Increased Time Difference between Imagined and Physical Walking in Older Adults at a High Risk of Falling
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement
2.3. Statistics Analysis
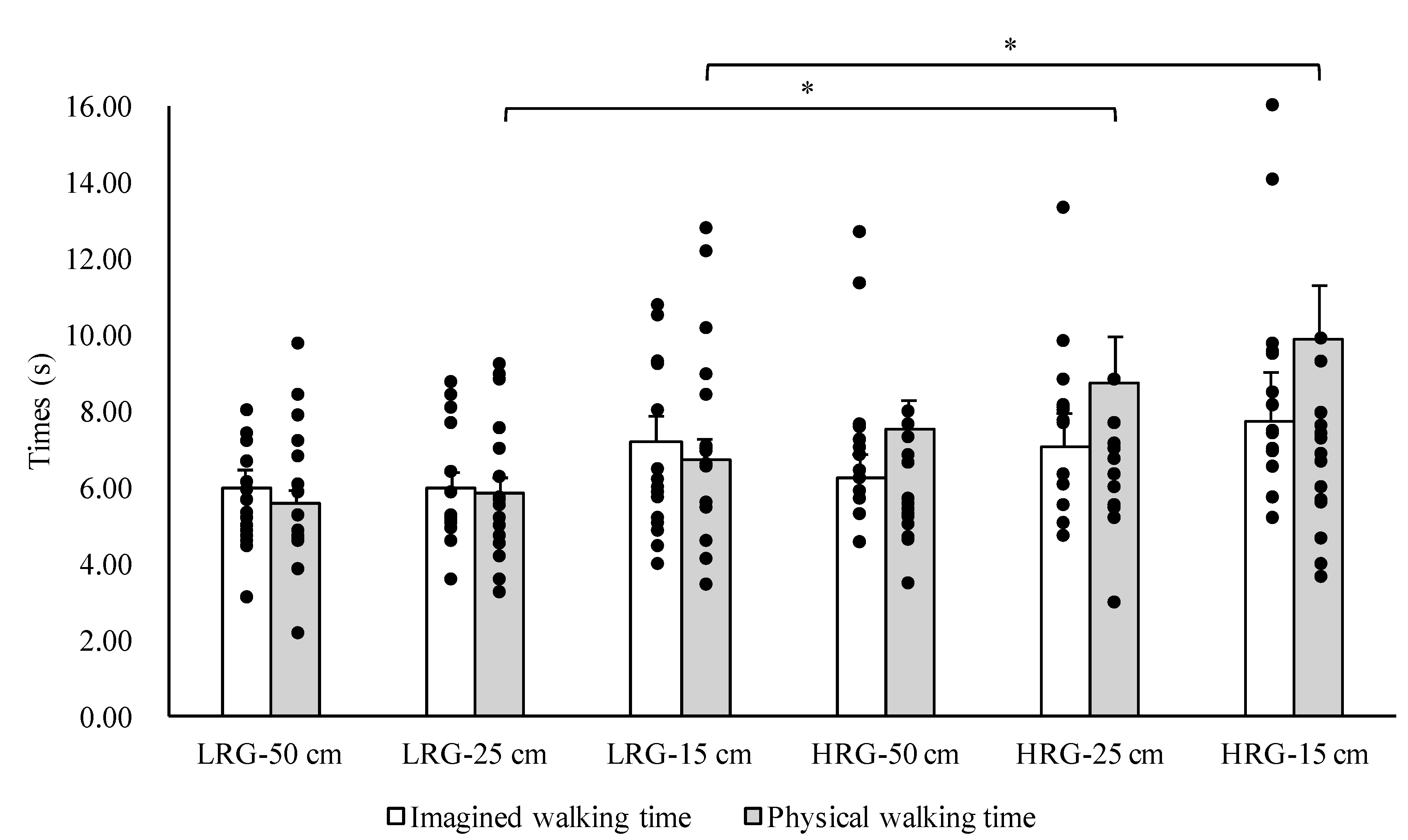
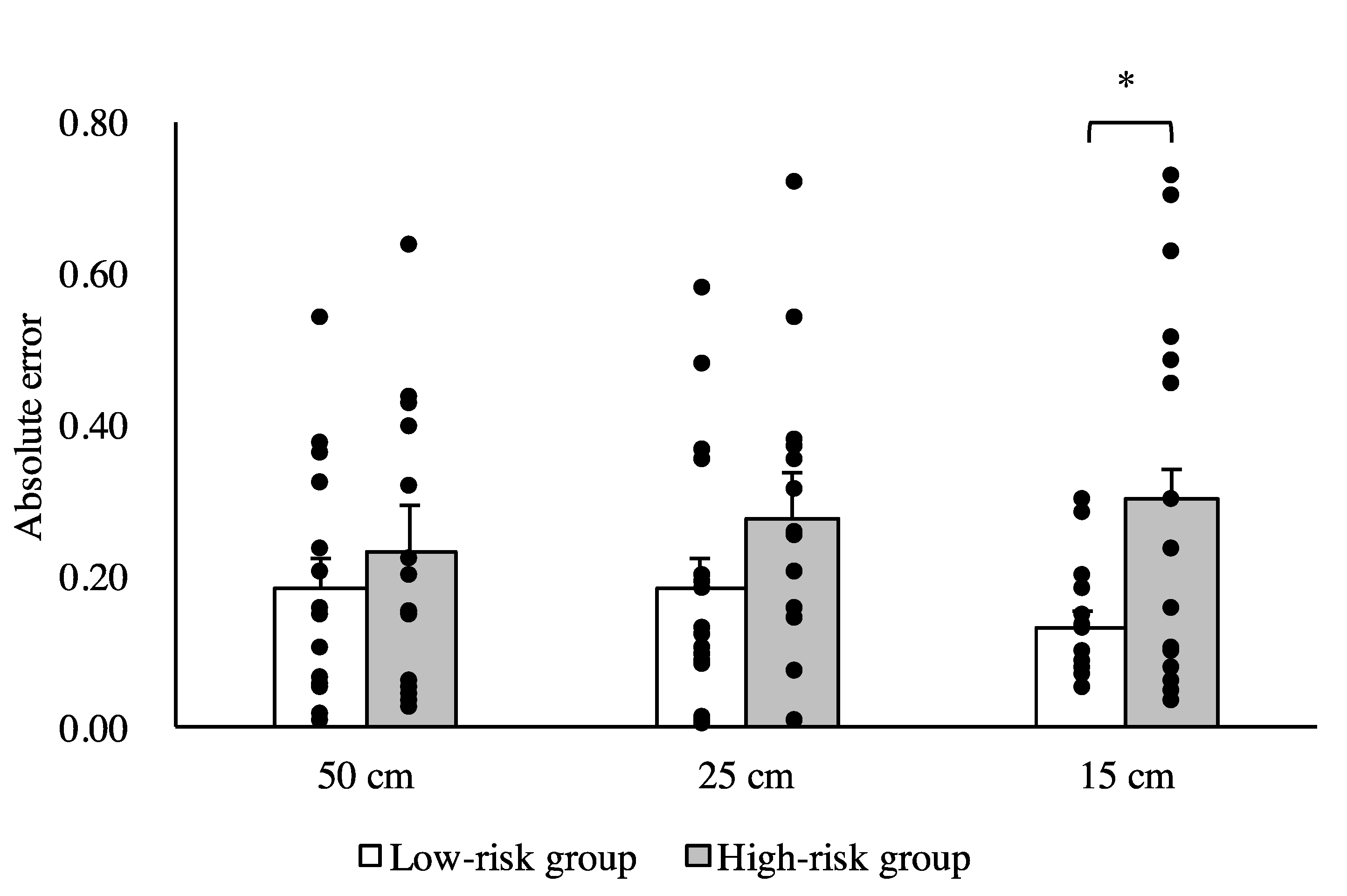
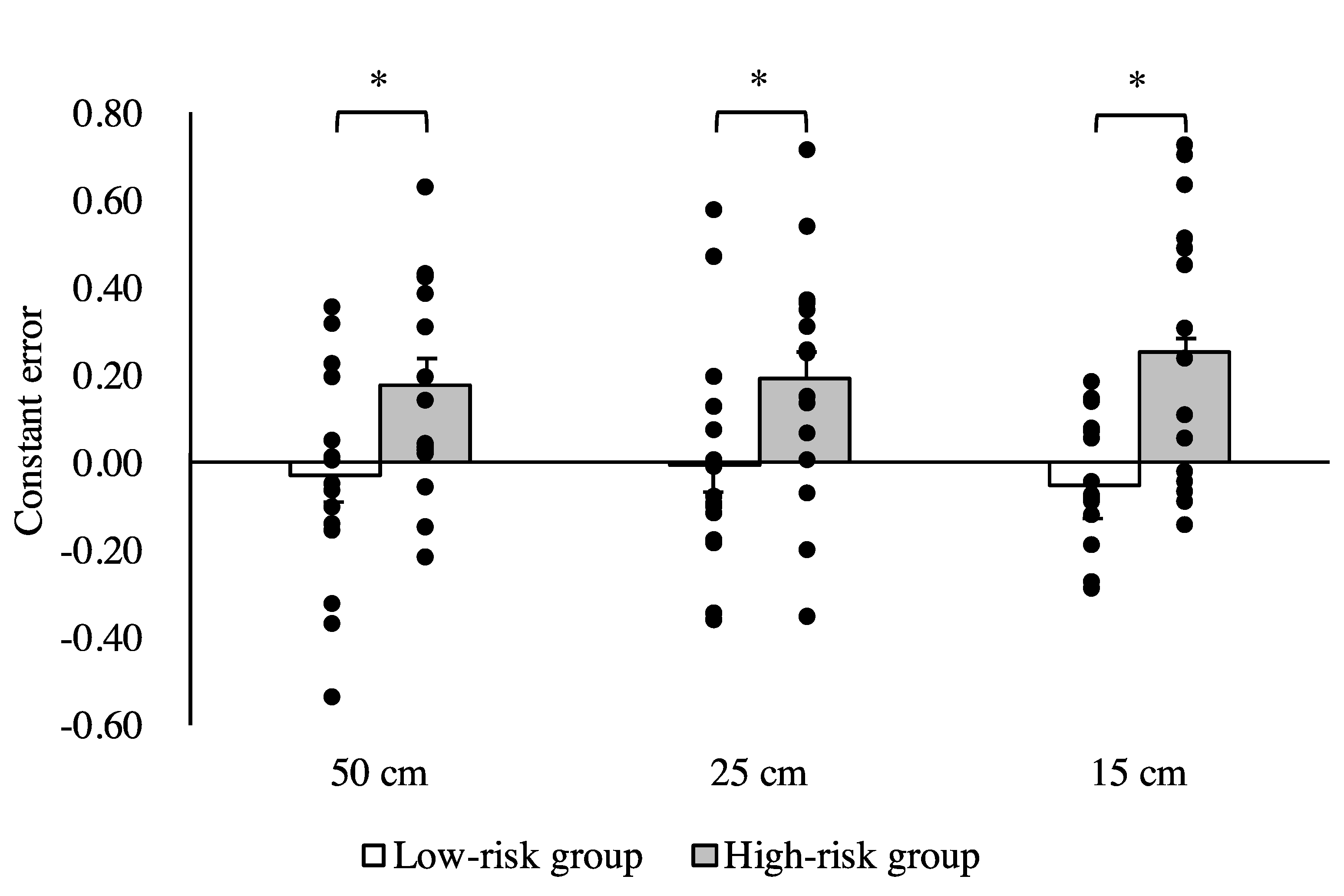
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeannerod, M. The representing brain: Neural correlates of motor intention and imagery. Behav. Brain Sci. 1994, 17, 187–245. [Google Scholar] [CrossRef]
- Grush, R. The emulation theory of representation: Motor control, imagery, and perception. Behav. Brain Sci. 2004, 27, 377–396. [Google Scholar] [CrossRef]
- Glover, S.; Baran, M. The motor-cognitive model of motor imagery: Evidence from timing errors in simulated reaching and grasping. J. Exp. Psychol. Hum. Percept. Perform. 2017, 43, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Jeannerod, M. Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage 2001, 14, 103–109. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef]
