Moderate Adolescent Ethanol Vapor Exposure and Acute Stress in Adulthood: Sex-Dependent Effects on Social Behavior and Ethanol Intake in Sprague–Dawley Rats
Abstract
1. Introduction
2. Methods
2.1. Animals
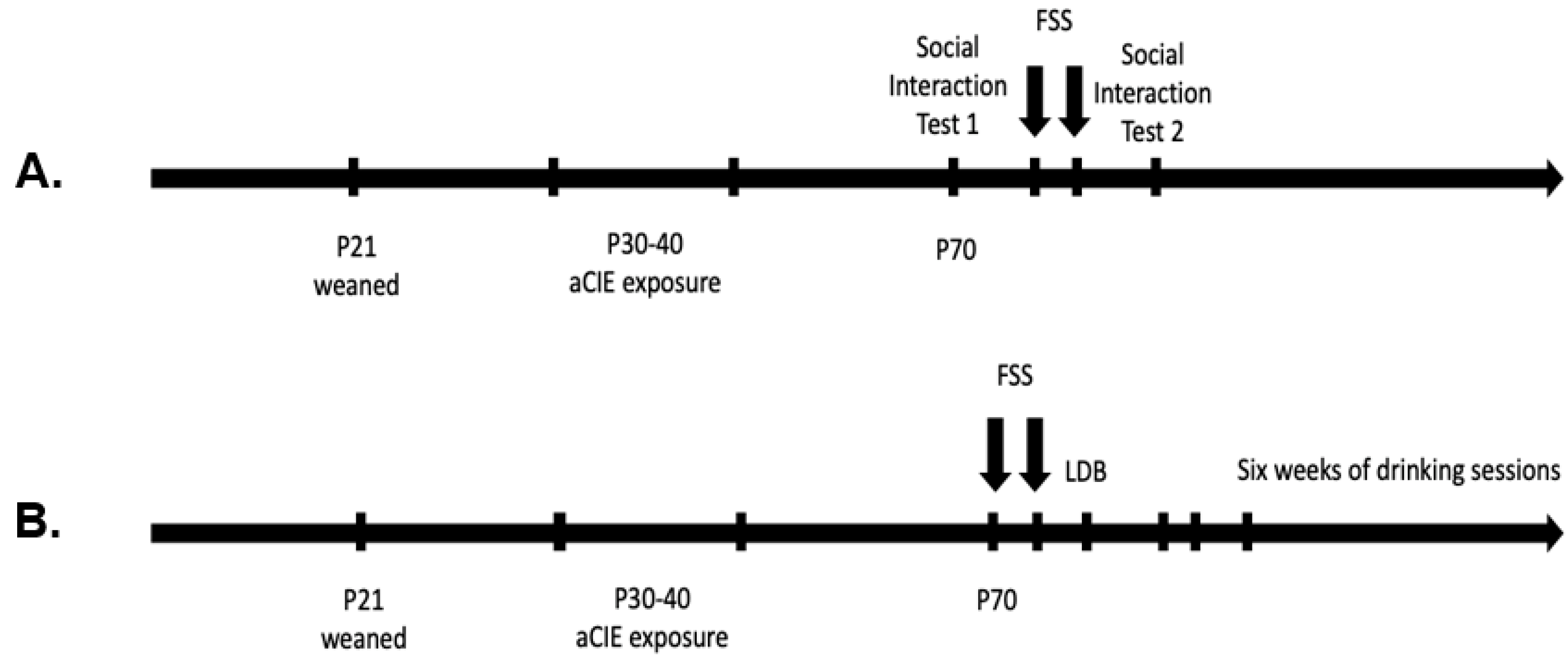
2.2. Experimental Design
2.3. Moderate Adolescent Chronic Intermittent Ethanol (aCIE) Exposure
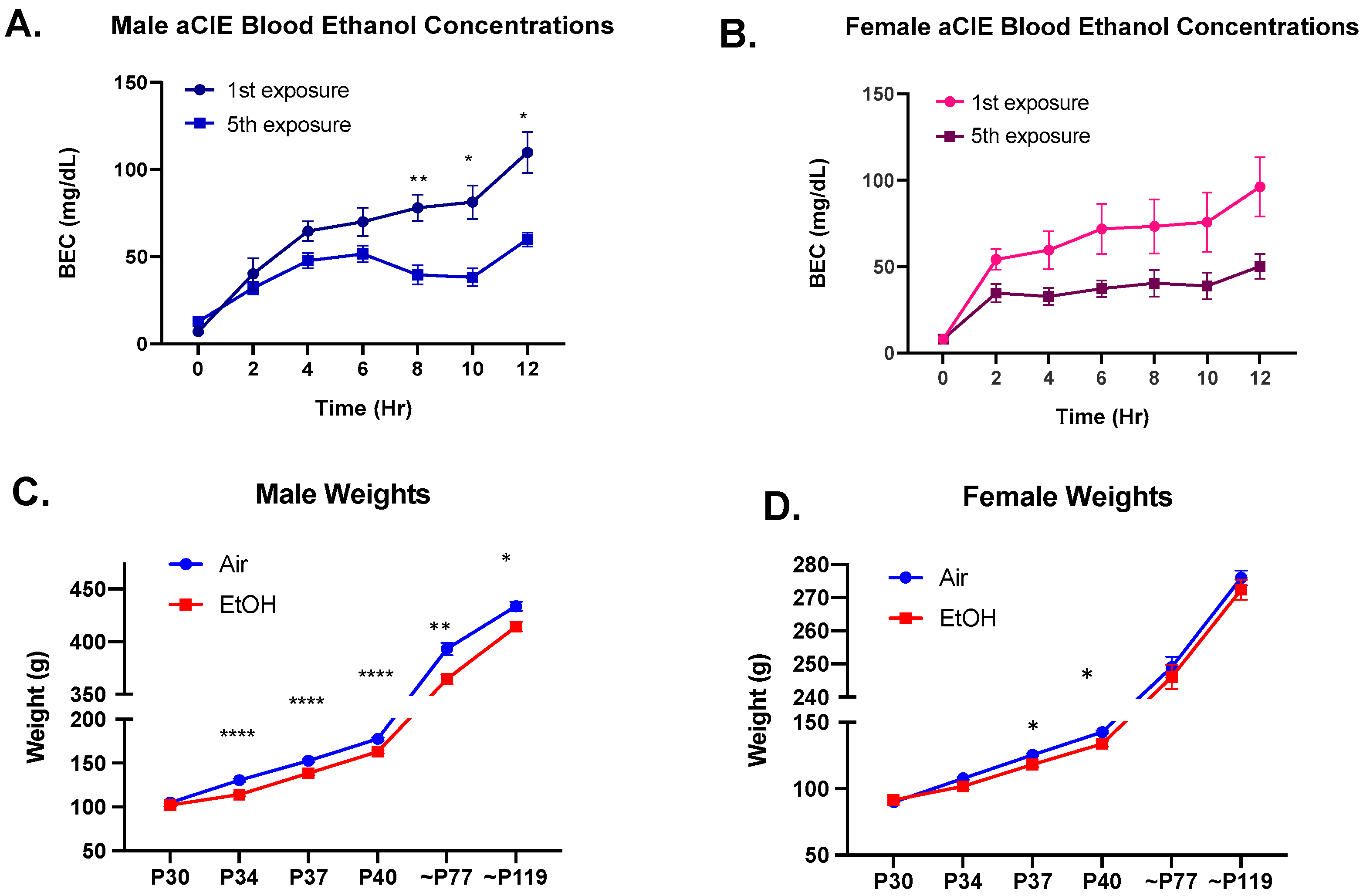
2.4. Blood Ethanol Concentrations
2.5. Forced Swim Stress
2.6. Experiment 1: Social Interaction
2.7. Experiment 2: Light–Dark Box and Voluntary Ethanol Drinking
2.7.1. Light–Dark Box
2.7.2. Voluntary Ethanol Drinking
2.8. Statistics and Data Analyses
3. Results
3.1. Characterization of the aCIE Model
3.2. Forced Swim Stress
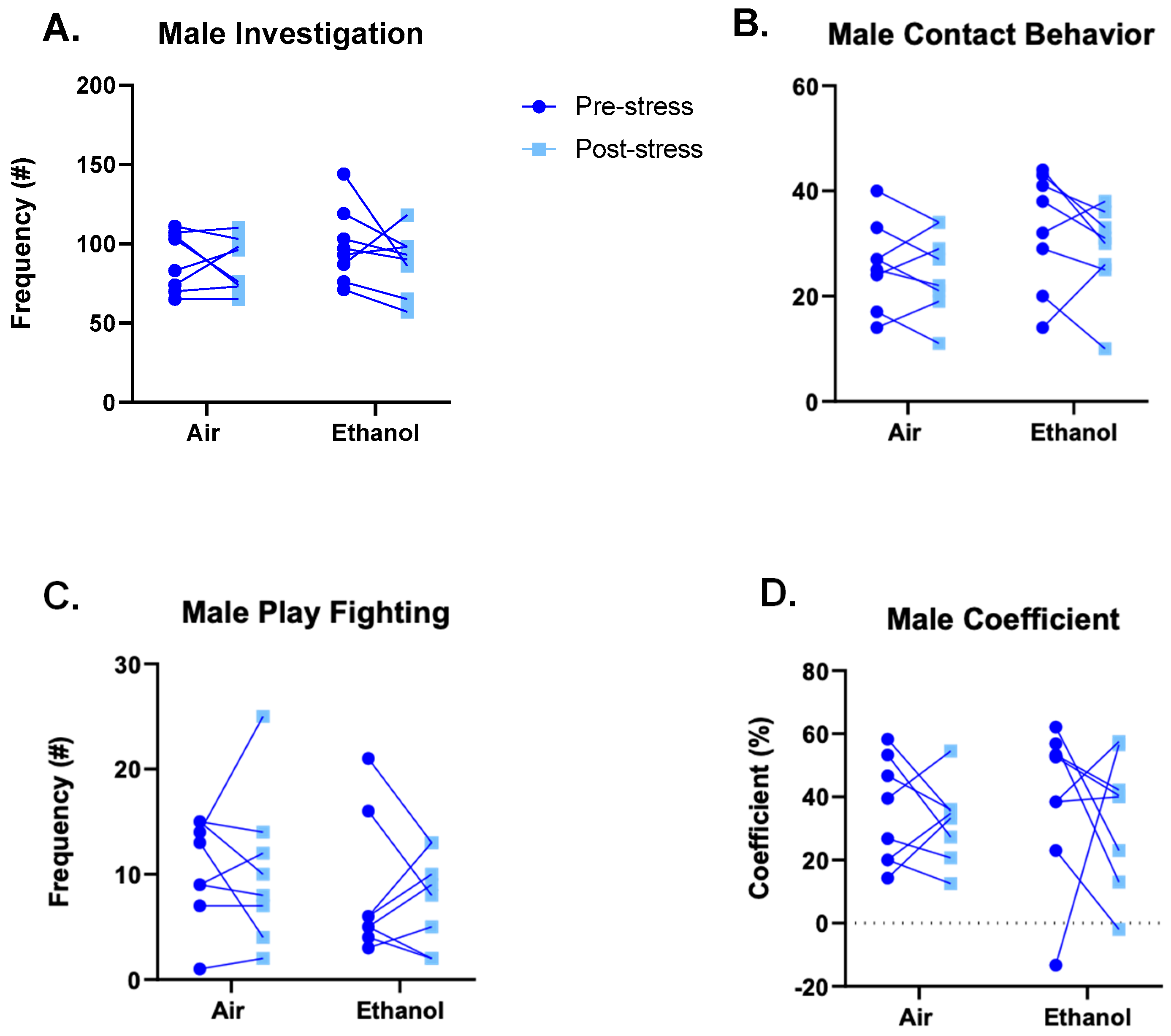
3.3. Experiment 1: Social Interaction
3.4. Experiment 2: Light–Dark Box and Voluntary Ethanol Drinking
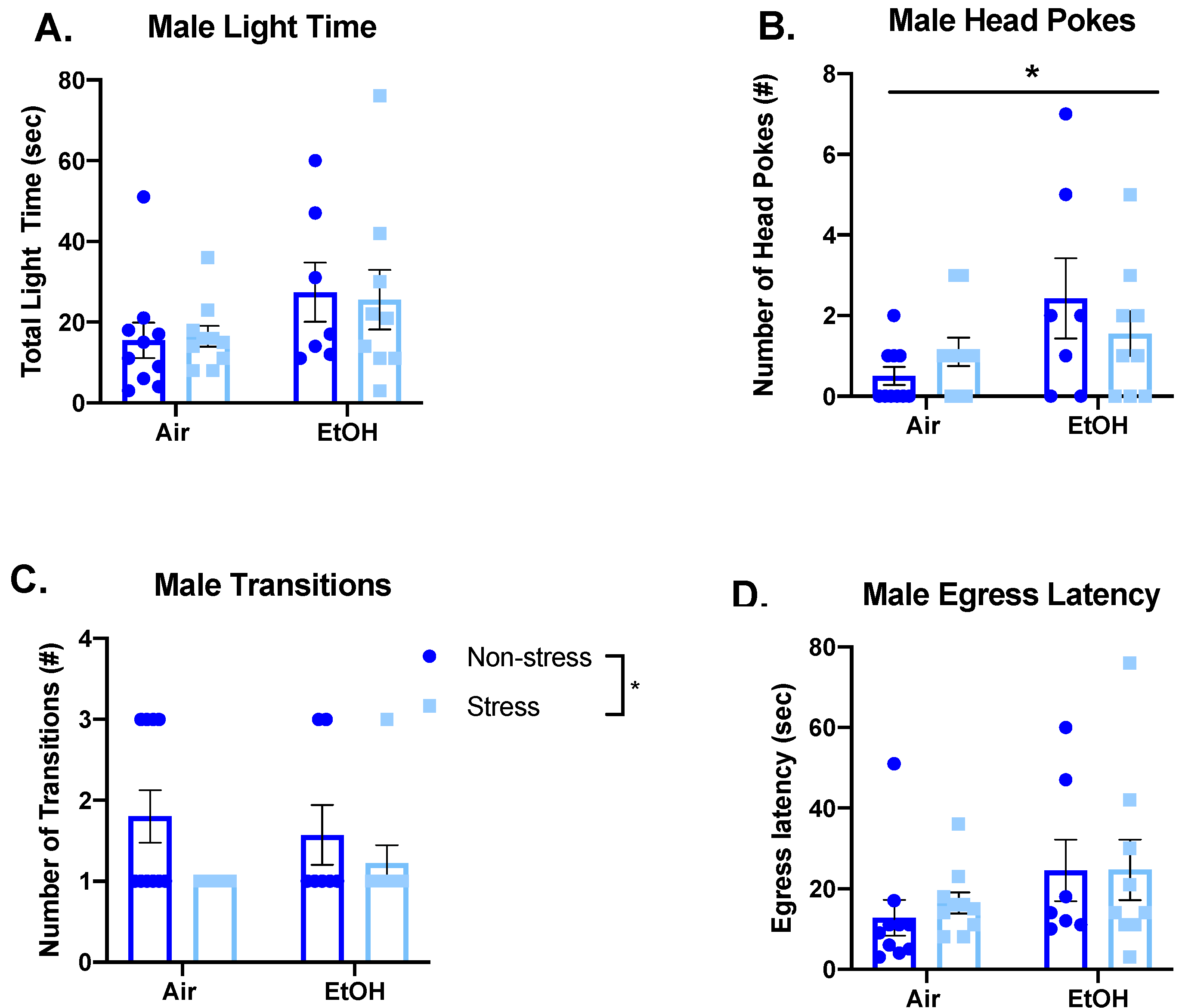
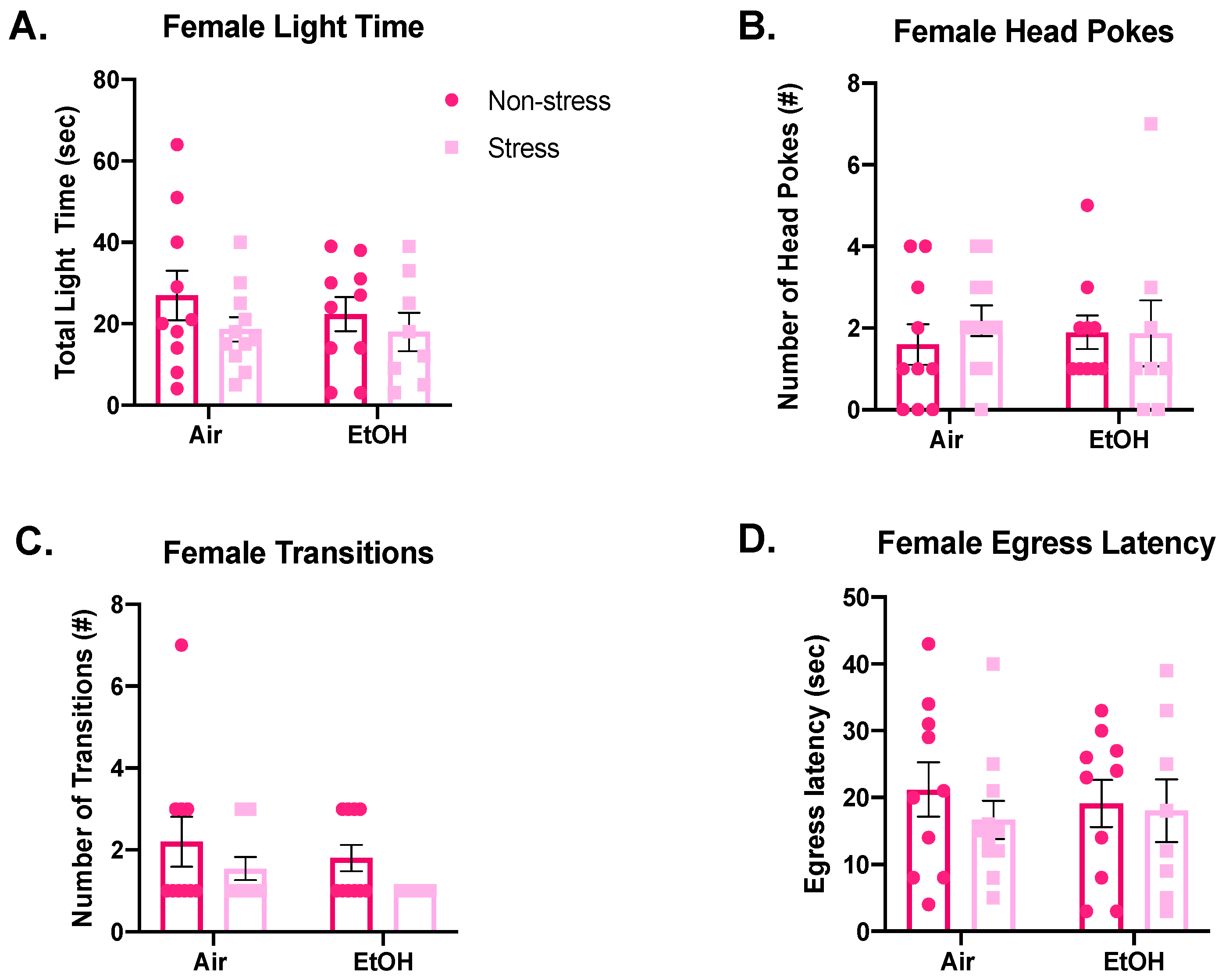
3.4.1. Light-Dark Box
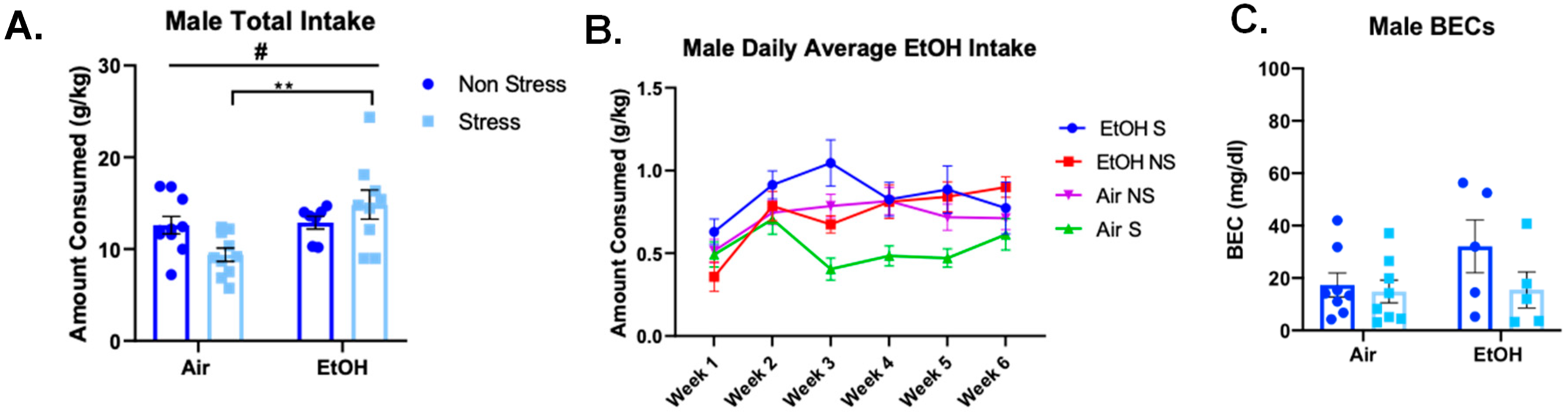
3.4.2. Voluntary Ethanol Drinking
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spear, L. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Casey, B.J.; Getz, S.; Galvan, A. The adolescent brain. Dev. Rev. 2008, 28, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Substance Abuse and Mental Health Services Administration (SAMHSA). Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Sruvey on Drug Use and Health, in NSDUH; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2019.
- Forman-Hoffman, V.L.; Edlund, M.; Glasheen, C.; Ridenour, T. Alcohol Initiation and Progression to Use, Heavy Episodic Use, and Alcohol Use Disorder Among Young Adolescents Ages 12–14 Living in U.S. Households. J. Stud. Alcohol Drugs 2017, 78, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.F.; Dawson, D.A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. J. Subst. Abus. 1997, 9, 103–110. [Google Scholar] [CrossRef]
