Effects of Combined Transcranial Direct Current Stimulation with Cognitive Training in Girls with Rett Syndrome
Abstract
1. Introduction
2. Method
2.1. Participants
2.2. Study Design
2.3. Assessment
2.4. Integrated Intervention: tDCS and Cognitive Empowerment
3. Measures
3.1. Attention Measure
3.2. Production of Vowels, Consonants and Words with Elicited Denomination
3.3. Quantitative EEG Analysis
3.4. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Amir, R.; Van den Veyver, I.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Zoghbi, H.Y. Rett syndrome: Methyl-CpG-binding protein 2 mutations and phenotype–genotype correlations. Am. J. Med. Genet. 2000, 97, 147–152. [Google Scholar] [CrossRef]
- Kaufmann, W.E.; Johnston, M.V.; Blue, M.E. MECP2 expression and function during brain development: Implications for Rett syndrome’s pathogenesis and clinical evolution. Brain Dev. 2005, 25, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Pini, G.; Bigoni, S.; Engerström, I.W.; Calabrese, O.; Felloni, B.; Scusa, M.F.; Di Marco, P.; Borelli, P.; Bonuccelli, U.; Julu, P.O.; et al. Variant of Rett Syndrome and CDKL5 Gene: Clinical and Autonomic Description of 10 Cases. Neuropediatrics 2012, 43, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodoulou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.S.; Schanen, N.C.; Zappella, M.; et al. Rett syndrome: Revised diagnostic criteria and nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef]
- Castelli, I.; Antonietti, A.; Fabio, R.A.; Lucchini, B.; Marchetti, A. Do rett syndrome persons possess theory of mind? Some evidence from not-treated girls. Life Span. Disab. 2013, 16, 157–168. [Google Scholar]
- Fabio, R.A.; Caprì, T.; Buzzai, C.; Pittalà, V.; Gangemi, A. Auditory and Visual Oddball Paradigm Evaluated Through P300 in Five Girls with Rett Syndrome. Neuroquantology 2019, 17, 40–49. [Google Scholar] [CrossRef]
- Fabio, R.A.; Giannatiempo, S.; Oliva, P.; Murdaca, A.M. The Increase of Attention in Rett Syndrome: A Pre-Test/Post-Test Research Design. J. Dev. Phys. Disab. 2011, 23, 99–111. [Google Scholar] [CrossRef]
- Fabio, R.A.; Giannatiempo, S.; Caprì, T. Attention and identification of the same and the similar visual stimuli in Rett Syndrome. Life Span. Disabil. 2019, 22, 113–127. [Google Scholar]
