Zhang, J.; Williams, J.C. Autologous attenuated T-cell vaccine (Tovaxin®) dose escalation in multiple sclerosis relapsing–remitting and secondary progressive patients nonresponsive to approved immunomodulatory therapies. Clin. Immunol. 2009, 131, 202–215. [Google Scholar] [CrossRef]
- Fox, E.; Wynn, D.; Cohan, S.; Rill, D.; McGuire, D.; Markowitz, C. A randomized clinical trial of autologous T-cell therapy in multiple sclerosis: Subset analysis and implications for trial design. Mult. Scler. J. 2012, 18, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Karussis, D.; Shor, H.; Yachnin, J.; Lanxner, N.; Amiel, M.; Baruch, K.; Keren-Zur, Y.; Haviv, O.; Filippi, M.; Petrou, P.; et al. T Cell vaccination benefits relapsing progressive multiple sclerosis patients: A randomized, double-blind clinical trial. PLoS ONE 2012, 7, e50478. [Google Scholar] [CrossRef] [PubMed]
- Van der, A.A.; Hellings, N.; Medaer, R.; Gelin, G.; Palmers, Y.; Rauss, J.; Stinissen, P. T cell vaccination in multiple sclerosis patients with autologous CSF-derived activated T cells: Results from a pilot study. Clin. Exp. Immunol. 2003, 131, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Rivera, V.M.; Tejada-Simon, M.V.; Yang, D.; Hong, J.; Li, S.; Haykal, H.; Killian, J.; Zang, Y.C.Q. T cell vaccination in multiple sclerosis: Results of a preliminary study. J. Neurol. 2002, 249, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Lunda, B.; McMillan, M.; Koa, D.Y.; McCarthy, K.; Weiner, L.P. T cell vaccination in secondary progressive multiple sclerosis. J. Neuroimmunol. 2000, 107, 130–139. [Google Scholar] [CrossRef]
- Achiron, A.; Lavie, G.; Kishner, I.; Stern, Y.; Sarova-Pinhas, I.; Ben-Aharon, T.; Barak, Y.; Raz, H.; Lavie, M.; Barliya, T.; et al. T cell vaccination in multiple sclerosis relapsing–remitting nonresponders patients. Clin. Immunol. 2004, 113, 155–160. [Google Scholar] [CrossRef]
- Zang, Y.C.Q.; Hong, J.; Rivera, V.M.; Killian, J.; Zhang, J.Z. Preferential recognition of TCR hypervariable regions by human anti-idiotypic T cells induced by T cell vaccination. J. Immunol. 2000, 164, 4011–4017. [Google Scholar] [CrossRef]
- Vandenbark, A.A.; Culbertson, N.E.; Bartholomew, R.M.; Huan, J.; Agotsch, M.; LaTocha, D.; Yadav, V.; Mass, M.; Whitham, R.; Lovera, J.; et al. Therapeutic vaccination with a trivalent T-cell receptor (TCR) peptide vaccine restores deficient FoxP3 expression and TCR recognition in subjects with multiple sclerosis. Immunology 2007, 123, 66–78. [Google Scholar] [CrossRef]


| Therapeutic Molecule | Commercial Name | Year of Approval | Admin. Route | Admin. Frequency | Mode of Action | Side Effects |
|---|---|---|---|---|---|---|
| IFN-β1a | Avonex® Rebif® | 1993 | i.m. s.c. | Once a week Three times a week | Decrease of proinflammatory and increase of anti-inflammatory cytokines; decreased migration of inflammatory cells across the BBB; decrease of Th17 cells; modulation of T and B cells. | Symptoms similar to those of flu; leukopenia; liver damage. |
| pegIFN-β1a | Plegridy® | s.c. | Once per two weeks | Decrease of proinflammatory and increase of anti-inflammatory cytokines; decreased migration of inflammatory cells across the BBB; decrease of Th17 cells; modulation of T and B cells | Symptoms similar to those of flu; leukopenia; liver damage. | |
| IFN-β1b | Betaseron® Extavia® | 1993 | s.c. | Once per two days | Decrease of proinflammatory and increase of anti-inflammatory cytokines; decreased migration of inflammatory cells across the BBB; decrease of Th17 cells; modulation of T and B cells; down regulation of MHC expression on APCs. | Symptoms similar to those of flu; leukopenia; liver damage. |
| Glatiramer acetate | Copaxone® | 1996 | s.c. | - | Decrease of proinflammatory and increase of anti-inflammatory cytokines; decrease of Th17 cells; increase of Th2 cells and Tregs; blocking of pMHC. | Erythema; induration; heart palpitations; dyspnea; tightness of chest; flushes/anxiety. |
| Dimethyl fumarate | Tecfidera® | 2013 | oral | Twice or three times per day | Anti-inflammatory-Increase of Th2 cells; anti-oxidative stress; neuroprotection through activation of Nrf-2 pathway. | Flushes; vomit; diarrhea; nausea; decrease of WBC. |
| Teriflunomide | Aubagio® | 2012 | oral | Once per day | Inhibition of dihydroorotate dehydrogenase; inhibition of T and B cells; | Lymphopenia; nausea; hypertension; fatigue; headache; diarrhea; peripheral neuropathy; acute renal failure; alopecia. |
| Fingolimod | Glenya® | 2010 | oral | Once per day | S1P receptor modulator; preventing the circulation of lymphocytes in non-lymphoid tissues including the CNS. | Weakening of heart rate; hypertension; macular edema; increased liver enzymes; decreased lymphocyte levels. |
| Siponimod [55] | Mayzent® | 2019 | oral | Binding to S1P-1 and S1P-5 | ||
| Ozanimod [56] | Zeposia® | 2020 USA | oral | S1P receptor agonist | ||
| Laquinimod | Oral | Immunomodulation of T cells, DCs and monocytes; neuroprotection of astrocytes; decrease of proinflammatory and increase of anti-inflammatory cytokines; reduced infiltration of cells into the CNS. | No severe cardiac adverse effects were detected during Phase III clinical trials. | |||
| Cladribine [57] | Mavenclad® | 2017 EU 2019 USA | Reduction of circulating T and B cells. | Risk of cancer | ||
| Mitoxantrone | Novatrone® | 2000 USA | i.v. | Once per three months | Cytotoxic for B and T cells; reduction of Th1 cytokines; inhibition of type II topoisomerase. | Cardiotoxicity; leukemia |
| Methylprednisolone | i.v. | - | Immunosuppression; anti-inflammatory effects. | Risk of infections; retention of sodium; glucose intolerance; mood disturbances. | ||
| Dalfampridine | Ampyra® | oral | Twice per day | Blocking of potassium channel; improvement of motor symptoms. | ||
| Natalizumab | Tysabr® | 2004 | i.v. | Once per 28 days | Targeting α4-integrin | Progressive multifocal leukoencephalopathy. |
| Ofatumumab | Arzerra® | i.v. | Once per two weeks | Targeting CD20 | ||
| Ocrelizumab | Ocrevus® | i.v. | Once per six months | Targeting CD20 | ||
| Alemtuzumab | Lemtrada® | 2013 EU | i.v. | Once a year | Targeting CD52 | High risk of infections Graves’ disease |
| Daclizumab | Zinbryta® | s.c. | Once per month | Targeting CD25 | ||
| Rituximab | Rituxan® | i.v. | - | Targeting CD20 | Chills; nausea; hypotension | |
| Obinutuzumab | Gazyva® | i.v. | - | Direct cell death | Risk of infections; nausea; thrombocytopenia; neutropenia |
| Vaccine | Antigen | Targeting Ligand/Drug | Vaccination Type | Admin. Route | Admin. Dose | Animal Model | Vaccination Outcome |
|---|---|---|---|---|---|---|---|
| Myelin Proteins/Peptides | |||||||
| MBP [112] | Guinea pig MBP | - | Prophylactic: seven days b.i. | e.c. | SJLxB10.PL female mice (6–8 weeks old) with EAE induced with MBP | Protection from RR form of EAE Reduction of disease incidence to 58% | |
| MBP [113] | Guinea pig MBP | - | Prophylactic: seven and three days b.i. Therapeutic: at initial signs of EAE and after four days | e.c. | B10.PL female mice (6–8 weeks old) with EAE induced with MBP | Prophylactic vaccine: protection from EAE Therapeutic vaccine: suppression of EAE | |
| MBP [114] | Guinea pig MBP | - | Prophylactic: seven and three days b.i. | e.c. | B10.PL and SJLxB10.PL female mice (6–8 weeks old) with acute or RR EAE respectively, induced with MBP Knock out mice: TCRδ_/_, CD1d_/_ and β2m_/_ on H-2u background. | Vaccination with MBP prior to EAE induction prevented the development of the disease (incidence reduction by 50%) and reduced the severity of the clinical symptoms in the mice that developed EAE. Experiments with knock out mice showed that the disease could not be completely suppressed only in β2m_/_ mice. | |
| MOG35–55 [115] | MOG35–55 | - | Preclinical/Therapeutic: 3, 5, and 7 days p.i. | i.v. | C57BL/6 female mice (8–10 weeks old) with EAE induced with MOG35–55 | Dramatic suppression of EAE development | |
| c-MOG35–55 [116] | MOG35–55 and cyclic- MOG35–55 | - | Preclinical/Therapeutic on the same day with immunization and seven days p.i. | s.c. | C57BL/6 female mice (6–10 weeks old) with EAE induced with MOG35–55 | Amelioration of EAE clinical course and pathology. Reduction of clinical severity of acute phase of EAE and reduction of overall EAE burden. | |
