Prognosis after Mild Traumatic Brain Injury: Influence of Psychiatric Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Patients
2.2. Clinical Evaluation
2.3. Brain Imaging
2.4. Psychiatric Evaluation
2.5. Return to Work Evaluation
2.6. Statistical Analyses
3. Results
3.1. Clinical Evaluation
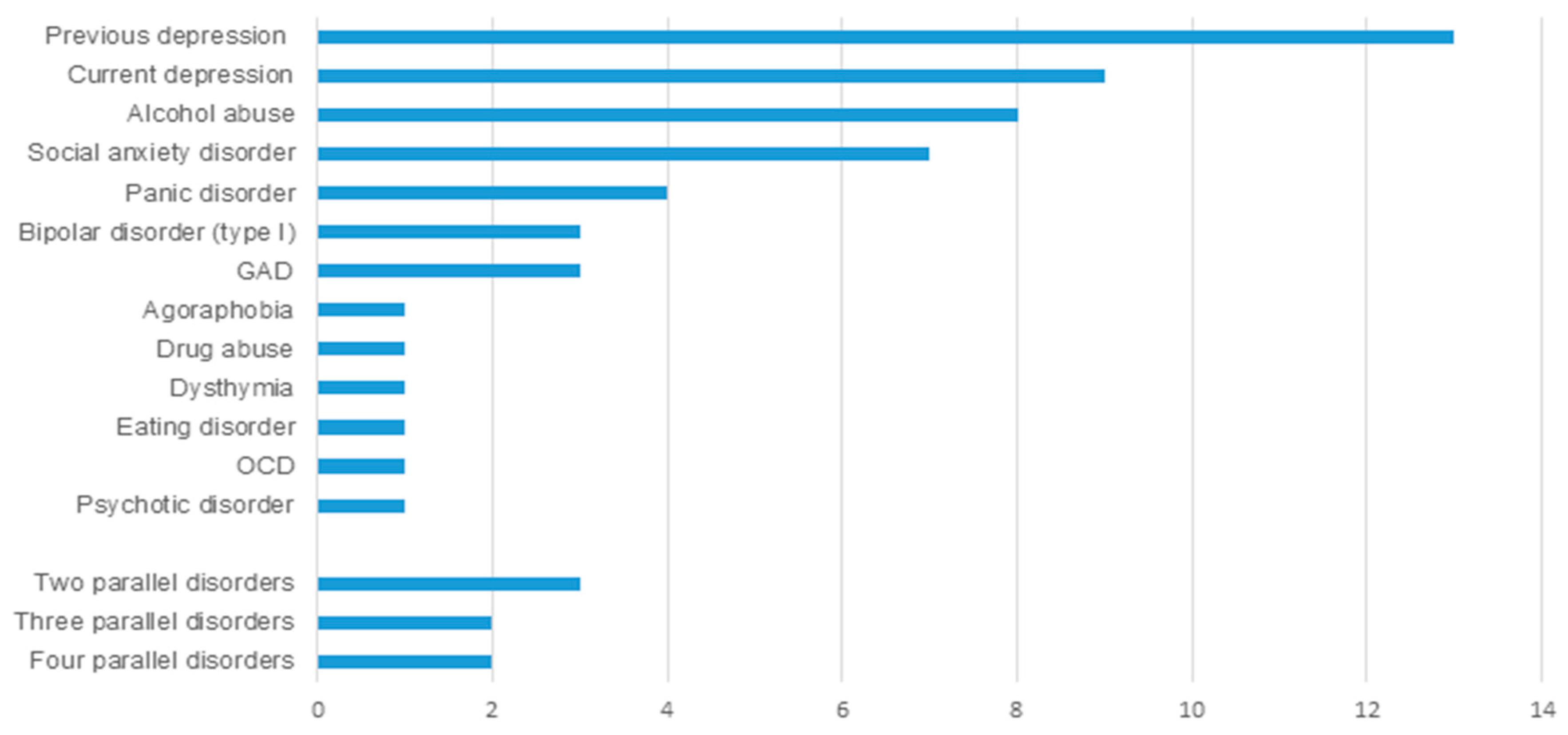
3.2. Psychiatric Evaluation
3.3. Return to Work
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FLAIR | Fluid attenuated inversion recovery |
| GOSE | Extended Glasgow Outcome Scale |
| HADS | Hospital Anxiety and Depression Scale |
| IQR | Interquartile ranges |
| MRI | Magnetic resonance imaging |
| MTBI | Mild traumatic brain injury |
| NINDS | National Institute of Neurological Disorders and Stroke |
| NOS-TBI | Neurological Outcome Scale for TBI |
| PHQ-9 | Patient Health Questionnaire 9-item depression scale |
| PTSD | Post-traumatic stress disorder |
| RPQ | Rivermead Post-Concussion Symptom Questionnaire |
| rs-FMRI | Resting state magnetic resonance imaging |
| RTW | Return to work |
| SCID-I | Structured Clinical Interview for DSM-IV Axis I disorders |
| WHO | World Health Organization |
References
- Van der Naalt, J.; Timmerman, M.E.; de Koning, M.E.; van der Horn, H.J.; Scheenen, M.E.; Jacobs, B.; Hageman, G.; Yilmaz, T.; Roks, G.; Spikman, J.M. Early predictors of outcome after mild traumatic brain injury (UPFRONT): An observational cohort study. Lancet Neurol. 2017, 16, 532–540. [Google Scholar] [CrossRef]
- Corrigan, J.D.; Horn, S.D.; Barrett, R.S.; Smout, R.J.; Bogner, J.; Hammond, F.M.; Brandstater, M.E.; Majercik, S. Effects of Patient Preinjury and Injury Characteristics on Acute Rehabilitation Outcomes for Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2015, 96 (Suppl. 8), S304–S329. [Google Scholar] [CrossRef]
- Bryant, R.A.; O’Donnell, M.L.; Creamer, M.; McFarlane, A.C.; Clark, C.R.; Silove, D. The psychiatric sequelae of traumatic injury. Am. J. Psychiatry 2010, 167, 312–320. [Google Scholar] [CrossRef]
- Perry, D.C.; Sturm, V.E.; Peterson, M.J.; Pieper, C.F.; Bullock, T.; Boeve, B.F.; Miller, B.L.; Guskiewicz, K.M.; Berger, M.S.; Kramer, J.H.; et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: A meta-analysis. J. Neurosurg. 2016, 124, 511–526. [Google Scholar] [CrossRef]