- Fabio, R.A.; Magaudda, C.; Caprì, T.; Towey, G.; Martino, G. Choice Behavior in Rett Syndrome: The consistency parameter. Life Span. Disab. 2018, 21, 47–62. [Google Scholar]
- Hagberg, B. Clinical manifestations and stages of Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Fabio, R.A.; La Briola, F.; Giannatiempo, S.; Antonietti, A.; Maggiolini, S.; Canevini, M.P. Correlations between neurophysiological, behavioral, and cognitive function in Rett syndrome. Epilepsy Behav. 2010, 17, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Pelentsov, L.J.; Laws, T.A.; Esterman, A.J. The supportive care needs of parents caring for a child with a rare disease: A scoping review. Disabil. Health J. 2015, 8, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Boban, S.; Wong, K.; Epstein, A.; Anderson, B.; Murphy, N.; Downs, J.; Leonard, H. Determinants of sleep disturbances in Rett syndrome: Novel findings in relation to genotype. Am. J. Med. Genet. 2016, 170, 2292–2300. [Google Scholar] [CrossRef]
- Fabio, R.A.; Colombo, B.; Russo, S.; Cogliati, F.; Masciadri, M.; Foglia, S.; Antonietti, A.; Tavian, D. Recent Insights into Genotype-Phenotype Relationships in Patients with Rett Syndrome Using a Fine Grain Scale. Res. Dev. Disabil. 2014, 35, 2976–2986. [Google Scholar] [CrossRef]
- Fabio, R.A.; Caprì, T.; Lotan, M.; Towey, G.E.; Martino, G. Motor abilities are related to the specific genotype in Rett Syndrome. In Advances in Genetics Research; Urbano, K.V., Ed.; Nova Science Publisher: New York, NY, USA, 2018; Volume 1, Chapter 1; p. 1. [Google Scholar]
- Fabio, R.A.; Giannatiempo, S.; Antonietti, A.; Budden, S. The role of stereotypies in overselectivity process in Rett syndrome. Res. Dev. Disabil. 2009, 30, 136–145. [Google Scholar] [CrossRef]
- Ardolino, G.; Bossi, B.; Barbieri, S.; Priori, A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J. Physiol. 2005, 568, 653–663. [Google Scholar] [CrossRef]
- Marangolo, P.; Fiori, V.; Calpagnano, M.A.; Campana, S.; Razzano, C.; Caltagirone, C.; Marini, A. tDCS over the left inferior frontal cortex improves speech production in aphasia. Front. Hum. Neurosci. 2013, 7, 539. [Google Scholar] [CrossRef]
- Gangemi, A.; Colombo, B.; Fabio, R.A. The role of Transcranial direct current stimulation in patients with moderate cognitive impairment. In Advances in Medicine and Biology; Berhardt, L.V., Ed.; Nova Science Publisher: New York, NY, USA, 2019; Volume 131. [Google Scholar]