| ATX-MS-1467 [117] | Mixture of MBP30–44, MBP 131–145, MBP140–154, MBP83–99 | - | Prophylactic Preclinical/Therapeutic | s.c. | 100 μL of ATX-MS-1467 twice a week | (ObxDR2)F1 mice with EAE induced with spinal cord homogenate | ATX-MS-1467 was shown to effectively prevent and treat EAE. The inhibition of the disease was found to be dose-dependent. |
| Pool of MBP peptides [118] | MBP68–86 and MBP87–99 | Therapeutic: secen and 11 days p.i. | i.n. | 500 μg of each MBP peptide /rat | Lewis female rats (9 weeks old) with EAE induced with MBP68–86 | Tolerization to a pool of MBP peptides was found to result in amelioration of clinical symptoms of EAE. | |
| MOG35–55 [119] | MOG35–55 | - | Prophylactic: every other day, for 10 days b.i. | oral | 200 μg of MOG35–55 | C57BL/6 male mice (6–8 weeks old) with EAE induced with MOG35–55. | Oral vaccination with MOG35–55 was found capable of efficiently suppressing pathogenic cells. |
| MBP [120] | MBP | - | Prophylactic: one day b.i. | oral | 100 mg of MBP | Euthymic and adult thymectomized Tg mice with EAE induced with MBP. | Euthymic Tg mice were shown to be protected from EAE after oral administration of MBP contrary to thymectomized mice, thus indicating the key role of thymus in oral tolerance induction. |
| Altered peptide ligands (APLs) | |||||||
| APL [121] | P1: MBP87–99, P2: (Ala91,Ala96)MBP87–99 P3: cyclo(87–99) (Ala91,Ala96)MBP87–99 | - | Prophylactic: on the day of immunization | s.c. | Female Lewis rats (6–8 weeks old) with EAE induced with MBP74–85 | Suppression of EAE was detected 8 days post P2 and P3 administration. P1 was not found to suppress EAE. P2 was shown to suppress EAE between 8–16 days whereas P3 suppressed EAE until the end of the experiment (e.g., day 18 or 20). | |
| APL [87] | [Ala41]MOG35–55, [Ala41,46]MOG35–55 and [TyrOMe40]MOG35–55 cyclo(46–55)MOG35–55 and cyclo(41–55)MOG35–55 | - | Prophylactic: on the day of immunization. | s.c. | C57BL/6 female mice (12–18 weeks old) with EAE induced with rat MOG35–55 | Significant reduction of EAE incidence and symptons with the administration of [Ala41,46]MOG35–55 or [Ala41]MOG35–55 as compared with the delivery of [TyrOMe40]MOG35–55, cyclo(46–55)MOG35–55 and cyclo(41–55)MOG35–55 | |
| Y-MSPc | |||||||
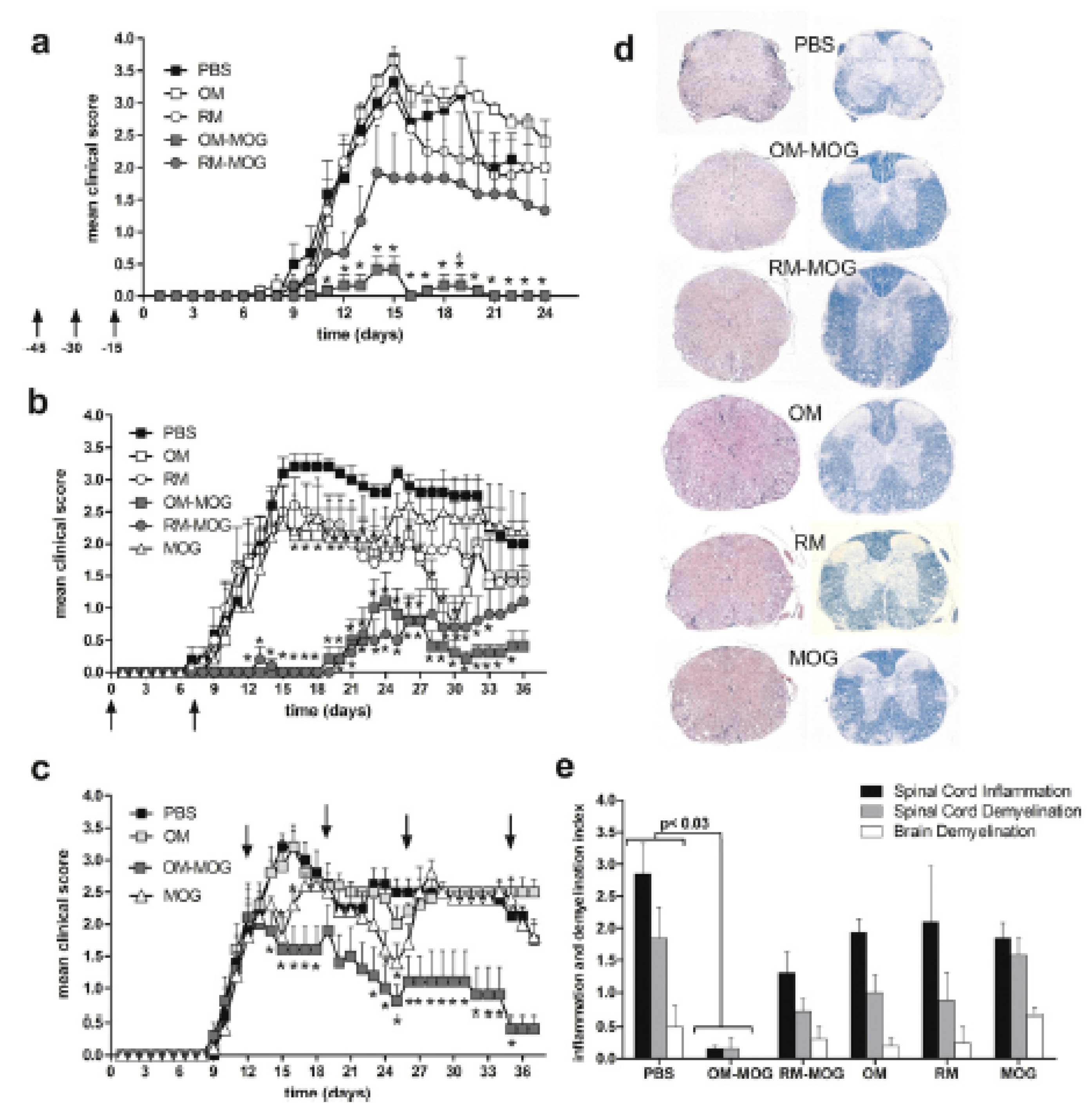
| Y-MSPc [94] | MOG34–56 MBP89–104 OSP55–80 OSP179–201 MOBP15–36 PLP139–151 PLP178–191 | - | Preclinical/Therapeutic: 3, 5, 7, and 21 days p.i. | i.v. | 75 μg of Y-MSPc/mouse | SJL/J female mice (2–3 months old) with EAE induced with PLP139–151 | Y-MSPc was revealed to be more efficient in inhibiting the development of the disease and suppressing its progression in comparison with a single encephalitogenic peptide or a cocktail of peptides. |
| Y-MSPc [93] | OSP55–74 MOBP55–77 MOBP15–36 MOG34–56 PLP175–194 PLP139–151 MBP89–104 | Preclinical/Therapeutic: administration post immunization | i.v. | 75 μg of Y-MSPc/mouse | (C57Bl/6J6SJL/J)F1 mice with EAE induced with PLP139–151 or rhMOG (active classical EAE), or a mixture of hMOG 34–56, hPLP 139–151, hMOBP15–36, hMBP89–104, hOSP55–80 (active complex EAE), or via transfer of line T cells specific for phMOG34–56 or phPLP139–151 (passive EAE) | Y-MSPc was shown to be more efficient in inhibiting the development of classical or complex EAE, suppressing the disease course and reversing the chronic disease, compared with a single encephalitogenic peptide or a cocktail of peptides. Additionally, Y-MSPc appeared to be more effective in suppressing passive EAE. | |
| Cytokine-neuroantigen (NAg) fusion proteins | |||||||
| GMCSF-NAg and MCSF-NAg [60] | Guinea pig MBP69–87 | GM-CSF M-CSF cytokines | Therapeutic: Exp.1: 9, 10, 12, and 14 days p.i.; exp. 2: 10, 11, and 13 days p.i.; exp. 3: eight and 11 days p.i. | s.c. | 1 nmol of fusion protein(s) per injection (exp. 1 and 2), 4 nmol on day 8 and 1 nmol on day 11 (exp. 3) | Lewis rats with EAE induced with DHFR-NAg fusion protein | GMCSF-NAg was found to potently target MBP69–87 to subsets of myeloid APCs and to successfully induce antigen-specific tolerance. |
| GMCSF-NAg MCSF-NAg [98] | MBP69–87 | GMC-SF MCSF | Prophylactic: 21, 1,4 and 7 days b.i. Therapeutic: 9, 10, 12 and 14 days p.i. (exp. 1), or 10, 11, and 13 days p.i. (exp. 2), or eight and 11 days p.i. (exp. 3) | s.c. | Prophylactic: 4 nmol of fusion protein(s) per injection Therapeutic: 1 nmol (exp. 1 & 2), 4 nmol on day 8 and 1 nmol on day 11 (exp. 3) | Lewis rats with EAE induced with DHFR-NAg fusion protein | Prophylactic vaccination with GMCSF-NAg resulted in attenuation of EAE severity. Furthermore, treatment with GMCSF-NAg successfully inhibited EAE progression to more severe stages. |
| GMCSF-NAg [122] | MOG35–55 | GM-CSF | Preclinical/Therapeutic: p.i. | s.c. | 2 or 1 nmol of GMCSF-NAg | C57BL/6 mice with EAE induced with MOG 35–55 (active EAE) or with activated MOG-specific Th1 T cells (passive EAE). SJL mice with EAE induced with PLP139–151. B cell deficient, CD4-deficient, IFN-γR1-deficient, and 2D2 | GMCSF-NAg was shown to suppress the established disease especially in passive EAE models. It also proved to be an efficient therapy for Cd4−defficient mice and to exhibit tolerogenic activity in B cell deficient mice. |
| Cytokine-NAg [97] | MOG35–55 PLP139–151 | GM-CSF | Prophylactic: 21, 14 and 7 days b.i. Therapeutic: 13, 15, 17, and 20 days p.i. | s.c. | Prophylactic: 2 nmol of cytokine-NAg Therapeutic: 4 nmol on days 9 and 11, and 2 nmol on day 14 p.i. | C57BL/6 with EAE induced with MOG35–55 (active EAE) or with transfer of activated MOG35–55-specific T lymphocytes. In order to provoke another bout of EAE on day 42, mice were challenged with MOG35–55. SJL mice with EAE induced with PLP139–151. | Fusion of GM-CSF with myelin protein epitopes was found to lead to efficient antigen uptake by myeloid APCs resulting in blocking of the development and progression of EAE. |
| Cytokine-NAg [96] | MBP69–87 MBP73–87 PLP139–151 MOG35–55 | GMCSF IFN-β IL16 IL2 | Prophylactic: 21, 14, and 7 days b.i. Therapeutic: 13, 15, 17, and 20 days p.i. or alternatively after the onset of paralysis | s.c. | C57BL/6 mice with EAE induced with MOG35–55. SJL mice with RR EAE induced with PLP139–151. Lewis rats with EAE (acute monophasic form) induced with MBP73–87 | The developed cytokine-NAg fusion proteins were shown to target APCs and to successfully prevent the induction of EAE when administered prophylactically as well as to suppress on-going EAE. | |
| Cytokine-NAg [123] | Guinea pig MBP | rat IL-2 or IL-4 | Prophylactic: 21, 14 and 7 days b.i. Preclinical/Therapeutic: five days p.i. and on every other day through days 9, 11, or 13 p.i. | s.c. | Prophylactic: 0.5-1 nmol per injection | Lewis rats with EAE induced with guinea pig MBP fusion protein | Prophylactic or therapeutic vaccination with IL-2/NAg resulted in attenuation of EAE course, whereas administration of IL4-NAg indicated lack of tolerogenic activity. |
| GMCSF-NAg [95] | MOG35–55 | GM-CSF | C57BL/6 mice: Prophylactic 21, 14, and 7 days b.i. 2D2-FIG mice: Preclinical/Therapeutic: 0, 7, and 14 days, or 7 and 14 days, or 14 days p.i. | C57BL/6 mice: s.c. 2D2-FIG mice: i.v. | C57BL/6 mice: 2 nmol GMCSF-MOG35–55 per injection 2D2-FIG mice: 4 nmol per injection | C57BL/6 mice with EAE induced with MOG 35–55 2D2-FIG mice with a transgenic MOG-specific repertoire of T cells and a GFP reporter of FOXP3 expression | The pretreatment with the GMCSF-MOG fusion protein elicited CD25+ Tregs which were required for the induction of tolerance. Vaccination of 2D2-FIG with GMCSF-MOG elicited circulating FOXP3+ Tregs the number of which was maintained with multiple boosters. |
| MOG35–55/I-Ab dimer [107] | MOG35–55 | I-Ab dimer | Therapeutic: nine days p.i. (treatment duration: four days). | i.p. | 12 nM MOG35–55/I-Ab dimer (1 μg/mouse/day) | C57BL/6 female mice (6–8 weeks old) with EAE induced with MOG35–55 | The administration of MOG35–55/I-Ab dimer resulted in the reduction of antigen-specific T cells and amelioration of EAE symptoms. |
| Antibodies coupled with myelin peptides | |||||||
| α-receptor–MOGp mAbs [100] | DNA for MOG29–59 (MOGp) | α-DEC mAbs α-Langerin mAb | Prophylactic: transfer of MOG-specific CD4+ T cells 15 days b.i. and admin. of α-receptor–MOGp mAbs 14 days b.i. | s.c. | 3 μg of α-receptor mAbs | C57BL/6 (B6) mice with EAE induced with MOG35–55 | Prophylactic vaccination with α-DEC- and a-Langerin–MOGp mAbs led to reduction of disease incidence, onset delay and amelioration of clinical scores. |
