The Source of Palm Orientation Errors in the Signing of Children with ASD: Imitative, Motoric, or Both?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant
2.1.1. Autism
2.1.2. Intelligence
2.1.3. Language
2.1.4. Prior report
2.2. Procedure
2.3. Coding
2.4. Reliability
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Shield, A.; Meier, R.P. Palm reversal errors in native-signing children with autism. J. Commun. Disord. 2012, 45, 439–454. [Google Scholar] [CrossRef] [Green Version]
- Shield, A.; Pyers, J.; Martin, A.; Tager-Flusberg, H. Relations between language and cognition in native-signing children with autism spectrum disorder. Autism Res. 2016, 9, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Naigles, L.R.; Cheng, M.; Xu Rattanasone, N.; Tek, S.; Khetrapal, N.; Fein, D.; Demuth, K. “You’re telling me!” The prevalence and predictors of pronoun reversals in children with autism spectrum disorders and typical development. Res. Autism Spectr. Disord. 2016, 27, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, K.E.; Demuth, K. Individual differences in pronoun reversal: Evidence from two longitudinal case studies. J. Child. Lang. 2012, 39, 162–191. [Google Scholar] [CrossRef] [PubMed]
- Kanner, L. Autistic disturbances of affective contact. Nerv. Child. 1943, 2, 217–250. [Google Scholar]
- Chiat, S. If I were you and you were me: The analysis of pronouns in a pronoun-reversing child. J. Child. Lang. 1982, 9, 359–379. [Google Scholar] [CrossRef]
- Oshima-Takane, Y. Analysis of pronominal errors: A case-study. J. Child. Lang. 1992, 19, 111–131. [Google Scholar] [CrossRef]
- Schiff-Myers, N.B. From pronoun reversals to correct pronoun usage: A case study of a normally developing child. J. Speech Hear. Disord. 1983, 48, 394–402. [Google Scholar] [CrossRef]
- Williams, J.H.G.; Whiten, A.; Singh, T. A systematic review of action imitation in autistic spectrum disorder. J. Autism Dev. Disord. 2004, 34, 285–299. [Google Scholar] [CrossRef]
- Brown, J.D. Imitation, Play and Theory of Mind in Autism: An Observational and Experimental study. Unpublished Ph.D. Thesis, Saint Andrew’s University, St Andrews, UK, 1996. [Google Scholar]