- Kang, E.K.; Kim, Y.K.; Sohn, H.M.; Cohen, L.G.; Paik, N.J. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca’s homologue area. Restor. Neurol. Neurosci. 2011, 29, 141–152. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cheon, H.J.; Yoon, K.J.; Chang, W.H.; Kim, Y.H. Effects of dual transcranial direct current stimulation for aphasia in chronic stroke patients. Ann. Rehabil. Med. 2013, 37, 603–610. [Google Scholar] [CrossRef]
- Shah, P.P.; Szaflarski, J.P.; Allendorfer, J.; Hamilton, R.H. Induction of neuroplasticity and recovery in post-stroke aphasia by non-invasive brain stimulation. Front. Hum. Neurosci. 2013, 7, 888. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Liebetanz, D.; Antal, A.; Lang, N.; Tergau, F.; Paulus, W. Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Suppl. Clin. Neurophysiol. 2003, 56, 255–276. [Google Scholar] [PubMed]
- Emara, T.H.; Moustafa, R.R.; Elnahas, N.M.; Elganzoury, A.M.; Abdo, T.A.; Mohamed, S.A.; Eletribi, M.A. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur. J. Neurol. 2010, 17, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Price, A.R.; McAdams, H.; Grossman, M.; Hamilton, R.H. A Meta-analysis of Transcranial Direct Current Stimulation Studies Examining the Reliability of Effects on Language Measures. Brain Stimul. 2015, 8, 1093–1100. [Google Scholar] [CrossRef]
- Roncero, C.; De Caro, M.; Thiel, A.; Probst, S.; Chertkow, H. Maximizing the Treatment Benefit of tDCS in Neurodegenerative Anomia. Front. Neurosci. 2019, 13, 1231. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, A.; Caprì, T.; Fabio, R.A.; Puggioni, P.; Falzone, A.M.; Martino, G. Transcranial Direct Current Stimulation (tDCS) and Cognitive Empowerment for the functional recovery of diseases with chronic impairment and genetic etiopathogenesis. In Advances in Research; Urbano, K.V., Ed.; Nova Science Publisher: New York, NY, USA, 2018; Volume 18, p. 1. [Google Scholar]
- Fabio, R.A.; Gangemi, A.; Caprì, T.; Budden, S.; Falzone, A. Neurophysiological and cognitive effects of Transcranial Direct Current Stimulation in three girls with Rett Syndrome with chronic language impairments. Res. Dev. Disabil. 2018, 76, 76–87. [Google Scholar] [CrossRef]
- Antal, A.; Paulus, W. Transcranial alternating current stimulation (tACS). Front. Hum. Neurosci. 2013, 317. [Google Scholar] [CrossRef]