| αDEC205-PLP139–151 mAb [Stern et al., 2010] | PLP139–151 | anti-DEC205 | Prophylactic: 10 or 15 days b.i. | i.p. | 1 μg of fusion mAb | SJL/J female mice (6–10 weeks old) with EAE induced with PLP 139–151 | Administration of αDEC205-PLP139–151 mAb was found to alleviate the disease symptoms. |
| scFv DEC:MOG [102] | MOG | scFv specific for DEC205 | Prophylactic: seven and three days b.i. Therapeutic: oje and four days after disease onset, signified by a clinical score equal to 1 | i.v. | 10 μg of scFvDEC:MOG | C57/Bl6 mice with EAE induced with WSCH | Almost complete prevention of EAE (90% of mice) was observed by administration of scFv DEC:MOG b.i. Moreover, vaccination with scFv DEC:MOG p.i. resulted in significant alleviation of the clinical symptoms in 90% of the mice. |
| αDCIR2-PLP139–151 fusion mAb [79] | PLP139–151 | αDCIR2 | Prophylactic: 10 days b.i. | i.p. | 1 μg of fusion mAbs | SJL/J female mice (6–10 weeks old) with EAE induced with PLP139–151 (active EAE) or via adoptive transfer of splenocytes from αDCIR2-PLP139–151-treated mice (passive EAE) | Vaccination with αDCIR2+-PLP139–151 fusion mAb was shown to decrease the severity of the disease and to delay its onset. Mice receiving splenocytes from αDCIR2-PLP139–151-treated mice exhibited substantially lower clinical scores in comparison to those receiving cells from αDCIR2 mAb-treated mice. |
| αCD4/CD8+PLP139–151 [103] | PLP139–151 | Anti-CD4, anti-CD8a Ab | Prophylactic: admin. of mAb 21 days b.i. followed by PLP139–151 delivery every other day for 16 days. Therapeutic: Mice treated with αCD4/CD8 Abs on day 11 p.i. were injected with αCD4/CD8+PLP139–151 every other day from day 12–26. | i.p. | 100 μg of CD4-/mouse) 100 μg of CD8a-/mouse 25 μg PLP139–151 per injection | SJL female mice (seven weeks old) with EAE induced with PLP139–151 | αCD4/CD8+PLP139–151-treated mice exhibited substantially lower EAE scores and reduced rate of relapses in chronic disease |
| Recombinant T-cell receptor ligands (RTLs) | |||||||
| RTL342M [124] | MOG35–55 | HLA-DR2 peptide-binding domains | Therapeutic (s.c. or i.v.): admin. on the day that the clinical score for each mouse was ≥ 2. Daily admin. for mice receiving multiple doses. Prophylactic (s.c.): admin. of 4, 9, or 14 doses within 15 days. EAE was induced 2 days after the admin. of the final dose. | i.v. s.c. | 50 μg of RTL342M | HLA-DR2 positive male/female mice (8–12 weeks old) with EAE induced with MOG35–55 | RTL treatment was revealed to be more efficient in reducing paralysis when administered in the form of multiple doses instead of a single dose, independently of the administration mode. Furthermore, the treatment with RTL342M could treat or prevent relapses. Pretreatment with RTL342M was shown to prevent the disease. |
| RTL401 [125] | PLP139–151 | α1 and β1 domains of the I-As class II molecule | Upon EAE onset, daily i) i.v. admin. for 3–4 days and ii) s.c. admin. for 8 days. | i.v. s.c. | 100 μg of RTL401 | SJL mice (6–7 weeks of age) with EAE induced with PLP139–151 or PLP178–191 or MBP84–104. C57BL/6 X SJL) F1 mice (6–7 weeks of age) with EAE induced with MOG35–55 or PLP139–151. | i.v. or s.c. vaccination with RTL401 resulted in prevention of relapses and long-term reduction of clinical severity only in SJL mice and C57BL/6 X SJL) F1 mice with EAE induced with PLP139–151. |
| RTL401 [126] | PLP139–151 | α1 and β1 domains of the I-As class II molecule | Upon EAE onset, daily (i) i.v. admin. for five days and (ii) s.c. for eight days. | i.v. s.c. | 100 μL of 1 mg/mL RTL401 | SJL female mice (7–8 weeks old) with EAE induced with PLP139–151 (active EAE) or via transfer of activated PLP139–150-specific T cells (passice EAE) | i.v. or s.c. vaccination with RTL401 was shown to effectively discontinue passive EAE progression, reverse its clinical severity and reduce the infiltration of cells into the CNS, as in the treatment of active EAE. Injury to axons was also prevented. |
| RTL551 [127] | MOG35–55 | α1 and β1 domains of the I-Ab class II molecule | Upon EAE onset (days 12–14 for active EAE and days 7–12 for passive EAE), daily i.v. admin. for five days. | i.v. | 100 μL of 1 mg/mL RTL551 | C57BL/6 male mice (6–7 weeks of age) with EAE induced with MOG35–55 (active EAE) or via transfer of activated cells (passive EAE). | RTL551 treatment of actively or passively induced EAE resulted in significant reduction of clinical symptoms and spinal cord lesions. |
| RTL401, RTL402, RTL403 [128] | PLP139–151 PLP178–191 MBP84–104 | α1 and β1 domains of the I-As class II molecule | At EAE onset (days 10-11), when the clinical score was ≥2, daily s.c. admin. for 8 days. | s.c. | 100 μL of 1 mg/mL RTL | SJL/J female mice (7–8 weeks old) with EAE induced with WSCH or with a mixture of PLP139–151 and PLP178–191. | A single RTL was found capable of successfully treating ongoing disease induced with a mixture of encephalitogenic epitopes as long as the cognate T cell specificity was present. |
| RTL551 [106] | rhMOG, hMOG35–55, mMOG35–55 | α1 and β1 domains of the I-Ab class II molecule | At EAE onset (days 10–13), when the clinical score was ≥2, daily i.v. admin. for eight days. | i.v. | 100 μL of 1 mg/mL RTL551 | C57BL/6 male mice (7–8 weeks old) with EAE induced with rhMOG or mMOG35–55. | Vaccination with RTL551 could reverse the progression of EAE, reduce demyelination and damage of axons without however induce suppression of anti-MOG Ab response. |
| RTL401 [129] | PLP139–151 | α1 and β1 domains of the I-As class II molecule | Upon EAE onset (days 10–11), daily admin. for 1, 2, or 5 days. | s.c. | 100 μL of 1 mg/mL RTL401 | SJL/J female mice (7–8 weeks old) with EAE induced with PLP139–151 (active EAE) or via transfer of activated cells (passive EAE). TCR Tg 5B6 mice with EAE induced with PLP139–151 B cell deficient (μMT knock-out, KO) mice on C57BL/6 background (7–8 weeks old) with EAE induced with MOG35–55. | A new interaction between cells was revealed via which the RTL-equipped myeloid APCs reverse EAE progression by transferring tolerogenic signals to cognate T lymphocytes. It was also found that splenocytes incubated with RTL401 exhibited reduced ability to passively transfer EAE. Finally, it was shown that EAE can be treated by RTL551 in the absence of B cells. |
| VG312, VG303, VG311 [108] | MOG35–55, MBP85-99, CABL | α1 and β1 domains of DR2 | Therapeutic: i.v. administration for eight consecutive days, 2–4 days after the disease onset. | i.v. | 100 μL of VG312, VG303, VG311 | Tg HLA-DR2 male and female mice (8–12 weeks old) with EAE induced with MOG35–55 | Vaccination with VG312 led to peptide- and dose-dependent induction of long-term tolerance to the encephalitogenic epitope MOG35–55 and reversal of the clinical/histological symptoms of EAE |
| RTL401 [130] | PLP139–151 | α1 and β1 domains of the I-As class II molecule | Therapeutic: (i) i.v. admin. for five consecutive days (days 20–24) and (ii) s.c. admin. for 3 days (days 32–34). | i.v. s.c. | 100 μg of RTL401 | SJL/J female mice (7–8 weeks old) with EAE induced with PLP139–151. | Administration of RTL401 post the relapsing EAE peak resulted in prevention of disease relapses, reduction of demyelination and axonal damage. |
| Bifunctional peptide inhibitor (BPI) | |||||||
| PLP-B7AP [131] | PLP139–151 | B7 antisense peptide (AP) derived from CD28 receptor | Prophylactic 11, 8, and 5 days b.i. Preclinical/Therapeutic: 4, 7, and 10 days p.i. | s.c. | Prophylactic: 50 or 100 nmol PLP-B7AP/injection Therapeutic: 100 nmol PBI/injection | SJL/J female mice (5–7 weeks old) with EAE induced with PLP139–151 | Both prophylactic and therapeutic vaccination with PLP-B7AP resulted in efficient suppression of EAE. Mice treated with PLP-B7AP exhibited significantly low demyelination. |
| PLP-LABL [132] | PLP139–151 | LABL | Prophylactic: 11, 8, and 5 days b.i. | s.c. | 100 nmol/injection/day | SJL/J female mice (5–7 weeks old) with EAE induced with PLP | The vaccination with PLP-LABL inhibited the inflammatory response resulting in prevention of BBB disruption and thus inhibition of EAE onset and progression. |