- Hawkins, J.D.; Graham, J.W.; Maguin, E.; Abbott, R.; Hill, K.G.; Catalano, R.F. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J. Stud. Alcohol 1997, 58, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P. Effects of adolescent alcohol consumption on the brain and behaviour. Nat. Rev. Neurosci. 2018, 19, 197–214. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Jacobus, J.; Tapert, S.F. The effect of alcohol use on human adolescent brain structures and systems. Handb. Clin. Neurol. 2014, 125, 501–510. [Google Scholar]
- Sung, M. Effects of age at first substance use and psychiatric comorbidity on the development of substance use disorders. Drug Alcohol Depend. 2004, 75, 287–299. [Google Scholar] [CrossRef]
- Winward, J.L.; Bekman, N.M.; Hanson, K.L.; Lejuez, C.W.; Brown, S.A. Changes in Emotional Reactivity and Distress Tolerance among Heavy Drinking Adolescents during Sustained Abstinence. Alcohol. Clin. Exp. Res. 2014, 38, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Wolford-Clevenger, C.; Cropsey, K.L. Depressive symptoms and age of alcohol use onset interact to predict suicidal ideation. Death Stud. 2019, 44, 540–546. [Google Scholar] [CrossRef]
- Duncan, S.C.; Alpert, A.; E Duncan, T.; Hops, H. Adolescent alcohol use development and young adult outcomes. Drug Alcohol Depend. 1997, 49, 39–48. [Google Scholar] [CrossRef]
- Kyzar, E.J.; Zhang, H.; Pandey, S.C. Adolescent Alcohol Exposure Epigenetically Suppresses Amygdala Arc Enhancer RNA Expression to Confer Adult Anxiety Susceptibility. Biol. Psychiatry 2019, 85, 904–914. [Google Scholar] [CrossRef]
- Pandey, S.C.; Sakharkar, A.J.; Tang, L.; Zhang, H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol. Dis. 2015, 82, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Alaux-Cantin, S.; Warnault, V.; Legastelois, R.; Botia, B.; Pierrefiche, O.; Vilpoux, C.; Naassila, M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology 2013, 67, 521–531. [Google Scholar] [CrossRef]
- Sakharkar, A.J.; Kyzar, E.J.; Gavin, D.P.; Zhang, H.; Chen, Y.; Krishnan, H.R.; Grayson, D.R.; Pandey, S.C. Altered amygdala DNA methylation mechanisms after adolescent alcohol exposure contribute to adult anxiety and alcohol drinking. Neuropharmacology 2019, 157, 107679. [Google Scholar] [CrossRef]