- Giordano, J.; Bikson, M.; Kappenman, E.S.; Clark, V.P.; Coslett, H.B.; Hamblin, M.R.; Hamilton, R.; Jankord, R.; Kozumbo, W.J.; McKinley, R.A.; et al. Mechanisms and Effects of Transcranial Direct Current Stimulation. Dose Response 2017, 15. [Google Scholar] [CrossRef]
- Fabio, R.A.; Castelli, I.; Marchetti, A.; Antonietti, A. Training communication abilities in Rett Syndrome through reading and writing. Front. Psychol. 2013, 4, 9–11. [Google Scholar] [CrossRef]
- Fabio, R.A.; Caprì, T.; Nucita, A.; Iannizzotto, G.; Mohammadhasani, N. Eye gaze digital games to improve motivational and attentional ability in Rett syndrome. J. Spec. Educ. Rehab. 2018, 19, 105–126. [Google Scholar] [CrossRef]
- Fabio, R.A.; Caprì, T.; Martino, G. Understanding Rett Syndrome. In Routledge Psychology Taylor and Francis; Routledge: Milton Park, Abingdon, 2019. [Google Scholar]
- Houwen, S.; van der Putten, A.; Vlaskamp, C. A systematic review of the effects of motor interventions to improve motor, cognitive, and/or social functioning in people with severe or profound intellectual disabilities. Res. Dev. Disabil. 2014, 35, 2093–2116. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Caprì, T.; Semino, M.; Bizzego, I.; Di Rosa, G.; Fabio, R.A. Gross Motor, Physical Activity and Musculoskeletal Disorder Evaluation Tools for Rett Syndrome: A Systematic Review. Dev. Neurorehab. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fabio, R.A.; Billeci, L.; Crifaci, G.; Troise, E.; Tortorella, G.; Pioggia, G. Cognitive training modifies frequency EEG bands and neuropsychological measures in Rett syndrome. Res. Dev. Disabil. 2016, 54, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, S.S.; Cicchetti, D.V.; Balla, D.A. Vineland Adaptive Behavior Scales: Second edition (Vineland II), The Expanded Interview Form. Livonia MN: Pearson Assessments, 2008. [CrossRef]
- Fabio, R.A.; Martinazzoli, C.; Antonietti, A. Costruzione e standardizzazione dello strumento ‘‘R.A.R.S.” (Rett Assessment Rating Scale). Ciclo Evol. Disabil. 2005, 8, 257–381. [Google Scholar]
- Fanzago, F. Test di valutazione dell’articolazione. Quad Acta Phoniatr Lat. 1983, 2, 80–85. [Google Scholar]