- Whiten, A.; Brown, J. Imitation and the reading of other minds: Perspectives from the study of autism, normal children and non-human primates. In Intersubjective communication and emotion in early ontogeny; Bråten, S., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 260–280. [Google Scholar]
- Ohta, M. Cognitive disorders of infantile autism: A study employing the WISC, spatial relationship conceptualization, and gesture imitations. J. Autism Dev. Disord. 1987, 17, 45–62. [Google Scholar] [CrossRef]
- Hobson, R.P.; Lee, A. Imitation and identification in autism. J. Child. Psychol. Psychiatry 1999, 40, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Shield, A.; Meier, R.P. Learning an embodied visual language: Four imitation strategies available to sign learners. Front. Psychol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ament, K.; Mejia, A.; Buhlman, R.; Erklin, S.; Caffo, B.; Mostofsky, S.; Wodka, E. Evidence for specificity of motor impairments in catching and balance in children with autism. J. Autism Dev. Disord. 2015, 45, 742–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, A.N.; Landa, R.J.; Galloway, J.C. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys. Ther. 2011, 91, 1116–1129. [Google Scholar] [CrossRef] [Green Version]
- Green, D.; Charman, T.; Pickles, A.; Chandler, S.; Loucas, T.; Simonoff, E.; Baird, G. Impairment in movement skills of children with autistic spectrum disorders. Dev. Med. Child. Neurol. 2009, 51, 311–316. [Google Scholar] [CrossRef]
- McPhillips, M.; Finlay, J.; Bejerot, S.; Hanley, M. Motor deficits in children with autism spectrum disorder: A cross-syndrome study. Autism Res. 2014, 7, 664–676. [Google Scholar] [CrossRef] [Green Version]
- Jansiewicz, E.M.; Goldberg, M.C.; Newschaffer, C.J.; Denckla, M.B.; Landa, R.; Mostofsky, S.H. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. J. Autism Dev. Disord. 2006, 36, 613–621. [Google Scholar] [CrossRef]