- Guillot, A.; Hoyek, N.; Louis, M.; Collet, C. Understanding the timing of motor imagery: Recent findings and future directions. Int. Rev. Sport Exerc. Psychol. 2012, 5, 3–22. [Google Scholar] [CrossRef]
- Decety, J.; Jeannerod, M.; Prablanc, C. The timing of mentally represented actions. Behav. Brain Res. 1989, 34, 35–42. [Google Scholar] [CrossRef]
- Collet, C.; Guillot, A.; Lebon, F.; MacIntyre, T.; Moran, A. Measuring motor imagery using psychometric, behavioral, and psychophysiological tools. Exerc. Sport Sci. Rev. 2011, 39, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Malouin, F.; Richards, C.L.; Durand, A.; Doyon, J. Reliability of mental chronometry for assessing motor imagery ability after stroke. Arch. Phys. Med. Rehabil. 2008, 89, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Saimpont, A.; Malouin, F.; Tousignant, B.; Jackson, P.L. Motor imagery and aging. J. Mot. Behav. 2013, 45, 21–28. [Google Scholar] [CrossRef]
- Schott, N.; Munzert, J. Temporal accuracy of motor imagery in older women. Int. J. Sport Psychol. 2007, 38, 304–320. [Google Scholar]
- Schott, N. Age-related differences in motor imagery: Working memory as a mediator. Exp. Aging Res. 2012, 38, 559–583. [Google Scholar] [CrossRef] [PubMed]
- Personnier, P.; Kubicki, A.; Laroche, D.; Papaxanthis, C. Temporal features of imagined locomotion in normal aging. Neurosci. Lett. 2010, 476, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Murata, S.; Shiraiwa, K.; Iwase, H.; Kodama, T. Temporal characteristics of imagined and actual walking in frail older adults. Aging Clin. Exp. Res. 2018, 30, 1453–1457. [Google Scholar] [CrossRef]
- Beauchet, O.; Annweiler, C.; Assal, F.; Bridenbaugh, S.; Herrmann, F.R.; Kressig, R.W.; Allali, G. Imagined Timed Up & Go test: A new tool to assess higher-level gait and balance disorders in older adults? J. Neurol. Sci. 2010, 294, 102–106. [Google Scholar] [PubMed]