- Varlinskaya, E.I.; Truxell, E.; Spear, L.P. Chronic intermittent ethanol exposure during adolescence: Effects on social behavior and ethanol sensitivity in adulthood. Alcohol 2014, 48, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Dannenhoffer, C.A.; Kim, E.U.; Saalfield, J.; Werner, D.F.; Varlinskaya, E.I.; Spear, L.P. Oxytocin and vasopressin modulation of social anxiety following adolescent intermittent ethanol exposure. Psychopharmacology 2018, 235, 3065–3077. [Google Scholar] [CrossRef] [PubMed]
- Torcaso, A.; Asimes, A.; Meagher, M.; Pak, T.R. Adolescent binge alcohol exposure increases risk assessment behaviors in male Wistar rats after exposure to an acute psychological stressor in adulthood. Psychoneuroendocrinology 2016, 76, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Nentwig, T.B.; Starr, E.M.; Chandler, L.J.; Glover, E. Absence of compulsive drinking phenotype in adult male rats exposed to ethanol in a binge-like pattern during adolescence. Alcohol 2019, 79, 93–103. [Google Scholar] [CrossRef]
- Gass, J.T.; Glen, W.B.; McGonigal, J.T.; Trantham-Davidson, H.; Lopez, M.F.; Randall, P.K.; Yaxley, R.; Floresco, S.B.; Chandler, L.J. Adolescent Alcohol Exposure Reduces Behavioral Flexibility, Promotes Disinhibition, and Increases Resistance to Extinction of Ethanol Self-Administration in Adulthood. Neuropsychopharmacology 2014, 39, 2570–2583. [Google Scholar] [CrossRef]
- Lamont, M.G.; McCallum, P.; Head, N.; Blundell, J.; Weber, J.T. Binge drinking in male adolescent rats and its relationship to persistent behavioral impairments and elevated proinflammatory/proapoptotic proteins in the cerebellum. Psychopharmacology 2020, 237, 1305–1315. [Google Scholar] [CrossRef]
- Sherrill, L.K.; Koss, W.A.; Foreman, E.S.; Gulley, J.M. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behav. Brain Res. 2011, 216, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.N.; Yoneyama, N.; Fretwell, A.M.; Snelling, C.; Tanchuck, M.A.; Finn, D.A. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm. Behav. 2010, 58, 82–90. [Google Scholar] [CrossRef]