- Mari, M.; Castiello, U.; Marks, D.; Marraffa, C.; Prior, M. The reach-to-grasp movement in children with autism spectrum disorder. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Biscaldi, M.; Rauh, R.; Irion, L.; Jung, N.H.; Mall, V.; Fleischhaker, C.; Klein, C. Deficits in motor abilities and developmental fractionation of imitation performance in high-functioning autism spectrum disorders. Eur. Child. Adolesc. Psychiatry 2014, 23, 599–610. [Google Scholar] [CrossRef]
- Gizzonio, V.; Avanzini, P.; Campi, C.; Orivoli, S.; Piccolo, B.; Cantalupo, G.; Tassinari, C.A.; Rizzolatti, G.; Fabbri-Destro, M. Failure in pantomime action execution correlates with the severity of social behavior deficits in children with autism: A praxis study. J. Autism Dev. Disord. 2015, 45, 3085–3097. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Dubey, P.; Jerath, V.K.; Jansiewicz, E.M.; Goldberg, M.C.; Denckla, M.B. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J. Int. Neuropsychol. Soc. JINS 2006, 12, 314–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, S.J.; Bennetto, L.; McEvoy, R.; Pennington, B.F. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child. Dev. 1996, 67, 2060–2073. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.M.; Bryson, S.E. Imitation and action in autism: A critical review. Psychol. Bull. 1994, 116, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.M.; Bryson, S.E. Gesture imitation in autism: II. Symbolic gestures and pantomimed object use. Cogn. Neuropsychol. 2007, 24, 679–700. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.N.; Srinivasan, S.M.; Woxholdt, C.; Shield, A. Differences in praxis performance and receptive language during fingerspelling between deaf children with and without autism spectrum disorder. Autism 2016, 22, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Njiokiktjien, C.; Driessen, M.; Habraken, L. Development of supination-pronation movements in normal children. Hum. Neurobiol. 1986, 5, 199–203. [Google Scholar]
- Brentari, D.; Poizner, H. A phonological analysis of a deaf Parkinsonian signer. Lang. Cogn. Process. 1994, 9, 69–99. [Google Scholar] [CrossRef]
- Padden, C.A.; Le Master, B. An alphabet on hand: The acquisition of fingerspelling in deaf children. Sign Lang. Stud. 1985, 14, 161–172. [Google Scholar] [CrossRef]