- Hart, T.; Brenner, L.; Clark, A.N.; Bogner, J.A.; Novack, T.A.; Chervoneva, I.; Nakase-Richardson, R.; Arango-Lasprilla, J.C. Major and minor depression after traumatic brain injury. Arch. Phys. Med. Rehabil. 2011, 92, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Ponsford, J.; Alway, Y.; Gould, K.R. Epidemiology and Natural History of Psychiatric Disorders After TBI. J. Neuropsychiatry Clin. Neurosci. 2018, 30, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Timonen, M.; Miettunen, J.; Hakko, H.; Zitting, P.; Veijola, J.; Von Wendt, L.; Räsänen, P. The association of preceding traumatic brain injury with mental disorders, alcoholism and criminality: The Northern Finland 1966 Birth Cohort Study. Psychiatry Res. 2002, 113, 217–226. [Google Scholar] [CrossRef]
- Madsen, T.; Erlangsen, A.; Orlovska, S.; Mofaddy, R.; Nordentoft, M.; Benros, M.E. Association between traumatic brain injury and risk of suicide. JAMA J. Am. Med. Assoc. 2018, 320, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Byers, A.L.; Li, Y.; Barnes, D.E.; Seal, K.H.; Boscardin, W.J.; Yaffe, K. A national study of TBI and risk of suicide and unintended death by overdose and firearms. Brain Inj. 2020, 34, 328–334. [Google Scholar] [CrossRef]
- Bloom, B.; Thomas, S.; Ahrensberg, J.M.; Weaver, R.; Fowler, A.; Bestwick, J.; Harris, T.; Pearse, R. A systematic review and meta-analysis of return to work after mild Traumatic brain injury. Brain Inj. 2018, 32, 1623–1636. [Google Scholar] [CrossRef]
- Mani, K.; Cater, B.; Hudlikar, A. Cognition and return to work after mild/moderate traumatic brain injury: A systematic review. Work 2017, 58, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, A.R.; Arnett, P.A. Positive psychology perspective on traumatic brain injury recovery and rehabilitation. Appl. Neuropsychol. 2018, 25, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Waldron, B.; Casserly, L.M.; O’Sullivan, C. Cognitive behavioural therapy for depression and anxiety in adults with acquired brain injury. What works for whom? Neuropsychol. Rehabil. 2013, 23, 64–101. [Google Scholar] [CrossRef] [PubMed]
- Soo, C.; Tate, R. Psychological treatment for anxiety in people with traumatic brain injury. Cochrane Database Syst. Rev. 2007, CD005239. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Iverson, G.L.; Lange, R.T.; Wäljas, M.; Liimatainen, S.; Dastidar, P.; Hartikainen, K.M.; Soimakallio, S.; Öhman, J. Outcome from Complicated versus Uncomplicated Mild Traumatic Brain Injury. Rehabil. Res. Pract. 2012. [Google Scholar] [CrossRef]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition (SCID-I/P, 11/2002 Revision); Springer: New York, NY, USA, 2002; ISBN 9780880489317. [Google Scholar]
- Carroll, L.; Cassidy, J.D.; Holm, L.; Kraus, J.; Coronado, V. Methodological issues and research recommendations for mild traumatic brain injury: The who collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 2004, 43, 113–125. [Google Scholar] [CrossRef]