| PLP-LABL derivatives [110] | PLP139–151 | LABL | Therapeutic: admin. on disease onset, signified by a clinical score ≥1, and for three consecutive days until the score was <1) | i.v. | 100 nmol/mouse | SJL/J (H-2S) female mice (5–7 weeks old) | Vaccination with the synthesized BPI derivatives was shown to efficiently inhibit EAE severity, and incidence. |
| PLP-LABL [133] | PLP139–151 | LABL | Preclinical/Therapeutic: 4, 7, 10, and 14 days p.i. | i.v. | 100 mol/mouse | SJL/J female mice (5–7 weeks old) with EAE induced with PLP139–151 | Low disease scores and incidence could be observed in mice vaccinated with PLP-LABL. |
| PLP-LABL derivatives [134] | PLP139–151 | LABL | Therapeutic: admin. on disease onset, signified by a clinical score ≥1, and for three consecutive days until the score was <1) | i.v. | 100 nmol/mouse | SJL/J female mice (5–7 weeks old) with EAE induced with PLP139–151 | The synthesized BPI derivatives were revealed to suppress EAE progression after intravenous administration more efficiently in comparison with unmodified BPI. |
| BPI-Fc fusion peptides LABL-Fc-ST-PLP and LABL-Fc-ST-MOG [109] | PLP139–151 MOG38–50 | LABL-Fc-ST | Preclinical/Therapeutic: four and seven days p.i. | i.v. | 25 nmol per dose | SJL/J mice (5–7 weeks old) with EAE induced with PLP139–151 | BPI-Fc fusion peptides were revealed to be highly efficient in suppressing EAE. The vaccinated mice were not found to exhibit weight loss, and featured benign clinical symptoms and reduced demyelination. |
| PLP–cIBR Derivatives [135] | PLP139–151 | cIBR7 peptide | Studies I and II: 4, 7, and 10 days p.i. Study III: admin. on disease onset, signified by a clin. score ≥1, and for 3 consecutive days until the score was <1 | i.v. | Study I: 100 nmol/injection/day Study II and III: 50 nmol/injection/day | SJL/J (H-2S) female mice (5–7 weeks old) with EAE induced with PLP139–151 | Vaccination with PLP–cIBR, even at low dose or less frequent i.v. injections, resulted in significant amelioration of EAE and protected CNS against demyelination. |
| Multivalent BPI (MVBMOG/PLP) [111] | MOG38–50 PLP139–151 | LABL | Preclinical/Therapeutic 4, 7, and 10 days p.i. | s.c. | 100 nmol/mouse | SJL/J female mice (5–7 weeks old) with EAE induced with PLP139–151 C57BL/6 mice (4–6 weeks old) with EAE induced with MOG38–50 | MVBMOG/PLP was found to significantly suppress EAE in both animal models despite the evidence of epitope spreading in the C57BL/6 mice. |
| Antigen-drug conjugates | |||||||
| PLP139−151-DEX [61] | PLP139−151 | DEX | Preclinical/Therapeutic: 4, 7, and 10 days p.i. | s.c. | SJL/J female mice (4–6 weeks old) with EAE induced with PLP139–151 | Vaccination with PLP139–151-DEX efficiently protected the SJL/J mice from the onset of clinical symptoms compared with DEX treatment. | |
| Vaccine | Antigen/Immunosuppr. | Vaccination Type | Admin. Route | Admin. Dose | Animal Model | Vaccination Outcome |
|---|---|---|---|---|---|---|
| pDNA encoding IL-4 pDNA encoding PLP139–151 pDNA encoding MOG [142] | PLP139–151 | Prophylactic: 17 and 10 days b.i. Therapeutic: 14 and 21 days p.i Co-vaccination with IL-4 plasmid and MOG plasmid on days 18 and 27 p.i. | i.m | 100 μg of plasmid per injection | SJL/J mice with EAE induced with PLP139–151 C57BL/6 mice with EAE induced with MOG35–55 | Co-vaccination with IL-4 and PLP139–151 plasmids significantly protected against induction of EAE. Co-vaccination with IL-4 plasmid and MOG plasmid reversed ongoing EAE. |
| pMOG 91–108 pK0-MOG91–108 (lacking CpG motifs) [143] | MOG91–108 | Prophylactic: three weeks b.i. | i.m. | 200 μg DNA/injection | LEW.1AV1 (RT1av1) female rats (4–5 weeks old) with EAE induced with MOG91–108 | Vaccinated rats were protected against EAE. |
| pDNA encoding IL-10 pDNA encoding MBP68–86 [144] | MBP68–86 | Admin. at the disease onset | Female Lewis rats (~6 weeks old) with EAE induced with MBP68–86 or MBP87–99, or with EAN induced with P257–81 | Rats co-vaccinated with IL-10 and MBP68–86 plasmids went into rapid remission. Co-administration of pDNA encoding IL-10 and pDNA encoding MBP68–86 were shown to suppress EAE in rats induced either with MBP68–86 or MBP87–99 but not EAN. | ||
| pZZ/MOG91–108 pMOG91–108 pK0-MOG91–108 pK3-MOG91–108 [145] | MOG91–108 | Prophylactic: 3–4 weeks b.i. | i.m. | 200 μg DNA/injection 100 μg of CpG DNA were added to pMOG91–108 before the injection | Female LEW.1AV1 (RT1av1) rats (4–5 weeks old) and female DA rats with EAE induced with MOG91–108 | Vaccination with pDNA encoding MOG91–108 (lacking the ZZ gene) reduced clinical symptoms of EAE and mortality in rats with different genetic background sharing the same MHC. |
| DNA encoding MBP, PLP, MOG, MAG and IL-4- [10] | MBP, PLP, MOG, MAG/GpG ODN | Therapeutic: admin. at the peak of acute EAE, when mice exhibited paralysis | i.m. i.p. | 0.025 mg of each myelin peptide plasmid, 0.05 mg of IL-4 plasmid and 0.05 mg of GpG ODN | Female SJL/J and C57BL/6 (B6) mice (8–12 weeks old) with EAE induced with PLP139–151 or MOG35–55 | Administration of myelin cocktail/IL-4 plasmids and the immunosuppressant GpG ODN resulted in dramatic improvement of the disease in mice having either chronic relapsing or chronic progressive EAE. |
| pMOG 91–108 pMOG-IFN-β pMOG-scr [146] | MOG91–108 | Prophylactic: three weeks b.i. | i.m. | 200 μg DNA/injection | Female LEW.1AV1 (RT1av1) rats (4–5 weeks old) and female DA rats with EAE induced with MOG91–108 | The suppressive ability of DNA vaccination was found to be abrogated via silencing IFN-β. |
| p2MOG35 [147] | MOG35–55/Tacrolimus (FK506) | Preclinical/Therapeutic: three and 17 days p.i. | i.m. | 100 μg of p2MOG35/mouse 10 μg of FK506/mouse | Female C57BL/6 mice (6–8 weeks old) with EAE induced with MOG35–55 | Co-administration of p2MOG35 with FK506 was shown to effectively meliorate EAE in mice. |
| pVAX-PLP, pVAX-MOG [148] | PLP, MOG | Prophylactic: four or 12 weeks b.i. | i.m. | 20μg pVAX-PLP, pVAX-MOG | Female SJL/J (9H-2) mice (6 weeks old) with EAE induced with PLP139–151 C57/B6 mice with EAE induced with MOG35–55 | EAE was found to be exacerbated in mice vaccinated with pVAX-PLP 4 weeks prior to immunization whereas both clinical and pathological symptoms were suppressed in mice vaccinated 12 weeks prior to EAE induction. In mice vaccinated with pVAX-MOG, either four or 12 weeks prior to immunization, EAE was shown to be significantly suppressed. |
| Cells | Inductive Agent/Peptide | Vaccination Type | Admin. Route | Admin. Dose | Animal Model | Vaccination Outcome |
|---|---|---|---|---|---|---|
| Tolerogenic Dendritic cells (tolDCs) | ||||||
| BMDCs from C57BL/6 mice [161] | Atorvastatin/MOG35–55 | Preclinical/Therapeutic: days five and 13 p.i. | i.p. | 1 × 106 cells per injection | Female C57BL/6 mice (8–10 weeks old) with EAE induced with MOG35–55 | MOG35–55—specific tolDCs successfully ameliorated clinical Symptoms in mice with EAE. |
| BMDCs [162] | mytomycin C/MOG196–204 | Admin. of MOG196-pulsed Kb−/−Db−/− DCs to C57BL/6 (B6) mice one week b.i. and one p.i. Admin. of MOG196-pulsed B6 DCs to C57BL/6 mice three days b.i. and two and seven days p.i. | s.c. | 1 × 106 cells per injection | Female C57BL/6 (B6) (8–10 weeks old) with EAE induced with MOG35–55 | Administration of MOG196-pulsed Kb−/−Db−/− DCs or MOG196-pulsed DCs ameliorated EAE in mice. |
| Murine BMDCs [154] | 1α, 25-dihydroxy-vitamin D3/MOG-encoding mRNA or MOG35–55 | Therapeutic: 13, 17, and 21 days p.i. | i.v. | 1 × 106 cells per injection | Female C57BL/6JOlaHsd mice (8–10 weeks old) with EAE induced with MOG35–55 | Vaccination with tolDCs electroporated with MOG-encoding mRNA or MOG35–55 stabilized the clinical signs of the disease already from the first injection. MRI examination of hyperintense spots present along the spinal cord of mice was found to be in line with the clinical score (Figure 9). |
| BMDCs [163] | CD40-specific and p19-specific shRNA encoding lentiviral vectors/pyromycin/MOG35–55 | Preclinical/Thereapeutic: 3, 5, and 7 days p.i. | i.v. | 2 × 106 cells per injection | C57BL/6 mice with EAE induced with MOG35–55 | Administration of MOG35–55-pulsed and lentiviral transduced BMDCs led to significant decrease in the clinical symptoms of EAE in mice. The highest decrease in the clinical scores was observed with the administration of co-transduced BMDCs (BoLV-DCs). |