- Hochgesang, J.A.; Crasborn, O.; Lillo-Martin, D. ASL Signbank; Haskins Lab, Yale University: New Haven, CT, USA, 2020. [Google Scholar]
- Geer, L. Teaching ASL Fingerspelling to Second-language Learners: Explicit Versus Implicit Phonetic Training. Ph.D. Thesis, University of Texas at Austin, Austin, TX, USA, 2016. [Google Scholar]
- Shield, A. The signing of deaf children with autism: Lexical phonology and perspective-taking in the visual-spatial modality. Unpublished Ph.D. Thesis, University of Texas at Austin, Austin, TX, USA, 2010. [Google Scholar]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S.L. Autism Diagnostic Observation Schedule, 2nd ed.; (ADOS-2); Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Rutter, M.; Bailey, A.; Lord, C. Social Communication Questionnaire; Western Psychological Services: Los Angeles, CA, USA, 2003. [Google Scholar]
- Baron-Cohen, S.; Hoekstra, R.A.; Knickmeyer, R.; Wheelwright, S. The autism-spectrum quotient (AQ)—adolescent version. J. Autism Dev. Disord. 2006, 36, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.; Sherbenou, R.J.; Johnsen, S.K. Test. of Nonverbal Intelligence, 4th ed.; Pro-Ed: Austin, TX, USA, 2010. [Google Scholar]
- Chen Pichler, D.; Lee, J.; Lillo-Martin, D. Language development in ASL-English bimodal bilinguals. In Multilingual Aspects of Signed Language Communication and Disorder; Quinto-Pozos, D., Ed.; Multilingual Matters: Bristol, UK, 2014; pp. 235–260. [Google Scholar]
- Davidson, K.; Lillo-Martin, D.; Chen Pichler, D. Spoken English language development among native signing children with cochlear implants. J. Deaf Stud. Deaf Educ. 2014, 19, 238–250. [Google Scholar] [CrossRef]
- Bebko, J.M.; Calderon, R.; Treder, R. The language proficiency profile-2: Assessment of the global communication skills of deaf children across languages and modalities of expression. J. Deaf Stud. Deaf Educ. 2003, 8, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.M.; Dunn, D.M. Peabody Picture Vocabulary Test, 4th ed.; (PPVTTM-4); AGS: Circle Pines, MN, USA, 2007. [Google Scholar]
- Enns, C.J.; Zimmer, K.; Boudreault, P.; Rabu, S.; Broszeit, C. American Sign Language: Receptive skills test; Northern Signs Research, Inc.: Winnipeg, MB, USA, 2013. [Google Scholar]