- Maldonado-Devincci, A.M.; Makdisi, J.G.; Hill, A.M.; Waters, R.C.; Hall, N.I.; Jones, M.C.; Shobande, M.; Kumari, A. Adolescent intermittent ethanol exposure induces sex-dependent divergent changes in ethanol drinking and motor activity in adulthood in C57BL/6J mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Varlinskaya, E.I.; Kim, E.U.; Spear, L.P. Chronic intermittent ethanol exposure during adolescence: Effects on stress-induced social alterations and social drinking in adulthood. Brain Res. 2017, 1654, 145–156. [Google Scholar] [CrossRef]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). NIAAA Council Approves Definition of Binge Drinking; Department of Health and Human Services: Rockville, MD, USA, 2004.
- Center for Behavioral Health Statistics and Quality. 2018 National Survey on Drug Use and Health Public Use File Codebook; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2019.
- US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. Available online: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/ (accessed on 20 October 2020).
- Poli, A.; Marangoni, F.; Avogaro, A.; Barba, G.; Bellentani, S.; Bucci, M.; Cambieri, R.; Catapano, A.; Costanzo, S.; Cricelli, C.; et al. Moderate alcohol use and health: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 487–504. [Google Scholar] [CrossRef]
- Broadwater, M.; Varlinskaya, E.I.; Spear, L.P. Effects of Voluntary Access to Sweetened Ethanol during Adolescence on Intake in Adulthood. Alcohol. Clin. Exp. Res. 2013, 37, 1048–1055. [Google Scholar] [CrossRef]
- Amodeo, L.R.; Kneiber, D.; Wills, D.N.; Ehlers, C.L. Alcohol drinking during adolescence increases consumptive responses to alcohol in adulthood in Wistar rats. Alcohol 2017, 59, 43–51. [Google Scholar] [CrossRef]
- Leonardo, E.D.; Hen, R. Anxiety as a Developmental Disorder. Neuropsychopharmacology 2008, 33, 134–140. [Google Scholar] [CrossRef]
- José, B.S.; Van Oers, H.A.M.; Van De Mheen, H.D.; Garretsen, H.F.L.; MacKenbach, J.P. STRESSORS AND ALCOHOL CONSUMPTION. Alcohol Alcohol. 2000, 35, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Bertholomey, M.L.; Nagarajan, V.; Torregrossa, M.M. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology 2016, 233, 2277–2287. [Google Scholar] [CrossRef]