| BMDCs [164] | Vitamin D3/MOG40–55 | Preclinical/Therapeutic: two and five days p.i., or five and nine days p.i. or 15, 19, 23, and 33 days p.i. | i.v. | 2 or 4 × 106 cells | Female C57BL/6J mice (8–10 weeks old) with EAE induced with MOG40–55 | MOG40–55—specific TolDCs were found to succeed in reducing EAE incidence and ameliorating its clinical signs. |
| BMDCs [165] | Vitamin D3/MOG40–55/cryopreserved | i.v. | 2 or 4 × 106 cells | Female C57BL/6J mice (8–10 weeks old) with EAE induced with MOG40–55 | It was shown that MOG40–55—specific TolDCs maintain their tolerogenic properties and can efficiently ameliorate the clinical symptoms of EAE. | |
| Murine BMDCs [166] | Tofacitinib/MOG35–55 | Therapeutic: 7, 11, and 15 days p.i. | i.v. | Twelve-week Female C57BL/6 mice (12 weeks old) with EAE induced with MOG35–55 | MOG35–55—specific TolDCs efficiently dampened EAE severity and progression. | |
| BMDCs [167] | 1,25-dihydroxyvitamin D3/MOG35–55 | Therapeutic: 10, 13, and 16 days p.i. | i.v. | Female C57BL/6 mice (6–8 weeks old) with EAE induced with MOG35–55 | Vitamin D3 treated MOG35–55—specific. TolDCs succeeded in postponing the disease onset and reducing its clinical scores. | |
| DCs [168] | Estriol (E3)/MOG35–55 | Prophylactic: one day b.i. | i.v. | 8–10 × 106 cells per mouse | Female C57BL/6 (H-2b) mice (4–6 weeks old) with EAE induced with MOG35–55 | Mice vaccinated with E3 MOG35–55—specific TolDCs exhibited a reduced cumulative clinical score and EAE severity. They also avoided relapses and development of chronic disease. |
| BMDCs matured with TNF-α [169] | /MOG35–55 | Prophylactic: 7, 5, 3, and 1 days b.i. Preclinical: one day p.i. | i.v. | 2–2.5 × 106 cells per injection Rat anti–mouse IL-10R mAb: 0.5 mg equivalents per mouse | C57Bl/6 mice with EAE induced with MOG35–55 | Vaccination with MOG35–55—specific TNF/DCs improved the clinical disease score. Pulsing of TNF-α/DCs with an unrelated peptide did not succeed in preventing the disease. |
| DCs [170] | /in vivo pulsing in Lewis rats with EAE induced with MBP68–86 | Prophylactic: four weeks b.i. | s.c. | 1 × 106 cells per rat | Male Lewis rats with EAE induced with MBP68–86 | Injection of EAE DCs to rats resulted in induction of immune tolerance against the disease as demonstrated by delayed onset and marked decrease of the mean clinical score. |
| T cell-based vaccination | ||||||
| Ob2F3 Tregs [171] | Retrovirally transduced pre-stimulated naïve CD4+ Tcells from peripheral blood mononuclear cells (PBMCs) of healthy donors using Ob2F3. | Preclinical/Therapeutic: seven days p.i. | i.v. | 2 × 106 cells | Male and female HLA-DR15 transgenic mice (4.5–7.5 months old) with EAE induced with MOG35–55 | Ob2F3 Tregs were shown to significantly ameliorate MOG35–55 induced EAE via bystander suppression. |
| MBP-specific T-cell lines (e.g., B12 and B12-GFP) [157] | Prophylactic: admin. three times at weekly intervals, with the last injection 10 or seven days b.i. | s.c. | 1 × 107 activated and irradiated T cells | Female Lewis rats (6–8 weeks old) with EAE induced via i.v. injection of antigen stimulated T cells. | Vaccination with MBP-specific T cell lines inhibited the development of EAE clinical symptoms. | |
| Hematopoietic stem cells (HSCs) | ||||||
| DC-MOG vector-transduced BM-HSC [172] | Ex vivo modification of HSCs with SIN lentivirus vectors which transcriptionally target the expression of myelin peptides to DCs. | Prophylactic: Lethally Irradiated (10.5 Gy) mice were transplanted with DC-MOG transduced BM-HSCs eight weeks b.i. BM chimeras received neomycin treatment for three weeks post transplantation. | i.v. | 1–3 × 106 cells per mouse | C57BL/6 mice with EAE induced with MOG peptide. | The transplantation of DC-MOG vector-transduced BM-HSC was found to completely protect mice from developing EAE even in cases of transplantation 6 months b.i. In agreement with the clinical observations, no histological signs of the disease such as demyelination, damage of axons, etc. could be detected in the tolerized mice. |
| Bone marrow cells (BMC) | ||||||
| BMCs expressing MOG40–55 [173] | liMOG | Prophylactic: mice were transplanted with BMCs transduced with liMOG 21 days b.i. Therapeutic: mice were transplanted with transduced BMCs 15–17 days p.i. | i.v. | 0.7–1.6 × 106 cells per mouse | Female C57BL/6J mice (5–10 weeks old) with EAE induced with MOG40–55 | Transplantation of BMCs expressing MOG40–55 was shown to protect mice from developing EAE and reduce the disease severity in mice with established EAE. |
| Myeloid-derived suppressor cells (MDSCs) | ||||||
| MDSCs isolated via positive selection from BMCs expressing MOG40–55 [174] | liMOG | Prophylactic: mice were transplanted with MDSCs transduced with liMOG seven days b.i. Therapeutic: mice were transplanted with transduced MDSCs 13–14 days p.i. | i.v. | 0.5–1 × 106 cells per mouse | Female C57BL6/J mice (6–8 weeks old) with EAE induced with MOG40–55 | MOG40–55 -expressing MDSCs were found to exhibit both preventive and therapeutic effects in EAE induced with MOG40–55 |
| Antigen-cell conjugates | ||||||
| Ag-SP [158] | Chemically treated Ag-coupled SPs | Administration on day −7 b.i. or at peak of disease in actively induced EAE, or two days p.i. | i.v. | 50 × 106 Ag-SPs per mouse | Wild-type C57BL/6 (I-Ab), B10.S (I-As), and BALB/c (I-Ad) female mice (5–6 weeks old) with EAE induced with myelin peptide or via adoptive transfer. | It was revealed that syngeneic or allogeneic Ag-SPs can effectively protect mice against ongoing clinical EAE. |
| Ag-SP [159] | Chemically treated Ag-coupled SPs | Prophylactic: at indicated time points b.i. | i.v. | 50 × 106 Ag-SPs or 15–20 μg Ag per mouse | SJL and C57BL/6 mice with EAE induced with myelin peptide or via adoptive transfer. | i.v. infusion of peptide antigens coupled to syngeneic splenic leukocytes (Ag-SP) was found to efficiently induce antigen-specific T cell tolerance. |
| Ag-RBC [160] | Genetically engineerd Kell-LPETGG RBCs, coupled with MOG 35–55 through enzymatic surface modification with sortase transpeptidase. | Prophylactic: transfusion seven days b.i. Preclinical: transfusion five days p.i. Therapeutic: Transfusion on the day of EAE onset | i.v. | 200 μL RBC-MOG35–55 | C57BL/6J (CD45.2+), B6.SJL-Ptprc (CD45.1+), BALB/c Female C57BL/6 mice (10–12 weeks old) with EAE induced with MOG35–55 | The transfusion of RBC-MOG35–55 was shown to significantly improve the clinical signs of EAE in mice. |
| Carrier | Particle Size (nm) | Zeta Potential (mV) | Antigen | Ag Loading (wt%)/Enc. Eff. (%) | Immunomodul. Agent | Vaccination Type | Admin. Route | Dose | Animal Model | Vaccination Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Polymer particles | ||||||||||
| PLGA NPs [193] | - | - | MOG35–55 | - | (r) IL-10 | Prophylactic: 31 and 15 days b.i. Therapeutic: eight and 22 days p.i. | s.c. | Female C57BL/6 mice with EAE induced with MOG35–55 | Vaccination with mixed PLGA- MOG35–55 and PLGA-IL10 both in a prophylactic and therapeutic setting resulted in significant protection, decrease of EAE severity and reduction of histopathological lesions in spinal cord. | |
| PLGA NPs [194] | - | - | PLP139–151 | 8μg/mg NP | TGF-β (166ng/mg NP) | Prophylactic: seven days b.i. Therapeutic: 13 days p.i. | i.v. s.c. | 2.5, 1.25, 0.0625 mg NPs | Female SJL/J mice (6–8 weeks old) with EAE induced with PLP139–151 | i.v. vaccination with PLGA- PLP139–151-TGF-β demonstrated improved efficiency at lower doses. s.c. delivery of TGF-β-coupled to PLGA- PLP139–151 NPs reduced the severity of relapses in EAE. |
| PLGA MPs [195] | 800, 55,000 | MOG35–55 | -/48.6 | Vitamin D3 TGF-β1 Recombinant mouse GM-CSF | Preclinical/Therapeutic: 4, 7, and 10, days p.i. | s.c. | Female C57BL/6 mice (10–11 weeks old) with EAE induced with MOG35–55 | Delivery of various immunomodulators combined with MOG35–55 via a dual size MP platform resulted in the induction of enhanced antigen-specific autoimmune protection. | ||
| PLGA NPs [196] | 151.2, 521.7 | −14.1, −5.65 | MOG35–55 | 2.58, 0.96 /25.85, 9.65 | - | Prophylactic: seven days b.i. | i.v. s.c. | 2 mg NPs containing 20 μg MOG35–55 | Female C57BL/6 mice (6–8 weeks old) with EAE induced with MOG35–55 | The intravenous injection of PLGA- MOG35–55 was shown to delay EAE incidence and enhance antigen-specific immune tolerance. |