- Welch, P.D. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. Ieee Trans. Audio Electroacous 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wasserman, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Dayan, E.; Censor, N.; Buch, E.R. Noninvasive brain stimulation: From physiology to network dynamics and back. Nat. Neurosci. 2013, 16, 838–844. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Liebetanz, D.; Nitsche, M.A.; Tergau, F.; Paulus, W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 2002, 125, 2238–2247. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Souza Carneiro, M.I.; Bolognini, N.; Fregni, F. Safety Review of Transcranial Direct Current Stimulation in Stroke. Neuromodulation 2017, 20, 215–222. [Google Scholar] [CrossRef] [PubMed]
- San-Juan, D.; Morales-Quezada, L.; Orozco Garduño, A.J.; Alonso-Vanegas, M.; González-Aragón, M.F.; Espinoza López, D.A.; Vázquez Gregorio, R.; Anschel, D.J.; Fregni, F. Transcranial Direct Current Stimulation in Epilepsy. Brain Stimul. 2015, 8, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Vicario, C.M.; Salehinejad, M.A.; Felmingham, K.; Martino, G.; Nitsche, M.A. A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neurosc. Biobehav. R 2019, 96, 219–231. [Google Scholar] [CrossRef]
- Caprì, T.; Fabio, R.A.; Iannizzotto, G.; Nucita, A. The TCTRS Project: A Holistic Approach for Telerehabilitation in Rett Syndrome. Electronics 2020, 9, 491. [Google Scholar] [CrossRef]
- Fabio, R.A.; Martino, G.; Caprì, T.; Giacchero, R.; Giannatiempo, S.; La Briola, F.; Banderali, G.; Canevini, M.P.; Vignoli, A. Long chain poly-unsaturated fatty acid supplementation in Rett Syndrome: A randomized placebo-controlled trial. Asia. J. Clin. Nutr. 2018, 10, 37–46. [Google Scholar] [CrossRef]
| Group | Name | Clinical Stage | Age | MeCP2 Mutation | Level 1 of Severity | |
|---|---|---|---|---|---|---|
| Non-sham | 1 | M.R. | IV | 35 | T158M | Moderate |
| tDCS | 2 | A.S. | III | 13 | c.1566_1197 del41 | Moderate |
| 3 | S.B. | III | 20 | c.916C>T | Mild | |
| 4 | N.R. | IV | 29 | R255C | Moderate | |
| 5 | T.C. | III | 18 | R270X | Moderate | |
| 6 | G.V. | III | 13 | R255C | Mild | |
| 7 | S.B. | IV | 31 | R255C | Moderate | |
| 8 | S.C. | III | 16 | T158M | Moderate | |
| 9 | R.O. | III | 19 | R270X | Severe | |
| 10 | S.M. | III | 18 | C.916C>T | Moderate | |
| 11 | P.S. | III | 15 | R255X | Moderate | |
| 12 | C.A. | III | 19 | PT158M | Moderate | |