- Peltier, M.R.; Verplaetse, T.L.; Mineur, Y.S.; Petrakis, I.L.; Cosgrove, K.P.; Picciotto, M.R.; McKee, S.A. Sex differences in stress-related alcohol use. Neurobiol. Stress 2019, 10, 100149. [Google Scholar] [CrossRef]
- McCormick, C.M.; Mathews, I.Z. HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol. Biochem. Behav. 2007, 86, 220–233. [Google Scholar] [CrossRef]
- Kim, E.U.; Varlinskaya, E.I.; Dannenhoffer, C.A.; Spear, L.P. Adolescent intermittent ethanol exposure: Effects on pubertal development, novelty seeking, and social interaction in adulthood. Alcohol 2019, 75, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Vore, A.S.; Doremus-Fitzwater, T.; Gano, A.; Deak, T. Adolescent Ethanol Exposure Leads to Stimulus-Specific Changes in Cytokine Reactivity and Hypothalamic-Pituitary-Adrenal Axis Sensitivity in Adulthood. Front. Behav. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef]
- Allen, C.D.; Grigoleit, J.-S.; Hong, J.; Bae, S.; Vaughan, J.; Lee, S.Y. Exposure to alcohol during adolescence exerts long-term effects on stress response and the adult brain stress circuits. Neuroscience 2016, 339, 64–71. [Google Scholar] [CrossRef]
- Siegmund, S.; Vengeliene, V.; Singer, M.V.; Spanagel, R. Influence of Age at Drinking Onset on Long-Term Ethanol Self-Administration With Deprivation and Stress Phases. Alcohol. Clin. Exp. Res. 2005, 29, 1139–1145. [Google Scholar] [CrossRef]
- Füllgrabe, M.W.; Vengeliene, V.; Spanagel, R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol. Biochem. Behav. 2007, 86, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, M.J.; File, S.E.; Gessa, G.L.; Grant, K.A.; Guerri, C.; Hoffman, P.L.; Kalant, H.; Koob, G.F.; Li, T.-K.; Tabakoff, B. Effects of Moderate Alcohol Consumption on the Central Nervous System. Alcohol. Clin. Exp. Res. 1998, 22, 998–1040. [Google Scholar] [CrossRef]
- Morton, R.A.; Diaz, M.R.; Topper, L.A.; Valenzuela, C.F. Construction of Vapor Chambers Used to Expose Mice to Alcohol during the Equivalent of all Three Trimesters of Human Development. J. Vis. Exp. 2014, 2014, e51839. [Google Scholar] [CrossRef]