- Wiig, E.H.; Semel, E.; Secord, W.A. Clinical Evaluation of Language Fundamentals, 5th ed.; (CELF-5); NCS Pearson: Bloomington, MN, USA, 2013. [Google Scholar]
- Wittenburg, P.; Brugman, H.; Russel, A.; Klassmann, A.; Sloetjes, H. ELAN: A professional framework for multimodality research. In Proceedings of the 5th International Conference on Language Resources and Evaluation (LREC 2006), Genoa, Italy, 24–26 May 2006; pp. 1556–1559. [Google Scholar]
- Marentette, P.F.; Mayberry, R.I. Principles for an emerging phonological system: A case study of early ASL acquisition. In Language Acquisition by Eye; Chamberlain, C., Morford, J.P., Mayberry, R.I., Eds.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2000; pp. 71–90. [Google Scholar]
- Siedlecki, T.; Bonvillian, J.D. Location, handshape, and movement: Young children’s acquisition of the formational aspects of American Sign Language. Sign Lang. Stud. 1993, 78, 31–52. [Google Scholar] [CrossRef]
- Meier, R.P.; Mauk, C.; Mirus, G.R.; Conlin, K.E. Motoric constraints on early sign acquisition. In Proceedings of the Child Language Research Forum; CSLI Press: Stanford, CA, USA, 1998; Volume 29, pp. 63–72. [Google Scholar]
- Cheek, A.; Cormier, K.; Repp, A.; Meier, R.P. Prelinguistic gesture predicts mastery and error in the production of early signs. Language 2001, 77, 292–323. [Google Scholar] [CrossRef]
- Clibbens, J.; Harris, M. Phonological processes and sign language development. In Critical Influences on Child Language Acquisition and Development; Messer, D., Turner, G., Eds.; Macmillan Press: New York, NY, USA, 1993; pp. 197–208. [Google Scholar]
- Karnopp, L.B. Phonological acquisition in sign languages. Let. Hoje 1997, 32, 147–162. [Google Scholar]
- Meier, R.P. The form of early signs: Explaining signing children’s articulatory development. In Advances in the Sign Language Development of Deaf Children; Schick, B., Marschark, M., Spencer, P.E., Eds.; Oxford University Press: New York, NY, USA, 2006; pp. 202–230. [Google Scholar]