| PLGA-PEMA NPs [197] | 429.9 | −67.4 | PLP139–151PLP178–191 | 0.85/10.61 | - | Prophylactic: 7, 25, and 50 days b.i. Preclinical/Therapeutic: 4, 14, and 18 days p.i. | i.v. i.p. s.c. oral | 0.0625 0.125 0.625 1.25 | Female SJL/J mice (6-8 weeks old) with EAE induced with PLP178–191 | Vaccination with PLP epitope-coupled PLGA-PEMA NPs was shown to both prevent and treat relapsing-remitting EAE. Tolerance induction was antigen-specific. The i.v. administration route was the most effective. |
| PLGA/PLA-PEG NPs [198] | - | - | PLP139–151 | rapamycin | Prophylactic: 14 and 21 days b.i. Therapeutic: 13 days p.i. | s.c. i.v. | SJL mice with EAE induced with PLP139–151 | s.c. vaccination with the tolerogenic NPs inhibited paralysis. Therapeutic s.c. treatment completely inhibited EAE relapses. A single therapeutic dose of tolerogenic NP sadministered i.v. near the peak of EAE resulted in complete prevention of relapses. | ||
| PLGA-PEMA NPs [199] | 377.9, 621.5–834.8 | −72.8, −50 to −43.7 | PLP139–151 PLP178–191 | 0.58, 0.24–0.83/7.2, 4.4–16.5 | - | Prophylactic: seven days b.i. Therapeutic: 18 days p.i. | i.v. | SJL/J mice with EAE induced with PLP139–151 or PLP178–191 | Antigen-specific immune tolerance was successfully induced by PLP encephalitogenic epitopes, encapsulated in or conjugated with PLGA-PEMA NPs. | |
| PLGA NPs [181] | 217 | - | MOG40–54/H-2Db-Ig dimer, MOG35–55/I-Ab multimer | - | anti-Fas, PD-L1-Fc TGF-β1CD47-Fc | Therapeutic: 8, 18, 28, and 38 days p.i. | i.v. | 1 mg NPs/mouse/injection | Female C57BL/6J mice (8–10 weeks old) with EAE induced with MOG35–55 | Four i.v. injections of the developed NPs resulted in long-lasting amelioration of the disease by markedly reducing neuroinflammation, clinical EAE score and demyelination |
| PLGA NPs PLA NPs [200] | PLGA: 351.3–436.2 PLA: 443.2 | PLGA: −40.6 to −39.8 PLA: −40.2 | PLP139–151 | PLGA: 0.25–0.28 PLA: 0.25 | - | Preclinical/Therapeutic: seven days p.i. | i.v. | 2.5, 2.0, 1.5 or 1.0 mg NPs/mouse | Female SJL/J mice (8–10 weeks old) with EAE induced with PLP139–151 | Low dose vaccination with PLA NPs resulted in long-lasting (>200 days post immunization) significant reduction of the clinical score at the chronic stage of EAE contrary to vaccination with PLGA NPs. |
| PLGA MPs PEI-coated PLGA-MPs [201] | PLGA: 5080 | PLGA: 45.3 | MOG35–55 MOG40–54 MOG40–54/H-2Db-Ig dimer, MOG35–55/I-Ab multimer | anti-Fas, PD-L1-Fc TGF-β1 CD47-Fc | Therapeutic: 8, 18, 28, and 38 days p.i. | i.v. i.p. s.c. | Female C57BL/6J mice with EAE induced with MOG35–55 | Four injections of the multipotent particles resulted in long-lasting suppression of EAE and reduction of neuroinflammation in an antigen-specific manner. | ||
| PLGA MPs [202] | 8000 | Ac-PLP-BPI-NH2-2 | 1.4/8.2 | Preclinical/Therapeutic: 4, 7, 10, and 14 days p.i. | s.c. | Female SJL/J mice (5–7 weeks old) with EAE induced with PLP139–151 | Administration of PLGA MPs resulted in slightly less efficient reduction of EAE symptoms compared with the administration of the peptide solution, but without toxicity. | |||
| PLGA [203] | 400–656 | −51.3 to −38.0 | PLP 139–151 PLP 178–191 | 0.26–0.8 | Prophylactic: seven and one days b.i. | i.v. | Female SJL/J mice (6–8 weeks old) with EAE induced with PLP139–151 or both PLP139–151 and PLP178–191 | PLGA NPs coupled with a PLP encephalitogenic epitope were shown to efficiently induce antigen-specific tolerance in a mouse model of relapsing-remitting EAE induced either by PLP139–151 or by both PLP139–151 and PLP178–191 | ||
| PLGA NPs [204] | 363–420 | - | PLP139–151 | LABL | Preclinical/Therapeutic: 4, 7, and 10 days p.i. | s.c. | 100 nmol PLP per injection | SJL/J female mice (5–7 weeks old) with EAE induced with PLP139–151 | It was shown that efficient suppression of EAE required the co-administration of PLP peptide and LABL. | |
| PLGA [205] | 538 | −43 | PLP139–151 | 0.41–0.98 | - | Preclinical/Therapeutic: seven days p.i. | i.v. | 1 to 100 μg/mL NPs per injection | SJL/J mice with EAE induced with PLP139–151 | Antigen-specific, dose-dependent tolerance was successfully induced in an EAE model via the administration of PLGA NPs couple with a PLP peptide. |
| PLGA [206] | 500 | - | PLP139–151 | - | IL2 | Prophylactic: secen days b.i. Therapeutic: 11 days p.i. | i.v. | SJL/J mice with EAE induced with PLP139–151 | Vaccination with PLGA NPs loaded with PLP139–151 was found to prevent EAE onset and modulate its course. | |
| PLGA MPs [207] | 3900 | - | MOG35–55 | 0.73/38 | Rapamycin (loading: 0.17%/enc. eff. 42.1%) | Therapeutic: 10 days p.i. | direct intra-lymph node (LN) injection | 2 mg MPs per mouse or 1 mg MPs per LN | Female C57BL/6J mice (10–11 weeks old) with EAE induced with MOG35–55 | A single intra-LN injection (at the peak of EAE) of PLGA NPs containing a MOG peptide and rapamycin was revealed to permanently reverse paralysis. |
| Colloidal gel based on self-assembly of PLGA-CS and PLGA-Alginate NPs [208] | PLGA-CS: 400.1, PLGA-Alginate: 208.1 | PLGA-CS: 23.79 PLGA-Alginate: −38.85, | Ac-PLP-BPI-NH2-2 | - | - | Prophylactic: five days b.i. Preclinical/Therapeutic: four and 30 days p.i. | s.c. | 300 nmol of colloidal gel per injection | Mice (6–8 weeks old) with EAE PLP139–151 | A single injection of the colloidal gel containing the Ac-PLP-BPI-NH2-2 peptide led to long-term disease suppression. |
| Soluble antigen arrays (SAgAs) | ||||||||||
| HA-peptide conjugate [209] | HA | - | PLP139–151 | - | LABL, B7AP, CD80-CAP1, sF2 (cyclized) | Preclinical/Therapeutic: 4, 7, and 10 days p.i. | s.c. | 200 nmol PLP peptide | SJL/J (H-2s) female mice (4–6 weeks old) with EAE induced with PLP139–151 | SAgAs were shown to effectively reduce EAE incidence and suppress it via co-administration of an immunodominant myelin epitope and peptides targeting the B7 signaling pathway. |
| SAgAs [210] | HA | - | PLP139–151 | - | LABL | Preclinical/Therapeutic: 4, 7, and 10 days p.i. | s.c. | 200 nmol PLP139–151 | SJL/J female mice (4–6 weeks old) with EAE induced with PLP139–151 | Co-administration via conjugation of PLP139–151 and LABL improved the clinical scores of EAE |
| cSAgAs [184] | HA | - | PLP139–151 | LABL | Preclinical/Therapeutic: 4, 7, and 10 days p.i. | s.c. | 50, 133, or 200 nmol PLP139–151 | SJL/J female mice (4–6 weeks old) with EAE induced with PLP139–151 | cSAgAs was found to achieve equivalent efficiency with SAgAS regarding the suppression of EAE at a quarter of the SAgAS dose. | |
| cSAgAs (Figure 11) [185] | HA | - | PLP139–151 | - | LABL | Preclinical/Therapeutic: 4, 7, and 10 days p.i. | s.c. | 50, nmol PLP139–151 | SJL/J female mice (4–6 weeks old) with EAE induced with PLP139–151 | Low dose s.c. vaccination with cSAgAS resulted in successful suppression of EAE clinical symproms and minimization of body weight loss. |
| SAgAs [210] | HA | - | PLP139–151 | - | LABL | Preclinical/Therapeutic: 4, 7, and 10 days p.i. | pulmonary | 65.1–74.5 mg SAgAs/mouse kg | Female SJL/J mice (four weeks old) with EAE induced with PLP139–151 | The pulmonary administration of SAgAs was found to suppress the clinical score of the disease, decrease EAE incidence and improve weight gain. |
| SAgAs [183] | HA | - | PLP139–151 | - | LABL | Preclinical/Therapeutic: 4, 7, or 10 days p.i. | i.p., upper and lower i.m., upper and lower s.c., i.v. pulmonary | 200 nMol PLP per 100 μL injection volume 200 nMol PLP per 50 μL injection volume | Female SJL/J mice (6–8 weeks old) with EAE induced with PLP139–151 | i.v. administration demonstrated similar efficiency with the other routes. p.i. vaccination decreased completely clinical disease scores. Single injection-based treatment resulted in decreased efficiency compared with a triple injection treatment. Decrease of SAgAs dose and/or injection volume decreased the therapeutic efficiency. |
| Immune polyelectrolyte multilayers (iPEMs) | ||||||||||
| iPEMs [186] | - | - | MOG-R3 | 28.4–89.7% | GpG (0.7–10.3%) | Preclinical/Therapeutic: 5 and 10 p.i. | s.c. | 200 μg of (MOG-R3/GpG)3 iPEMs, per injection. | C57BL/6J mice with EAE induced with a myelin antigen | s.c. delivery of iPEMs restrained inflammation and promoted autoimmune tolerance in an EAE mouse models. |
| iPEMs [187] | 114.9–199.2 | −42.5 to 33.4 | MOGR1, MOGR2 | 0.57–9.18 μg of MOGRx | GpG 2.18 μg–4.88 μg | Preclinical/Therapeutic: seven days or 6, 12, and 18 days p.i. | s.c. | 200 μg MOGR2 (85.9 μg GpG) | Female C57BL/6J mice (10 weeks old) with EAE induced with MOG35–55 | iPEMs were shown to improve the severity, progression and incidence of EAE. |