- Varlinskaya, E.; Johnson, J.; Przybysz, K.; Deak, T.; Diaz, M.R. Adolescent forced swim stress increases social anxiety-like behaviors and alters kappa opioid receptor function in the basolateral amygdala of male rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 98, 109812. [Google Scholar] [CrossRef]
- Deak, T.; Larish, Y. Methods and Motives for use of the Forced Swim Test as an Animal Model of Behvaioral Despair/Depression. In Tasks and Techniques: A Sampling of the Methodologies for the Investigation of Animal Learning, Behavior and Cognition; Anderson, M.J., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2006; pp. 3–18. [Google Scholar]
- Ehlers, C.L.; Criado, J.R.; Wills, D.N.; Liu, W.; Crews, F.T. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: Correlation with behavioral pathology. Neuroscience 2011, 199, 333–345. [Google Scholar] [CrossRef]
- Van Skike, C.E.; Diaz-Granados, J.L.; Matthews, U.B. Chronic intermittent ethanol exposure produces persistent anxiety in adolescent and adult rats. Alcohol. Clin. Exp. Res. 2015, 39, 262–271. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Karanikas, C.A.; Richardson, H.N. Adolescent Binge Drinking Leads to Changes in Alcohol Drinking, Anxiety, and Amygdalar Corticotropin Releasing Factor Cells in Adulthood in Male Rats. PLoS ONE 2012, 7, e31466. [Google Scholar] [CrossRef] [PubMed]
- Varlinskaya, E.I.; Hosová, D.; Towner, T.; Werner, D.F.; Spear, L.P. Effects of chronic intermittent ethanol exposure during early and late adolescence on anxiety-like behaviors and behavioral flexibility in adulthood. Behav. Brain Res. 2020, 378, 112292. [Google Scholar] [CrossRef]
- Morato, S.; Castrechini, P. Effects of floor surface and environmental illumination on exploratory activity in the elevated plus-maze. Braz. J. Med. Biol. Res. 1989, 22, 707–710. [Google Scholar]
- Garcia, A.M.B.; Cardenas, F.P.; Morato, S. Effect of different illumination levels on rat behavior in the elevated plus-maze. Physiol. Behav. 2005, 85, 265–270. [Google Scholar] [CrossRef]
- Slawecki, C.J. Comparison of anxiety-like behavior in adolescent and adult sprague-dawley rats. Behav. Neurosci. 2005, 119, 1477–1483. [Google Scholar] [CrossRef]