- Grenier, S.; Richard-Devantoy, S.; Nadeau, A.; Payette, M.C.; Benyebdri, F.; Duhaime, M.B.; Gunther, B.; Beauchet, O. The association between fear of falling and motor imagery abilities in older community-dwelling individuals. Maturitas 2018, 110, 18–20. [Google Scholar] [CrossRef]
- Sakurai, R.; Fujiwara, Y.; Yasunaga, M.; Suzuki, H.; Sakuma, N.; Imanaka, K.; Montero-Odasso, M. Older adults with fear of falling show deficits in motor imagery of gait. J. Nutr. Health Aging 2017, 21, 721–726. [Google Scholar] [CrossRef]
- Greenberg, S.A. Analysis of measurement tools of fear of falling for high-risk, community-dwelling older adults. Clin. Nurs. Res. 2012, 21, 113–130. [Google Scholar] [CrossRef]
- Sakurai, R.; Fujiwara, Y.; Yasunaga, M.; Suzuki, H.; Murayama, Y.; Imanaka, K.; Kanosue, K.; Ishii, K. Neural correlates of older adults’ self-overestimation of stepping-over ability. Age 2016, 38, 351–361. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Health and Welfare Services for the Elderly. Available online: https://www.mhlw.go.jp/english/wp/wp-hw11/dl/10e.pdf (accessed on 8 January 2020).