| Inorganic particles and pMHC-nanoparticles (pMHC-NPs) | ||||||||||
| Quantum dots [211] | 15.0–21.0 | −17.6 to −4.2 | MOG | Up to 55 | - | Preclinical: two days p.i. | s.c. | Female C57BL/6 mice (10–12 weeks old) | Ten-fold reduction of EAE incidence. Increased numbers of QDs with lower peptide loading were more efficient regarding the induction of immune tolerance. | |
| Iron oxide NPs [212] | - | - | MOG38–49 | - | IAb | Therapeutic: 14 or 21 days p.i. | C57BL/6 mice with EAE induced with pMOG35–55 | By administration on day 14 the NPs were found to diminish the progression of the disease, whereas when administered on day 21 they were shown to restore the motor function of paralytic mice. | ||
| Iron oxide NPs [212] | - | - | hPLP175–192 hMOG97–108 | - | DR4-IE | Therapeutic: | HLA-DR4-IE-transgenic C57BL/6 IAbnull mice | Successful EAE suppression was observed. | ||
| Pegylated gold NPs [213] | 60 | - | MOG35–55 PLP139–151 PLP178–191 | AhR ligand ITE | Prophylactic: admin. on the day of EAE induction Therapeutic: Admin. on day 17 post immunization. Weekly treatment of mice | parenteral | 6 μg NPs per mouse | B6 mice with EAE induced with MOG35–55 SJL mice with EAE induced with EAE induced with PLP139–151 | Pegylated gold NPs loaded with MOG35-55 and ITE significantly suppressed the development of EAE, whereas those loaded with PLP epitopes reduced the clinical scores of the disease and the number of relapses. | |
| Mannan-conjugated myelin peptides | ||||||||||
| Mannan-peptide conjugates (Figure 12) [188] | - | - | MOG35–55, PLP139–151, PLP178–191, MBP83–99 | - | - | Prophylactic: 45, 30, and 15 days b.i. Preclinical/Therapeutic: Admin. on day 0 and 7 p.i. | i.d. | 30 μg peptide/injection 700 μg mannan/injection | C57BL/6 mice (12–14 weeks old) with EAE induced with MOG Female SJL/J mice (6–8 weeks old) with EAE induced with PLP. | Mannan-peptide conjugates were shown to generate robust antigen-specific protection of mice from the clinical disease symptoms. |
| Mannan-peptide conjugates [214] | - | - | Linear and cyclic MBP83–99 peptide analogues cyclo(83-99)[A91]MBP83-99 mutant peptide | Preclinical/Therapeutic: Admin. on day 0 and 14 p.i. | i.d. | 50 μg of linear and cyclic MBP83–99 peptide analogues | Female SJL/J mice (6–8 weeks old) with EAE induced with linear and cyclic MBP83–99 peptide analogues | It was shown that the mutant peptide cyclo(83–99)[A91]MBP83–99 more efficiently inhibited EAE development. | ||
| Liposomes | ||||||||||
| Liposomes [190] | 861.3 | −36.2 | MOG40–55 | -/91.5 | - | Preclinical/Therapeutic: 5 and 9 days p.i. | i.p. | 1.75 mg of lipid per injection | C57BL/6 female mice (8 weeks old) with EAE induced with MOG40–55 | Liposomes successfully delayed the onset, suppressed the severity and decreased the incidence of the disease. |
| (mannosylated) SUV [189] | ~85 | −7.5 to −10.5 | MBP46–62 MBP124–139 MBP147–170 | -/90 | - | Preclinical/Therapeutic: admin. on day 7 post immunization followed by five consecutive days. | s.c. | Female DA rats (8–9 weeks old) with EAE induced with a syngeneic spinal cord homogenate or with MBP63-81. | It was revealed that mSUVs loaded with immunodominant epitopes of MBP could significantly suppress EAE in DA rats. | |
| Exosomes | ||||||||||
| mTGF-β1-EXOs [215] | 50–100 | Prophylactic: 8, 5, and 2 days b.i. Therapeutic: 14, 17 and 21 days p.i. | i.v. | 10 μg/mouse/injection | Female C57BL/6 mice (6–8 weeks) with EAE induced with MOG35–55 Female BALB/c mice (6–8 weeks) with EAE induced with PLP180-199 | Treatment with mTGF-β1-EXOs from C57BL/6 mice successfully inhibited the development and progression of the disease in both mice strains. | ||||
| Antigen-presenting yeast cells | ||||||||||
| C. utilis expressing MOG35–55 on its surface [216] | - | - | MOG35–55 pCB13 pCB10 | Prophylactic: admin. on day 7 prior to immunization and for six consecutive days | Oral | 1.5 × 108 C. utilis | Female C57BL/6 mice (eight weeks old) with EAE induced with MOG35–55 | C. utilis expressing MOG35–55 on its surface appeared to be a promising approach to protect myelin against autoimmunity by effectively inducing oral tolerance. Fungal viability was not found to affect the induction of tolerance. | ||
| Objective | Phase | No. of Particip. | Antigen Immunotherapy | Admin. Route/Dose/Duration of Treatment | Results |
|---|---|---|---|---|---|
| To suppress disease activity in RRMS patients using CGP77116 [74] | II | 24 | CGP77116 | s.c. injection/50 mg CGP77116 per week; 5 mg per week; 5 mg per month/9 months | Decrease of dose because of adverse effects. Trial termination due to treatment-related disease exacerbation. |
| Evaluation of NBI 5788 safety, and effect on RRMS patients [217] | II | 144 | NBI5788 | s.c. injection/5, 20, or 50 mg NBI5788 per week/4 months | Trial suspension due to hypersensitivity reactions in some patients. No increase in relapses. Reduction of number and volume of enhancing lesions in patients who completed the trial receiving 5 mg of NBI5788 per week. |
| Assessment of safety, tolerability and clinical activity of AG284 in SPMS patients [218] | I | 33 | AG284 | /0.6, 2, 6, 20, 60, 105, and 150 mg AG284/kg body weight; each dose was received daily for three alternate days/ | No adverse events but also no significant therapeutic effect could be observed. |
| Assessment of the clinical efficiency of MBP82-98 in patients with progressive MS [219] | II | 32 | MBP82–98 | i.v./500 mg MBP82-98 per 6 months/24 months | Only patients with HLA haplotypes DR2 and/or DR4 appeared to have benefited from the treatment. |
| Evaluation of the safety and efficiency of MBP82-98 in SPMS patients with HLA haplotypes DR2 and/or DR4 [220] | III | 612 | MBP82–98 | i.v./500 mg MBP82-98 per 6 months/2 years | The administration of was safe and well tolerated. The treatment was not effective in SPMS patients with HLA DR2+ or DR4+ |
| Evaluation of RTL1000 safety in MS patients [221] | I | 34 | RTL1000 | i.v./2, 6, 20, 60, 200, and 100 mg of RTL/ | RTL1000 was safe at doses ≤ 60 mg |
| Determination of the maximum tolerable dose and safety of RTL1000 in MS patients [222] | I | 36 | RTL1000 | i.v./2, 6, 20, 60, 200, and 100 mg of RTL/ | The maximum tolerable dose of RTL100 was 60 mg. |
| Examination of the effect of high dose MBP82-98 on the number of regulatory T cells in CPMS patients [223] | 10 | MBP82–98 | i.v./500 mg of MBP82-98 per 6 months/ | Increase in the number of regulatory T cells in patients’ PBMCs six weeks and six6 months after treatment. Renversement of the state of T cell anergy. | |
| Assessment of safety and tolerability of autologous PBMCs coupled with 7 myelin peptides in RRMS and SPMS patients [224] | I | 9 | PBMCs chemically coupled with the following 7 myelin peptides: MOG1–20, MOG35–55, MBP13–32, MBP83–99, MBP111–129, MBP146–170, and PLP139–154 | Single infusion/1 × 103, 1 × 105, 1 × 107, 1 × 108, 1 × 109, 2.5 × 109 and 3 × 109 antigen-coupled PBMCs/3 months | The treatment was found to be safe and well-tolerated. Antigen-specific T cell responses were shown to decrease after treatment in patients who received doses ≥1 × 109 of antigen coupled PBMCs. |
| Examination of BHT-3009 safety and feasibility for immune nodulation in RRMS and SPMS patients [225] | I/II | 30 | BHT-3009 | i.m./0.5, 1.5, and 3 mg of BHT-3009 at weeks 1, 3, 5, and 9 after patients’ randomization into the clinical trial/The administration of BHT-3009 was combined or not with daily oral administration of 80 mg atorvastatin. | BHT-3009 was found to be safe and to induce antigen-specific immune tolerance in MS patients. The co-administration of atorvastatin was not considered substantially beneficial. |
| Assessment of the transdermal delivery of a mixture of three myelin peptides to induce immune tolerance in RRMS patients [226] | 30 | Mixture of the following 3 myelin peptides: MBP85–99, PLP139–151, and MOG35–55 | Transdermal (via an adhesive skin patch)/1 or 10 mg of each myelin peptide per week (for 4 weeks) and per month (for 11 months)/1 year | The transdermal administration of myelin peptides was proven to be tolerogenic in RRMS patients. | |