- Takkinen, R. Variation of handshape features in the acquisition process. In Cross-Linguistic Perspectives in Sign Language Research: Selected Papers From TISLR 2000; Baker, A., van den Bogaerde, B., Crasborn, O., Eds.; Signum: Hamburg, Germany, 2003; pp. 81–94. [Google Scholar]
- Von Tetzchner, S. First signs acquired by a Norwegian deaf child with hearing parents. Sign Lang. Stud. 1984, 44, 225–257. [Google Scholar] [CrossRef]
- Charney, R. Pronoun errors in autistic children: Support for a social explanation. Int. J. Lang. Commun. Disord. 1980, 15, 39–43. [Google Scholar] [CrossRef]
- Tager-Flusberg, H. Dissociations in form and function in the acquisition of language by autistic children. In Constraints on Language Acquisition: STUDIES of Atypical Children; Tager-Flusberg, H., Ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1994; pp. 175–194. [Google Scholar]
- Humphrey, J.K. One, Two, Buckle Your Shoe: Numbering Systems in ASL.; H & H Publishers: Seattle, WA, USA, 1989. [Google Scholar]
- Klima, E.S.; Bellugi, U. The Signs of Language; Harvard University Press: Cambridge, MA, USA, 1979. [Google Scholar]
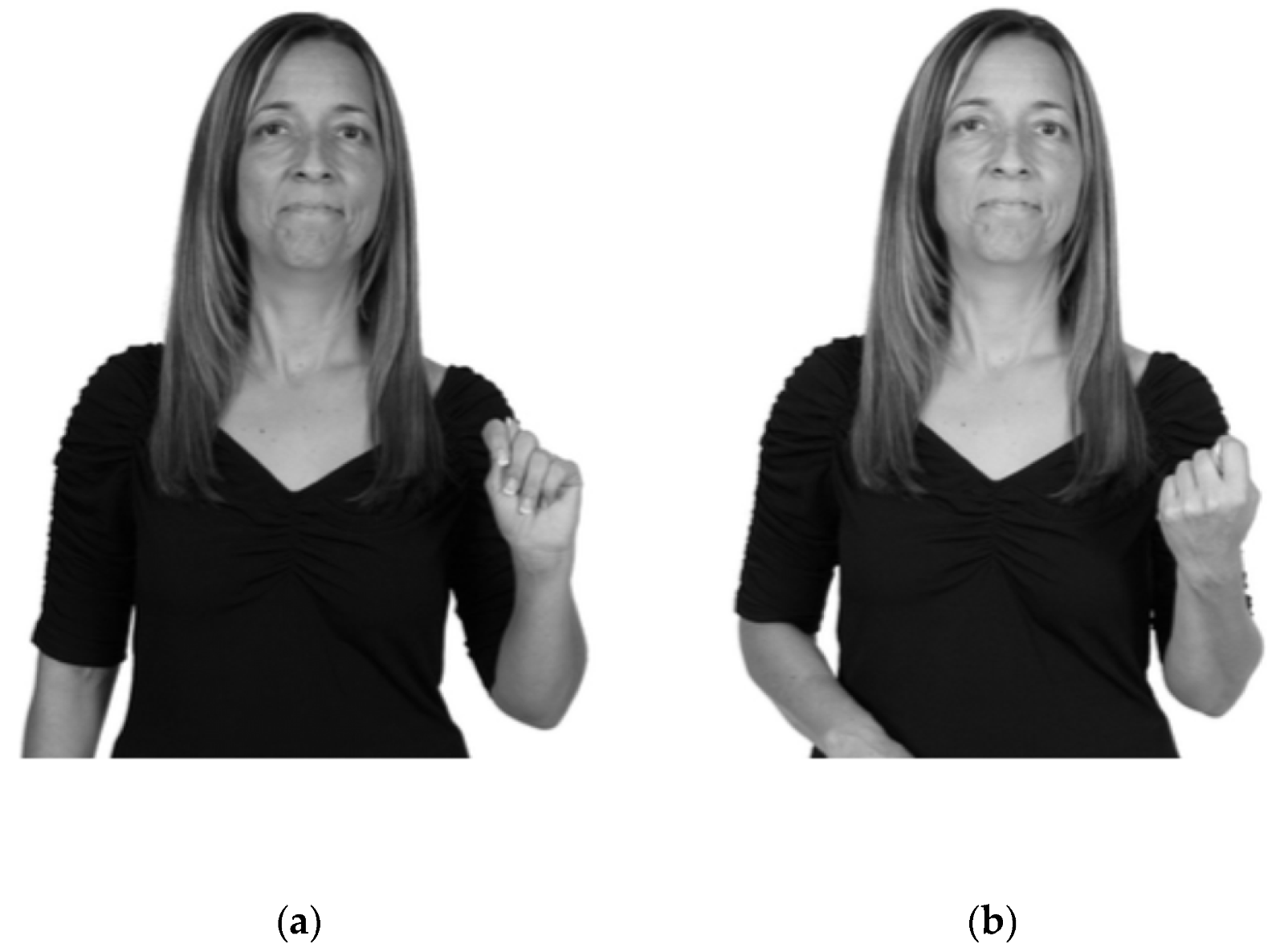
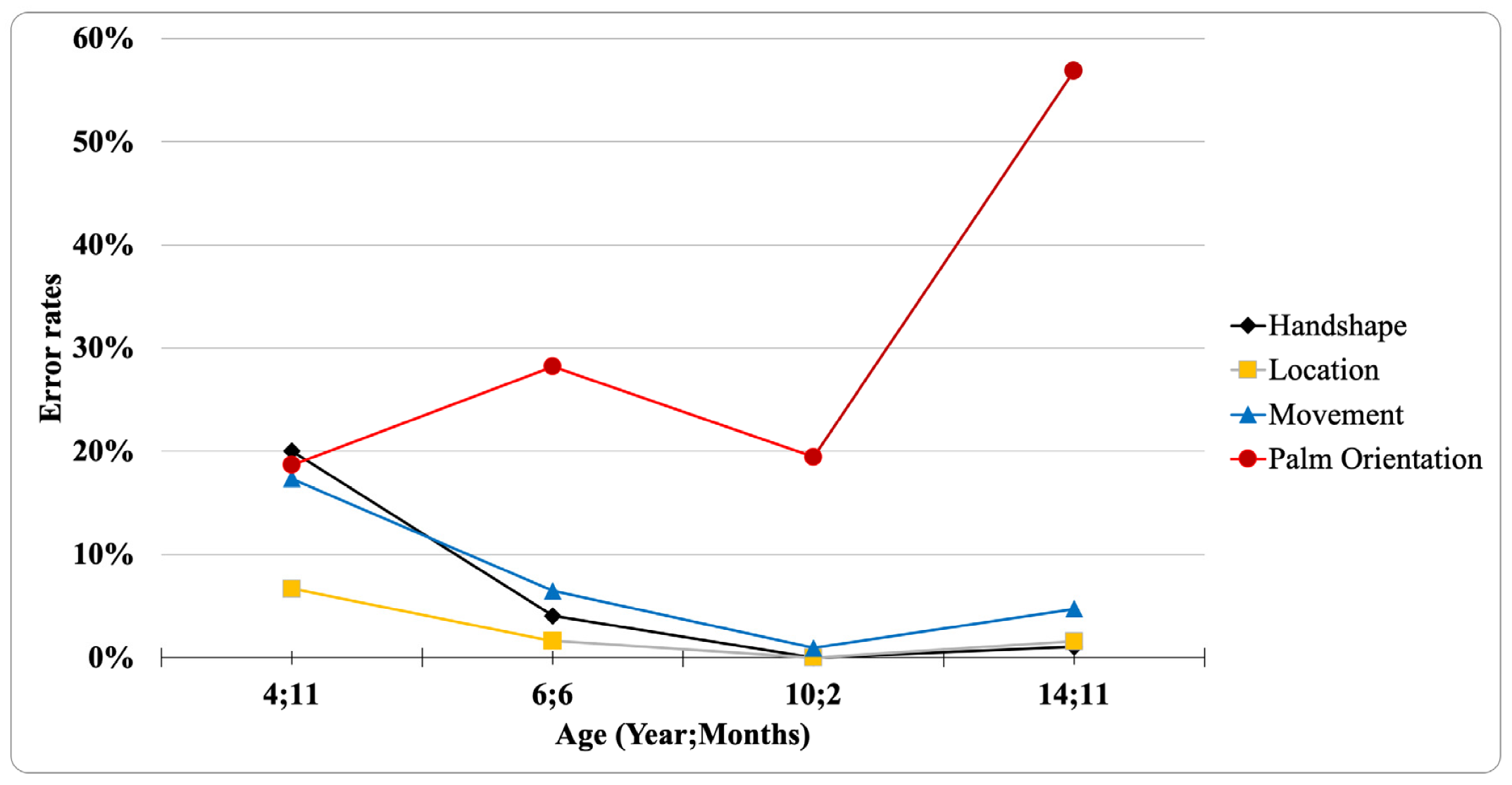
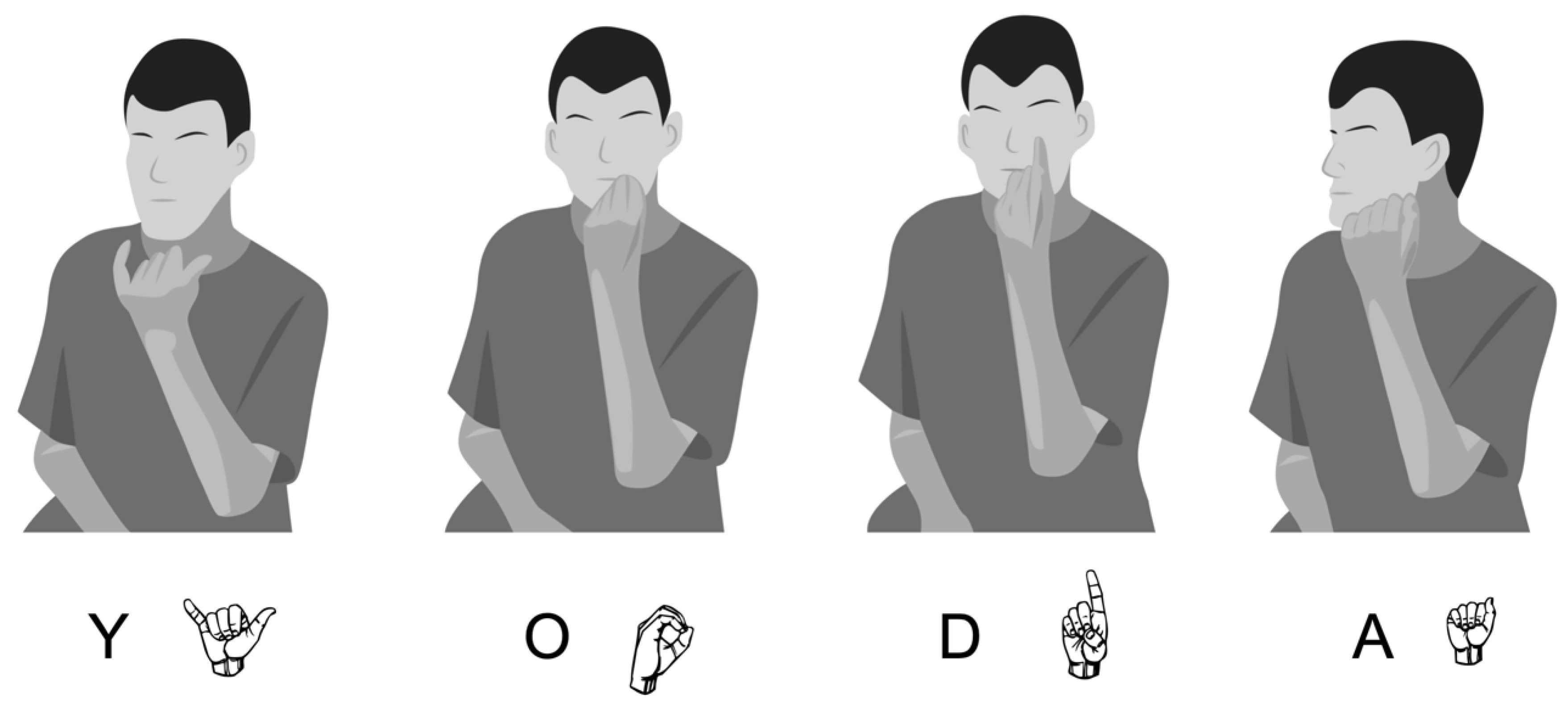

| Parameter | Number of Errors | Description of Errors |
|---|---|---|
| Location | 3 | On the sign orange, he failed to raise his hand from the resting position in his lap and therefore made the sign in contact with his knee rather than his chin (confirmed by maternal repetition immediately afterward) and produced the sign ice-cream in neutral space rather than at the chin. Finally, he produced the sign star without contact between the hands, at chest level rather than chin/head level |
| Handshape | 9 | He produced a 4-handshape instead of an h-dot handshape (i.e., a handshape with the first and second fingers extended and together, third and fourth fingers closed, and thumb extended) on rabbit, a 5-handshape instead of a v-handshape on dance (4 tokens), a baby-c-handshape instead of a g-handshape on the sign green, an a-handshape instead of an x-handshape on the sign apple, an 8-handshape instead of a g-handshape on the sign chicken, and a 5-handshape instead of an h-dot handshape on the sign horse |