- Wilde, E.A.; McCauley, S.R.; Kelly, T.M.; Levin, H.S.; Pedroza, C.; Clifton, G.L.; Robertson, C.S.; Moretti, P. Feasibility of the Neurological Outcome Scale for Traumatic Brain Injury (NOS-TBI) in Adults. J. Neurotrauma 2010, 27, 991–997. [Google Scholar] [CrossRef]
- King, N.S.; Crawford, S.; Wenden, F.J.; Moss, N.E.G.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef]
- Jennett, B.; Snoek, J.; Bond, M.R.; Brooks, N. Disability after severe head injury: Observations on the use of the Glasgow Outcome Scale. J. Neurol. Neurosurg. Psychiatry 1981, 44, 285–293. [Google Scholar] [CrossRef]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B.W. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-I-CV); American Pasychiatric Press Inc.: New York, NY, USA, 1997; ISBN 9780880489317. [Google Scholar]
- Hicks, R.; Giacino, J.; Harrison-Felix, C.; Manley, G.; Valadka, A.; Wilde, E.A. Progress in developing common data elements for traumatic brain injury research: Version two-the end of the beginning. J. Neurotrauma 2013, 30, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Kahan, M.; Jones, K.M.; Balalla, S.; McPherson, K.; Stedman, E.; Feigin, V.L. Return to Pre-Injury Work Following Mild Traumatic Brain Injury. Brain Impair. 2018, 19, 153–165. [Google Scholar] [CrossRef]
- Wäljas, M.; Iverson, G.L.; Lange, R.T.; Liimatainen, S.; Hartikainen, K.M.; Dastidar, P.; Soimakallio, S.; Öhman, J. Return to work following mild traumatic brain injury. J. Head Trauma Rehabil. 2014, 29, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Bigler, E.D. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J. Int. Neuropsychol. Soc. 2008, 14, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Booker, J.; Sinha, S.; Choudhari, K.; Dawson, J.; Singh, R. Predicting functional recovery after mild traumatic brain injury: The SHEFBIT cohort. Brain Inj. 2019, 33, 1158–1164. [Google Scholar] [CrossRef]
- Barker-Collo, S.; Theadom, A.; Jones, K.; Starkey, N.; Kahan, M.; Feigin, V. Depression and anxiety across the first 4 years after mild traumatic brain injury: Findings from a community-based study. Brain Inj. 2018, 32, 1651–1658. [Google Scholar] [CrossRef]
- Singh, R.; Mason, S.; Lecky, F.; Dawson, J. Prevalence of depression after TBI in a prospective cohort: The SHEFBIT study. Brain Inj. 2018, 33, 584–591. [Google Scholar] [CrossRef]
- Bombardier, C.H.; Fann, J.R.; Temkin, N.R.; Esselman, P.C.; Barber, J.; Dikmen, S.S. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA J. Am. Med. Assoc. 2010, 303, 1938–1945. [Google Scholar] [CrossRef]