- Saito, T.; Izawa, K.P.; Watanabe, S. The relative and absolute reliability of the Functional Independence and Difficulty Scale in community-dwelling frail elderly Japanese people using long-term care insurance services. Aging Clin. Exp. Res. 2017, 29, 549–556. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G * Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Ogawa, M.; Loenneke, J.P.; Thiebaud, R.S.; Loftin, M.; Mitsukawa, N. Association between site-specific muscle loss of lower body and one-leg standing balance in active women: The HIREGASAKI study. Geriatr. Gerontol. Int. 2014, 14, 381–387. [Google Scholar] [PubMed]
- Vellas, B.J.; Wayne, S.J.; Romero, L.; Baumgartner, R.N.; Rubenstein, L.Z.; Garry, P.J. One-leg balance is an important predictor of injurious falls in older persons. J. Am. Geriatr. Soc. 1997, 45, 735–738. [Google Scholar] [CrossRef]
- American Geriatrics Society; British Geriatrics Society; American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J. Am. Geriatr. Soc. 2001, 49, 664–672. [Google Scholar] [CrossRef]
- Allali, G.; van der Meulen, M.; Beauchet, O.; Rieger, S.W.; Vuilleumier, P.; Assal, F. The neural basis of age-related changes in motor imagery of gait: An fMRI study. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1389–1398. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: London, UK, 1988. [Google Scholar]
- Kalicinski, M.; Kempe, M.; Bock, O. Motor imagery: Effects of age, task complexity, and task setting. Exp. Aging Res. 2015, 41, 25–38. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc. 2010, 58, 248–255. [Google Scholar] [CrossRef]
- Sakurai, R.; Fujiwara, Y.; Yasunaga, M.; Suzuki, H.; Kanosue, K.; Montero-Odasso, M.; Ishii, K. Association between hypometabolism in the supplementary motor area and fear of falling in older adults. Front. Aging Neurosci. 2017, 9, 251. [Google Scholar] [CrossRef]
- Sakurai, R.; Fujiwara, Y.; Sakuma, N.; Suzuki, H.; Ishihara, M.; Higuchi, T.; Imanaka, K. Influential factors affecting age-related self-overestimation of step-over ability: Focusing on frequency of going outdoors and executive function. Arch. Gerontol. Geriatr. 2014, 59, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Hori, H.; Tamura, T.; Hirai, T.; Umemura, T.; Iguchi, F.; Sawa, S.; Ogawa, K.; Sato, K.; Kusaka, Y. Relationships between estimation errors and falls in healthy aged dwellers. Gerontology 2015, 61, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ridderinkhof, K.R.; Ullsperger, M.; Crone, E.A.; Nieuwenhuis, S. The role of the medial frontal cortex in cognitive control. Science 2004, 306, 443–447. [Google Scholar] [PubMed]
- Shany-Ur, T.; Lin, N.; Rosen, H.J.; Sollberger, M.; Miller, B.L.; Rankin, K.P. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain 2014, 137, 2368–2381. [Google Scholar] [PubMed]
- O’Shea, H.; Moran, A. Does Motor Simulation Theory Explain the Cognitive Mechanisms Underlying Motor Imagery? A Critical Review. Front. Hum. Neurosci. 2017, 11, 72. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, H.; Murata, S.; Shiraiwa, K.; Nonaka, K. Increased Time Difference between Imagined and Physical Walking in Older Adults at a High Risk of Falling. Brain Sci. 2020, 10, 332. https://doi.org/10.3390/brainsci10060332
Nakano H, Murata S, Shiraiwa K, Nonaka K. Increased Time Difference between Imagined and Physical Walking in Older Adults at a High Risk of Falling. Brain Sciences. 2020; 10(6):332. https://doi.org/10.3390/brainsci10060332
Chicago/Turabian StyleNakano, Hideki, Shin Murata, Kayoko Shiraiwa, and Koji Nonaka. 2020. "Increased Time Difference between Imagined and Physical Walking in Older Adults at a High Risk of Falling" Brain Sciences 10, no. 6: 332. https://doi.org/10.3390/brainsci10060332
APA StyleNakano, H., Murata, S., Shiraiwa, K., & Nonaka, K. (2020). Increased Time Difference between Imagined and Physical Walking in Older Adults at a High Risk of Falling. Brain Sciences, 10(6), 332. https://doi.org/10.3390/brainsci10060332