| Movement | 23 | Forward movement (outward) rather than inward on the sign lion (two tokens) and computer (one token). He did not execute a path movement on several signs that normally exhibit path movement (such as elephant and giraffe), and reduced movement on several signs that typically exhibit repeated cycles of movement (e.g., horse, duck, monkey, bear and chicken). He deleted the movement segment entirely on the sign ice-cream. Other simplifications included the loss of the non-dominant hand on the sign dance as well as the dropping of one hand from a two-handed sign (bear, monkey). Several signs exhibited wild, uncontrolled movement, which were only interpretable because the parent repeated the sign with the correct form; these included dance and blue. Finally, he produced the sign yes with a forearm rotation rather than with a nodding movement of the wrist (two tokens) |
| Palm orientation | 4 | Two substitutions of an inward orientation for an outward orientation (on the sign flashing-light as well as a wave gesture) and the substitution of a downward orientation for a midline-facing orientation (turtle) and for an inward orientation (three) |
| Age | 4;11 | 6;6 | 10;2 | 14;11 |
|---|---|---|---|---|
| Number of sign tokens produced | 76 | 124 | 108 | 197 |
| Sign tokens/min | 6.33 | 10.33 | 9.0 | 16.42 |
| AGE | 4;11 | 6;6 | 10;2 | 14;11 | ||||
|---|---|---|---|---|---|---|---|---|
| Lexical (N = 72) | Fingerspelling (N = 4) | Lexical (N = 69) | Fingerspelling (N = 55) | Lexical (N = 43) | Fingerspelling (N = 65) | Lexical (N= 76) | Fingerspelling (N = 121) | |
| Handshape error | 15 (20.8%) | 0 | 5 (7.2%) | 0 | 0 | 0 | 2 (2.6%) | 0 |
| Location error | 5 (6.9%) | 0 | 2 (2.9%) | 0 | 0 | 0 | 3 (3.9%) | 0 |
| Movement error | 13 (18.1%) | 0 | 8 (11.6%) | 0 | 1 (2.3%) | 0 | 9 (11.8%) | 0 |
| Palm orientation error | 14 19.4%) | 0 | 5 (7.2%) | 30 (54.5%) | 2 (4.7%) | 19 (29.2%) | 2 (2.6%) | 110 (90.9%) |
| Error Type | 4;11 | 6;6 | 10;2 | 14;11 | Total |
|---|---|---|---|---|---|
| 180-degree reversal errors | 1 (7.1%) | 15 (42.9%) | 8 (36.4%) | 58 (50.9%) | 82 (45.1%) |
| Midline errors | 2 (14.3%) | 18 (51.4%) | 13 (59.1%) | 54 (47.4%) | 87 (47.8%) |