| 13 | T.M. | III | 26 | R270X | Moderate | |
| 14 | V.A. | III | 15 | R294X | Moderate | |
| 15 | E.M. | III | 14 | R270X | Moderate | |
| 16 | E.P. | IV | 29 | P.R135C | Mild | |
| 17 | M.L. | III | 25 | p.Pro322Leu | Moderate | |
| 18 | C.T. | III | 17 | R270X | Moderate | |
| Sham | 19 | S.L. | III | 18 | p.R133C | Mild |
| tDCS | 20 | M.G. | III | 17 | R168X | Mild |
| 21 | V.A. | IV | 35 | R270X | Mild | |
| 22 | M.B. | III | 18 | C.916C>T | Moderate | |
| 23 | A.L. | III | 17 | T158M | Severe | |
| 24 | R.S. | III | 21 | R168X | Moderate | |
| 25 | S.P. | III | 16 | 168RX | Moderate | |
| 26 | R.B. | III | 18 | R270X | Moderate | |
| 27 | M.C. | III | 19 | R168X | Moderate | |
| 28 | C.S. | III | 13 | R306C | Moderate | |
| 29 | V.S | III | 16 | R133C | Moderate | |
| 30 | Z.F | III | 14 | P322A | Moderate | |
| 31 | G.S. | III | 19 | R255C | Moderate |
| Parameters | Non-Sham tDCS Group | Sham tDCS Group |
|---|---|---|
| M (S.D.) | M (S.D.) | |
| Attention | ||
| Pre-test | 4.00 (2.40) | 1.70 (2.07) |
| Post-test | 8.15 (2.66) | 2.62 (2.08) |
| Follow-up | 8.00 (2.38) | 2.52 (2.12) |
| Vowels | ||
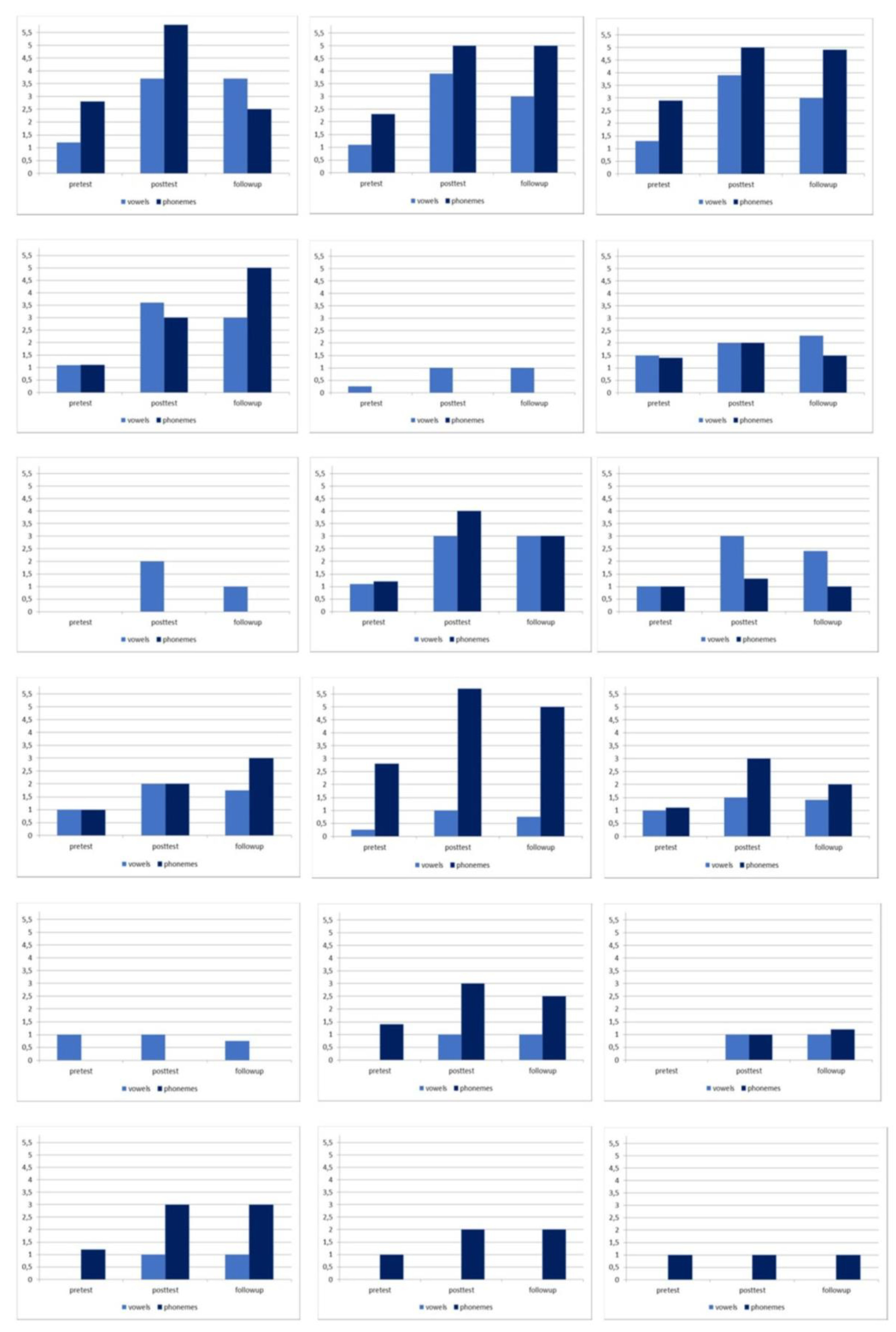
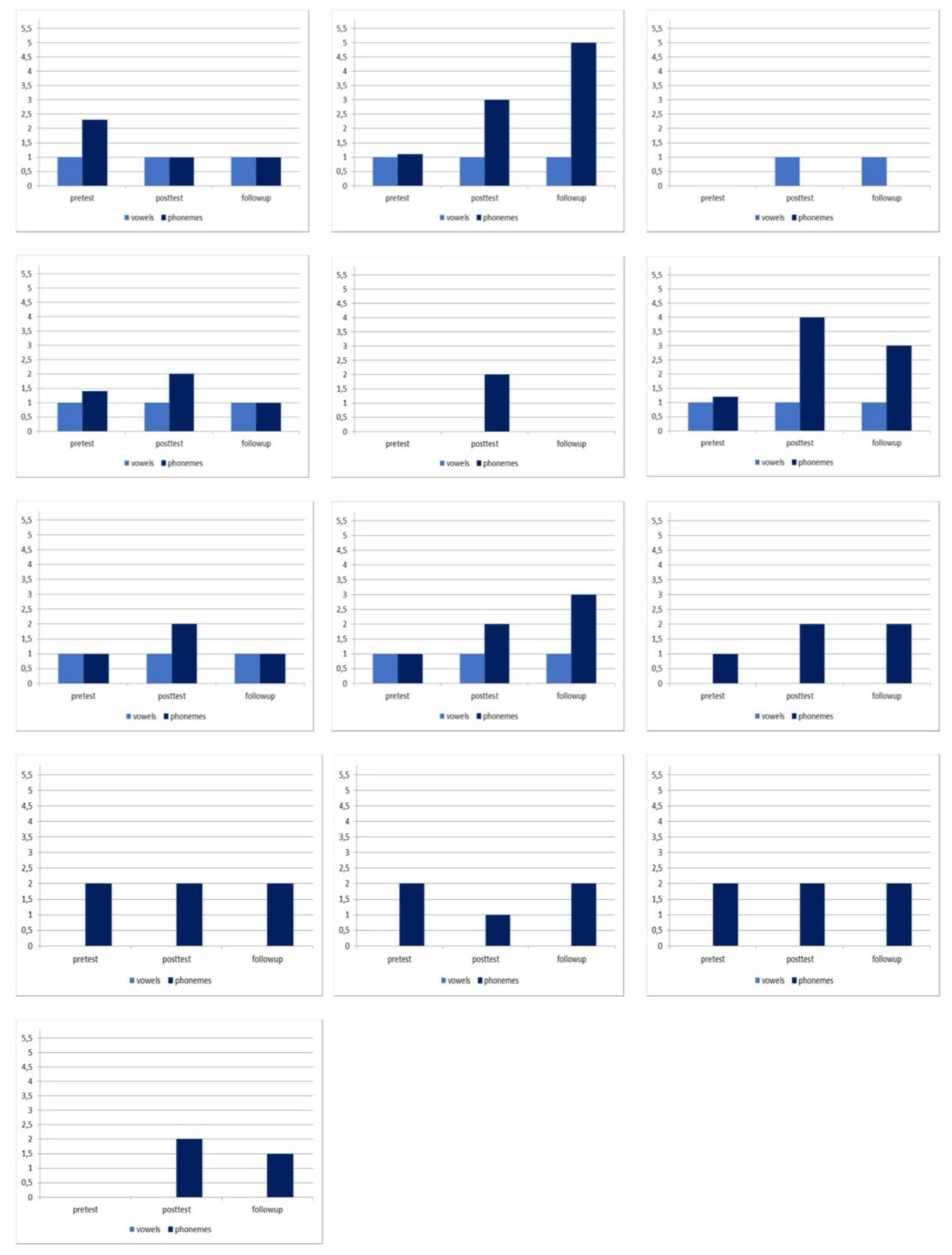
| Pre-test | 0.65 (0.54) | 0.46 (1.03) |
| Post-test | 1.92 (1.23) | 0.53 (1.50) |
| Follow-up | 1.66 (0.79) | 0.53 (1.70) |
| Phonemes | ||
| Pre-test | 1.23 (0.99) | 1.15 (0.89) |
| Post-test | 2.60 (1.89) | 1.92 (1.85) |
| Follow-up | 2.36 (1.71) | 1.80 (1.67) |
| Words | ||
| Pre-test | 0.32 (0.54) | 0.15 (0.58) |
| Post-test | 0.80 (1.08) | 0.40 (1.05) |
| Follow-up | 1.00 (1.71) | 0.35 (1.78) |
| Alpha (8–13 Hz) | ||
| Pre-test | 8.60 (1.75) | 8.51 (1.74) |
| Post-test | 9.45 (1.73) | 8.51 (1.73) |
| Follow-up | 8.80 (1.72) | 8.23 (1.73) |
| Beta (14–29 Hz) | ||
| Pre-test | 14.12 (3.60) | 14.02 (3.40) |
| Post-test | 21.43 (3.30) | 16.34 (3.30) |
| Follow-up | 19.71 (3.38) | 14.05 (3.40) |
| Theta (3.5–7 Hz) | ||
| Pre-test | 6.11 (1.90) | 6.01 (1.89) |
| Post-test | 6.22 (1.79) | 6.03 (1.80) |
| Follow-up | 6.18 (1.79) | 6.05 (1.80) |
| Parameters | Non-Sham tDCS Group | Sham tDCS Group |
|---|---|---|
| t (p) | t (p) | |
| Attention | ||
| Pre-test vs post-test | 16.32 (0.001) | 1.90 (0.07) |
| Pre-test vs follow-up | 13.12 (0.001) | 1.87 (0.08) |
| Post-test vs follow-up | 2.34 (0.05) | 1.75 (0.12) |
| Vowels | ||