- Mora, S.; Dussaubat, N.; Díaz-Véliz, G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology 1996, 21, 609–620. [Google Scholar] [CrossRef]
- Morales, M.; McGinnis, M.M.; Robinson, S.L.; Chappell, A.M.; McCool, B.A. Chronic Intermittent Ethanol Exposure Modulation of Glutamatergic Neurotransmission in Rat Lateral/Basolateral Amygdala is Duration-, Input-, and Sex-Dependent. Neuroscience 2018, 371, 277–287. [Google Scholar] [CrossRef]
- Jury, N.J.; Pollack, G.A.; Ward, M.J.; Bezek, J.L.; Ng, A.J.; Pinard, C.R.; Bergstrom, H.C.; Holmes, A. Chronic Ethanol during Adolescence Impacts Corticolimbic Dendritic Spines and Behavior. Alcohol. Clin. Exp. Res. 2017, 41, 1298–1308. [Google Scholar] [CrossRef]
- Slawecki, C.J.; Betancourt, M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol 2002, 26, 23–30. [Google Scholar] [CrossRef]
- Chappell, A.M.; Carter, E.; McCool, B.A.; Weiner, J.L. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol. Clin. Exp. Res. 2012, 37, E394–E403. [Google Scholar] [CrossRef]
- Cozzoli, D.K.; Tanchuck-Nipper, M.A.; Kaufman, M.N.; Horowitz, C.B.; Finn, D.A. Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol 2014, 48, 741–754. [Google Scholar] [CrossRef]
- Li, J.; Chen, P.; Han, X.; Zuo, W.; Mei, Q.; Bian, E.Y.; Umeugo, J.; Ye, J.-H. Differences between male and female rats in alcohol drinking, negative affects and neuronal activity after acute and prolonged abstinence. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 163–176. [Google Scholar]
- Vetter-O’Hagen, C.; Varlinskaya, E.; Spear, L. Sex Differences in Ethanol Intake and Sensitivity to Aversive Effects during Adolescence and Adulthood. Alcohol Alcohol. 2009, 44, 547–554. [Google Scholar] [CrossRef]
- Kelly, S.J.; Bonthius, D.J.; West, J.R. Developmental Changes in Alcohol Pharmacokinetics in Rats. Alcohol. Clin. Exp. Res. 1987, 11, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.M.; Ehlers, C.L. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol. Biochem. Behav. 2009, 91, 560–565. [Google Scholar] [CrossRef]
- McCormick, C.M.; Hodges, T.E.; Simone, J.J. Peer pressures: Social instability stress in adolescence and social deficits in adulthood in a rodent model. Dev. Cogn. Neurosci. 2014, 11, 2–11. [Google Scholar] [CrossRef]