| Other errors | 11 (78.6%) | 2 (5.7%) | 0 | 0 | 13 (7.1%) |
| Total | 14 | 35 | 21 | 112 | 182 |
| 4;11 | 6;6 | 10;2 | 14;11 | |
|---|---|---|---|---|
| R | W | B-A-L-L | S-C-H | |
| B | F | P-A-P-E-R | S-C-H-O-O-L | |
| D-W | V | G-I-R-L | S-W-I-N-G | |
| B-O-O-K | S-H-O-O-L | [redacted] | ||
| D-O-O-R | B-I-R-D | [redacted] | ||
| C-H-A-I-R | T-E-A-C-H | [redacted] | ||
| W-A-T-C-H | P-P-H-O-N-E | Y | ||
| S-H-O-E-S | D-E-S-K | [redacted] | ||
| T-A-B-I-E | C-H-A-I-R | A | ||
| C-A-P | D-O-L-L | D-I-D | ||
| B-E-D | F-A-T-H-E-R | B-E-D-A | ||
| S-C-I-S-S-O-R-S | M-O-T-H-E-R | [redacted] | ||
| T-E-I-E-P-H-O-N-E | W-V-A-N | [redacted] | ||
| K | B-U-G | B-E | ||
| D-L-F-W | ||||
| M-D | ||||
| N-A-D-D-W | ||||
| M-D | ||||
| P-A-R-K | ||||
| P-A-R-I-S | ||||
| C | ||||
| [redacted] | ||||
| D-A-S-R | ||||
| D-A | ||||
| T-H-E-I-N-C-R-E-D-I-B-L-E-S | ||||
| A-R-L | ||||
| P-E-T-E-R-R | ||||
| R | ||||
| R | ||||
| D | ||||
| Y-O-D-A | ||||
| Total number of fingerspelled letters produced | 4 | 55 | 65 | 121 |
| Total number of midline errors | 0 | 18 (32.7%) | 11 (16.9%) | 53 (43.8%) |
| Total number of reversed letters | 0 | 12 (21.8%) | 8 (12.3%) | 57 (47.1%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shield, A.; Igel, M.; Randall, K.; Meier, R.P. The Source of Palm Orientation Errors in the Signing of Children with ASD: Imitative, Motoric, or Both? Brain Sci. 2020, 10, 268. https://doi.org/10.3390/brainsci10050268
Shield A, Igel M, Randall K, Meier RP. The Source of Palm Orientation Errors in the Signing of Children with ASD: Imitative, Motoric, or Both? Brain Sciences. 2020; 10(5):268. https://doi.org/10.3390/brainsci10050268
Chicago/Turabian StyleShield, Aaron, Megan Igel, Kristina Randall, and Richard P. Meier. 2020. "The Source of Palm Orientation Errors in the Signing of Children with ASD: Imitative, Motoric, or Both?" Brain Sciences 10, no. 5: 268. https://doi.org/10.3390/brainsci10050268
APA StyleShield, A., Igel, M., Randall, K., & Meier, R. P. (2020). The Source of Palm Orientation Errors in the Signing of Children with ASD: Imitative, Motoric, or Both? Brain Sciences, 10(5), 268. https://doi.org/10.3390/brainsci10050268