| Pre-test vs post-test | 6.27 (0.001) | 1.45 (0.03) |
| Pre-test vs follow-up | 5.60 (0.001) | 0.85 (0.07) |
| Post-test vs follow-up | 3.01 (0.001) | 1.70 (0.08) |
| Phonemes | ||
| Pre-test vs post-test | 6.09 (0.001) | 2.25 (0.08) |
| Pre-test vs follow-up | 4.63 (0.001) | 2.67 (0.07) |
| Post-test vs follow-up | 1.18 (0.07) | 3.56 (0.12) |
| Words | ||
| Pre-test vs post-test | 3.57 (0.01) | 2.40 (0.06) |
| Pre-test vs follow-up | 3.16 (0.05) | 1.90 (0.11) |
| Post-test vs follow-up | 1.85 (0.05) | 2.45 (0.22) |
| Alpha (8–13 Hz) | ||
| Pre-test vs post-test | 14.71 (0.001) | 1.12 (0.17) |
| Pre-test vs follow-up | 13.50 (0.001) | 2.08 (0.07) |
| Post-test vs follow-up | 12.56 (0.05) | 2.70 (0.07) |
| Beta (14–29 Hz) | ||
| Pre-test vs post-test | 6.02 (0.001) | 2.14 (0.09) |
| Pre-test vs follow-up | 6.12 (0.001) | 1.23 (0.08) |
| Post-test vs follow-up | 1.90 (0.05) | 2.18 (0.08) |
| Theta (3.5–7 Hz) | ||
| Pre-test vs post-test | 3.50 (0.09) | 1.05 (0.12) |
| Pre-test vs follow-up | 3.55 (0.07) | 2.13 (0.08) |
| Post-test vs follow-up | 3.40 (0.07) | 1.21 (0.09) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabio, R.A.; Gangemi, A.; Semino, M.; Vignoli, A.; Priori, A.; Canevini, M.P.; Di Rosa, G.; Caprì, T. Effects of Combined Transcranial Direct Current Stimulation with Cognitive Training in Girls with Rett Syndrome. Brain Sci. 2020, 10, 276. https://doi.org/10.3390/brainsci10050276
Fabio RA, Gangemi A, Semino M, Vignoli A, Priori A, Canevini MP, Di Rosa G, Caprì T. Effects of Combined Transcranial Direct Current Stimulation with Cognitive Training in Girls with Rett Syndrome. Brain Sciences. 2020; 10(5):276. https://doi.org/10.3390/brainsci10050276
Chicago/Turabian StyleFabio, Rosa Angela, Antonio Gangemi, Martina Semino, Aglaia Vignoli, Alberto Priori, Maria Paola Canevini, Gabriella Di Rosa, and Tindara Caprì. 2020. "Effects of Combined Transcranial Direct Current Stimulation with Cognitive Training in Girls with Rett Syndrome" Brain Sciences 10, no. 5: 276. https://doi.org/10.3390/brainsci10050276
APA StyleFabio, R. A., Gangemi, A., Semino, M., Vignoli, A., Priori, A., Canevini, M. P., Di Rosa, G., & Caprì, T. (2020). Effects of Combined Transcranial Direct Current Stimulation with Cognitive Training in Girls with Rett Syndrome. Brain Sciences, 10(5), 276. https://doi.org/10.3390/brainsci10050276