- Moore, E.L.; Terryberry-Spohr, L.; Hope, D.A. Mild traumatic brain injury and anxiety sequelae: A review of the literature. Brain Inj. 2006, 20, 117–132. [Google Scholar] [CrossRef]
- Mallya, S.; Sutherland, J.; Pongracic, S.; Mainland, B.; Ornstein, T.J. The Manifestation of Anxiety Disorders after Traumatic Brain Injury: A Review. J. Neurotrauma 2015, 32, 411–421. [Google Scholar] [CrossRef]
- Bigler, E.D.; Tsao, J.W. Mild traumatic brain injury in soldiers returning from combat. Neurology 2017, 88, 1490–1492. [Google Scholar] [CrossRef] [PubMed]
- Neria, Y.; Nandi, A.; Galea, S. Post-traumatic stress disorder following disasters: A systematic review. Psychol. Med. 2008, 38, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Gillikin, C.; Habib, L.; Evces, M.; Bradley, B.; Ressler, K.J.; Sanders, J. Trauma exposure and PTSD symptoms associate with violence in inner city civilians. J. Psychiatr. Res. 2016, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.E.; Larson, E.R.; Hunt, J.C.; Lorber, W.G.; DeRoon-Cassini, T.A. Interaction Between Psychiatric Symptoms and History of Mild TBI When Evaluating Postconcussion Syndrome in Veterans. Mil. Med. 2019, 185 (Suppl. 1), 161–167. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, A.; Marinkovic, I.; Isokuortti, H.; Vanni, S.; Melkas, S. Intracranial Traumatic Lesions Dealy Return to Work in Mild Traumatic Injury Patients. Brain Inj. 2019, 33, 130–131. [Google Scholar]
- Lobbestael, J.; Leurgans, M.; Arntz, A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II). Clin. Psychol. Psychother. 2011, 18, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Paananen, R.; Ristikari, T.; Merikukka, M.; Gissler, M. Social determinants of mental health: A finnish nationwide follow-up study on mental disorders. J. Epidemiol. Community Health 2013, 67, 1025–1031. [Google Scholar] [CrossRef]
- Gary, F.A. Stigma: Barrier to mental health care among ethnic minorities. Issues Ment. Health Nurs. 2005, 26, 979–999. [Google Scholar] [CrossRef]
- Pirkola, S.P.; Isometsä, E.; Suvisaari, J.; Aro, H.; Joukamaa, M.; Poikolainen, K.; Koskinen, S.; Aromaa, A.; Lönnqvist, J.K. DSM-IV mood-, anxiety- and alcohol use disorders and their comorbidity in the Finnish general population. Results from the Health 2000 Study. Soc. Psychiatry Psychiatr. Epidemiol. 2005, 40, 1–10. [Google Scholar] [CrossRef]

| Dropped Out n = 28 | Included n = 103 | p Value | |
|---|---|---|---|
| Mean age | 37.5 (SD 12.5) | 40.5 (SD 13.2) | 0.265 |