| Assessment of safety and efficiency of transdermal administration of myelin peptides in RRMS patients [227] | 30 | Mixture of the following three myelin peptides: MBP85–99, MOG35–55, and PLP139–151 | Transdermal (via an adhesive skin patch)/1 or 10 mg of each myelin peptide per week (for four weeks) and per month (for 11 months)/1 year | The transdermal delivery of myelin peptides was found to be safe, well tolerated and to reduce clinical symptoms and number of Gadolinium lesions in RRMS patients. | |
| Evaluation of BHT-3009 regarding its safety and efficiency to induce immune tolerance in RRMS patients [228,229] | II | 289 | BHT-3009 | i.m./ 0.5 and 1.5 mg of BHT-3009 at weeks 0, 2, 4, and every four weeks until week 44/The administration of BHT-3009 was combined or not with daily oral administration of 80 mg atorvastatin. | It was shown that treatment with the lower dose of BHT-3009 (e.g., 0.5 mg) succeeded in inducing antigen-specific immune tolerance in some patients in contrast with the higher dose (e.g., 1.5 mg) which was found to be ineffective. |
| Evaluation of ATX-MS-1467 safety in SPMS patients [117] | I | 6 | ATX-MS-1467 | i.d/25, 50, 100, 400, and 800 μg of ATX-MS-1467/ | The safety and tolerability of ATX-MS-1467 at a dose ≤ 800 μg, was successfully demonstrated in SPMS patients. |
| Evaluation of ATX-MS-1467 safety, tolerability and efficiency to induce tolerance in RRMS patients [230] | Ib, IIa | 43, 37 | ATX-MS-1467 | Ib: i.d. (cohort 1) or s.c. (cohort 2)/25, 50, 100, 400 and 800 μg of ATXMS-1467 per two weeks (for eight weeks) and 800 μg per two weeks (for eight more weeks)/one year (including 32 weeks medication off study). IIa: i.d./50 μg of ATXMS-1467 (on day 1), 200 μg (on day 15), 800 μg (on day 29), and 800 μg per two weeks (for 16 more weeks)/one year (including 16 weeks medication off study). | Both treatment protocols were found to be safe. The relatively slow i.d. titration of ATX-MS-1467 followed by a longer high dose treatment period resulted in reduced GdE lesions which remained so even post treatment. |
| Tolerogenic DCs (tolDCs) | |||||
| Evaluation of the safety of myelin peptide loaded tolDCs and their ability of to induce immune tolerance in MS patients. [231] | I | 8 | Autologous tolDCs loaded with myelin peptides | i.v./50 × 106, 100 × 106, 150 × 106, and 300 × 106 tolDCs divided in three independent doses administered every two weeks/ | Myelin peptide loaded tolDCs were proven to be safe and well tolerated, and to induce tolerogenic responses in MS patients. |
| Evaluation of the safety of intradermal and intranodal delivery myelin peptide loaded tolDCs and their efficacy regarding the induction of antigen-specific tolerization in MS patients [232] | I | 9–15 | Autologous peptide-mix loaded tolDCs | i.d. or intranodal/six repetitive doses of 5 × 106, 10 × 106 and 15 × 106 autologous peptide-mix loaded tolDCs: administration of doses 1–4 once every two weeks and of doses 5–6 once every month. | - |
| T-cell vaccination (TCVs) | |||||
| Assessment of safety and immune efficiency of a polyclonal T cell vaccine in chronic MS patients in advanced diseases stages [233] | 39 | autological polyclonal TCVs | s.c./1.5–3 × 107 polyclonal T cells; four weekly injections followed by monthly injections. | Polyclonal TCV was proven safe and capable of inducing long-lasting, anti-inflammatory immune effects in progressive MS patients in advanced disease states. | |
| To establish a safe and efficient dose of Tovaxin® [234] | 9–15 | Attenuated T cells reactive to the following myelin peptides MBP83–99, MBP151–170, PLP30–49, PLP180–199, MOG1–17 and MOG19–39 | s.c./6–9 × 106, 30–45 × 106, and 60–90 × 106 administered at weeks 0, 4, 12, and 20/ | The study indicated the mid-dose as optimum with respect to safety, and efficiency in reducing peripheral blood myelin reactive T cells and showing a trend to improve clinical symptoms. | |
| Evaluation of safety and efficacy of Tovaxin in RRMS patients [235] | IIb | 150 | T cells reactive to different immunodominant peptides from three myelin proteins | s.c./five injections at weeks 0, 4, 8, 12, and 24 | s.c. administration of Tovaxin was shown to be safe. Evidence of clinical efficiency of Tovaxin® was observed during the analysis of subgroups of patients naïve to prior disease modifying therapies. |
| Examination of TCV safety and efficiency in progressive MS patients [236] | II | 26 | T-cell lines reactive to nine different peptides of MBP, MOG and PLP. | 19 patients received s.c. TCV/10–30 × 106 T cells, on days 1, 30, 90 and 180/7 patients received sham injections. | The clinical trial demonstrated the safety of TCV in progressive MS patients and indicated its clinical efficiency. |
| Assessment of TCV safety and immune modulation in RRMS and CPMS patients [237] | pilot | 5 | CSF derived activated CD4+T cells | 3 s.c. injections; 106 cells at months 2, 4, and 6. | TCV was safe and well tolerated. Patients were clinically stable or exhibited reduced EDSS without relapses during and post treatment. |
| Examine if the depletion of T cells reactive to MBP would have a clinical benefit for RRMS and SPMS patients [238] | Preliminary | 54 | Irradiated autologous T cells reactive to MBP- | 3 s.c. injections at 2 month intervals, 30 × 106–60 × 106 cells per injection. | A 40% decrease in the relapses rate and a minimal decrease in EDSS was observed in RRMS patients. On the other hand, a slight increase of EDSS was detected in SPMS patients. Finally, MRI scans indicated a stabilization of the lesion activity. |
| Assess the use of T cell lines reacting with a broad range of antigens regarding targeting and depletion of specific T cells reactive to a great number of myelin antigens in SPMS patients. [239] | Pilot | 4 | Peripheral blood derived T cell lines reactive to bovine myelin | TCV with T cells reactive to whole bovine myelin were shown to efficiently promote depletion of circulating T cells reactive to myelin protein. | |
| Evaluation of the TCV efficiency in patients with aggressive RRMS non-responding to DMTs [240] | 20 | Autologous attenuated T cell lines reactive to MBP and MOG encephalitogenic peptides. | Three s.c. injections in six- to eight-week intervals. | TCV was proven to be safe. A decrease in the relapse rate was observed. Additionally, significant decrease in the active lesions regarding number and volume as well as in T2 lesion burden was detected. | |
| Identification of the idiotypic determinants triggering CD81 cytotoxic anti-idiotypic responses by TCV in MS patients [241] | 3 | Irradiated autologous T cell clones reactive to MBP83–99 | s.c./repetitive injections of 2 × 107 of each cell clone every 2 months for 8 months. | CD3-specific T cells were recognized as a representative anti-idiotypic population of T cells induced by TCV. | |
| T-cell receptor (TCR) | |||||
| To examine the therapeutic potential of a trivalent TCR vaccine in MS patients [242] | 23 | A trivalent TCR vaccine containing the CDR2 peptides BV5S2, BV6S5 and BV13S1 | 12 monthly vaccinations | The therapeutic TCR vaccine induced an extended immunoregulatory network which could control complex self-reactive responses of MS. | |
| Liposomes | |||||
| Assessment of Xemys safety and efficiency in treating RRMS and SPMS patients non-responding to DMTs [191,192] | I | 20 | Xemys: Liposomes loaded with MBP46–62, MBP124–139 and MBP147–170 And targeting CD206 | s.c./six weekly injections of 50, 150, 225, 450, 900, and 900 μg Xemys | The administration of Xemys was proven to be safe and well tolerated, and to normalize cytokine levels in RRMS and SPMS patients. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kammona, O.; Kiparissides, C. Recent Advances in Antigen-Specific Immunotherapies for the Treatment of Multiple Sclerosis. Brain Sci. 2020, 10, 333. https://doi.org/10.3390/brainsci10060333
Kammona O, Kiparissides C. Recent Advances in Antigen-Specific Immunotherapies for the Treatment of Multiple Sclerosis. Brain Sciences. 2020; 10(6):333. https://doi.org/10.3390/brainsci10060333
Chicago/Turabian StyleKammona, Olga, and Costas Kiparissides. 2020. "Recent Advances in Antigen-Specific Immunotherapies for the Treatment of Multiple Sclerosis" Brain Sciences 10, no. 6: 333. https://doi.org/10.3390/brainsci10060333
APA StyleKammona, O., & Kiparissides, C. (2020). Recent Advances in Antigen-Specific Immunotherapies for the Treatment of Multiple Sclerosis. Brain Sciences, 10(6), 333. https://doi.org/10.3390/brainsci10060333