- Holson, R.; Pearce, B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol.Teratol. 1992, 14, 221–228. [Google Scholar] [CrossRef]
- Harvard Medical School. National Comorbidity Survey (NCS); Harvard Medical School: Boston, MA, USA, 2007. [Google Scholar]
- Vink, M.; Derks, J.M.; Bas-Hoogendam, J.M.; Hillegers, M.H.; Kahn, R.S. Functional differences in emotion processing during adolescence and early adulthood. NeuroImage 2014, 91, 70–76. [Google Scholar] [CrossRef]
- Walker, D.M.; Bell, M.R.; Flores, C.; Gulley, J.M.; Willing, J.; Paul, M.J. Adolescence and Reward: Making Sense of Neural and Behavioral Changes Amid the Chaos. J. Neurosci. 2017, 37, 10855–10866. [Google Scholar] [CrossRef] [PubMed]
- Del Piero, L.B.; Saxbe, D.E.; Margolin, G. Basic emotion processing and the adolescent brain: Task demands, analytic approaches, and trajectories of changes. Dev. Cogn. Neurosci. 2016, 19, 174–189. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamble, M.E.; Diaz, M.R. Moderate Adolescent Ethanol Vapor Exposure and Acute Stress in Adulthood: Sex-Dependent Effects on Social Behavior and Ethanol Intake in Sprague–Dawley Rats. Brain Sci. 2020, 10, 829. https://doi.org/10.3390/brainsci10110829
Gamble ME, Diaz MR. Moderate Adolescent Ethanol Vapor Exposure and Acute Stress in Adulthood: Sex-Dependent Effects on Social Behavior and Ethanol Intake in Sprague–Dawley Rats. Brain Sciences. 2020; 10(11):829. https://doi.org/10.3390/brainsci10110829
Chicago/Turabian StyleGamble, Meredith E., and Marvin R. Diaz. 2020. "Moderate Adolescent Ethanol Vapor Exposure and Acute Stress in Adulthood: Sex-Dependent Effects on Social Behavior and Ethanol Intake in Sprague–Dawley Rats" Brain Sciences 10, no. 11: 829. https://doi.org/10.3390/brainsci10110829
APA StyleGamble, M. E., & Diaz, M. R. (2020). Moderate Adolescent Ethanol Vapor Exposure and Acute Stress in Adulthood: Sex-Dependent Effects on Social Behavior and Ethanol Intake in Sprague–Dawley Rats. Brain Sciences, 10(11), 829. https://doi.org/10.3390/brainsci10110829