| Female sex | 60.7% | 42.7% | 0.091 |
| Median time to RTW | 7.0 (4.7–9.3) days | 10.0 (6.4–3.7) days | 0.175 |
| Median GOSE | 8.0 | 8.0 | 0.378 |
| Variable | No Psychiatric Disorder | Any Psychiatric Disorder | p | ||||
|---|---|---|---|---|---|---|---|
| Valid n | Mean/n | SD/IQR/% | Valid n | Mean/n | SD/IQR/% | ||
| Mean age | 77 | 41.6 | 13.0 | 26 | 38.5 | 3.2 | 0.293 |
| Female sex | 77 | 36 | 46.8 | 26 | 9 | 34.6 | 0.281 |
| Married or cohabited | 77 | 55 | 53.4 | 26 | 19 | 73.1 | 0.872 |
| Education >15 years | 77 | 47 | 61.0 | 26 | 12 | 45.6 | 0.185 |
| GCS < 15 (= 13 or 14) | 42 | 13 | 31.0 | 13 | 5 | 38.5 | 0.614 |
| Median LOC hh:min | 53 | 0:01 | 0:01–0:04 | 16 | 0:01 | 0:01–0:02 | 0.488 |
| Median retrograde PTA hh:min | 68 | 0:00 | 0:00–0:05 | 24 | 0:05 | 0:00–0:30 | 0.478 |
| Median anterograde PTA hh:min | 68 | 1:00 | 0:20–4:00 | 24 | 1:17 | 0:29–2:22 | 0.476 |
| Traumatic lesion in MRI | 77 | 26 | 33.8 | 26 | 10 | 38.5 | 0.418 |
| Mechanism | N | % |
|---|---|---|
| Car accident | 4 | 3.9 |
| Motorcycle accident | 2 | 1.9 |
| Traffic accident as a pedestrian | 2 | 1.9 |
| Sports | 13 | 12.6 |
| Bicycle accident | 28 | 27.2 |
| Ground-level fall | 28 | 27.2 |
| Fall from a height | 20 | 19.4 |
| Violence | 3 | 2.9 |
| Other | 2 | 1.9 |
| Unknown | 1 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinkovic, I.; Isokuortti, H.; Huovinen, A.; Trpeska Marinkovic, D.; Mäki, K.; Nybo, T.; Korvenoja, A.; Rahul, R.; Vataja, R.; Melkas, S. Prognosis after Mild Traumatic Brain Injury: Influence of Psychiatric Disorders. Brain Sci. 2020, 10, 916. https://doi.org/10.3390/brainsci10120916
Marinkovic I, Isokuortti H, Huovinen A, Trpeska Marinkovic D, Mäki K, Nybo T, Korvenoja A, Rahul R, Vataja R, Melkas S. Prognosis after Mild Traumatic Brain Injury: Influence of Psychiatric Disorders. Brain Sciences. 2020; 10(12):916. https://doi.org/10.3390/brainsci10120916
Chicago/Turabian StyleMarinkovic, Ivan, Harri Isokuortti, Antti Huovinen, Daniela Trpeska Marinkovic, Kaisa Mäki, Taina Nybo, Antti Korvenoja, Raj Rahul, Risto Vataja, and Susanna Melkas. 2020. "Prognosis after Mild Traumatic Brain Injury: Influence of Psychiatric Disorders" Brain Sciences 10, no. 12: 916. https://doi.org/10.3390/brainsci10120916
APA StyleMarinkovic, I., Isokuortti, H., Huovinen, A., Trpeska Marinkovic, D., Mäki, K., Nybo, T., Korvenoja, A., Rahul, R., Vataja, R., & Melkas, S. (2020). Prognosis after Mild Traumatic Brain Injury: Influence of Psychiatric Disorders. Brain Sciences, 10(12), 916. https://doi.org/10.3390/brainsci10120916





