Functional MRI Reveals Locomotion-Control Neural Circuits in Human Brainstem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Subjects
2.3. Walking Speed Test
2.4. Motor Task
2.5. MRI Data Acquisition
2.6. fMRI Activation Analysis
2.7. Network Analysis
3. Results
3.1. Changing-Speed Bipedal Movements Evoked Brainstem Activations
3.2. Slow, Fast and Free-Speed Movements also Evoked Brainstem Activations
3.3. Brainstem Contained Two Modules
3.4. Walking Automaticity Existed during Fast and Free-Speed Walking
3.5. Changing-Speed Condition Had Lower Cortical Load but Higher Brainstem Load Than the Free-Speed Task
3.6. Interactions between Topmost Brain Regions and Brainstem Existed Only during Motor Conditions
3.7. CN Initiated High-Speed Locomotion but PPN Appeared to Be a “Metronome”
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Montero-Odasso, M.M.; Sarquis-Adamson, Y.; Speechley, M.; Borrie, M.J.; Hachinski, V.C.; Wells, J.; Riccio, P.M.; Schapira, M.; Sejdic, E.; Camicioli, R.M.; et al. Association of Dual-Task Gait with Incident Dementia in Mild Cognitive Impairment. JAMA Neurol. 2017, 74, 857–865. [Google Scholar] [CrossRef]
- Commandeur, D.; Klimstra, M.; Macdonald, S.; Inouye, K.; Cox, M.; Chan, D.; Hundza, S.R. Difference scores between single-task and dual-task gait measures are better than clinical measures for detection of fall-risk in community-dwelling older adults. Gait Posture 2018, 66, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, N.; Abbas, J.J.; Shill, H.A.; Krishnamurthi, N. Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review. Sensors 2019, 19, 5468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-C.; Yang, Y.-R.; Tsai, Y.-A.; Wang, R.-Y. Cognitive and motor dual task gait training improve dual task gait performance after stroke—A randomized controlled pilot trial. Sci. Rep. 2017, 7, 4070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caggiano, V.; Leiras, R.; Goñi-Erro, H.; Masini, D.; Bellardita, C.; Bouvier, J.; Caldeira, V.; Fisone, G.; Kiehn, O. Midbrain circuits that set locomotor speed and gait selection. Nat. Cell Biol. 2018, 553, 455–460. [Google Scholar] [CrossRef]
- Ferreira-Pinto, M.; Ruder, L.; Capelli, P.; Arber, S. Connecting Circuits for Supraspinal Control of Locomotion. Neuron 2018, 100, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Ryczko, D.; Dubuc, R. Dopamine and the Brainstem Locomotor Networks: From Lamprey to Human. Front. Neurosci. 2017, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, L.C.; Matis, A.; Lindau, N.T.; Felder, P.; Gullo, M.; Schwab, M.E. Deep Brain Stimulation of the Midbrain Locomotor Region Improves Paretic Hindlimb Function after Spinal Cord Injury in Rats. Sci. Transl. Med. 2013, 5, 208ra146. [Google Scholar] [CrossRef]
- Alam, M.; Schwabe, K.; Krauss, J.K. The pedunculopontine nucleus area: Critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain 2010, 134, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Goetz, L.; Piallat, B.; Bhattacharjee, M.; Mathieu, H.; David, O.; Chabardes, S. On the Role of the Pedunculopontine Nucleus and Mesencephalic Reticular Formation in Locomotion in Nonhuman Primates. J. Neurosci. 2016, 36, 4917–4929. [Google Scholar] [CrossRef] [Green Version]
- La Fougère, C.; Zwergal, A.; Rominger, A.; Förster, S.; Fesl, G.; Dieterich, M.; Brandt, T.; Strupp, M.; Bartenstein, P.; Ejahn, K. Real versus imagined locomotion: A [18F]-FDG PET-fMRI comparison. NeuroImage 2010, 50, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Jahn, K.; Deutschländer, A.; Stephan, T.; Kalla, R.; Wiesmann, M.; Strupp, M.; Brandt, T. Imaging human supraspinal locomotor centers in brainstem and cerebellum. NeuroImage 2008, 39, 786–792. [Google Scholar] [CrossRef]
- Sacheli, L.M.; Zapparoli, L.; De Santis, C.; Preti, M.; Pelosi, C.; Ursino, N.; Zerbi, A.; Banfi, G.; Paulesu, E. Mental steps: Differential activation of internal pacemakers in motor imagery and in mental imitation of gait. Hum. Brain Mapp. 2017, 38, 5195–5216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, C.; Chowdhury, R.; Fitzgerald, T.; Fleming, S.M.; Lutti, A.; Hutton, C.; Draganski, B.; Frackowiak, R.S.; Ashburner, J. Characterizing Aging in the Human Brainstem Using Quantitative Multimodal MRI Analysis. Front. Hum. Neurosci. 2013, 7, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napadow, V.; Dhond, R.; Kennedy, D.; Hui, K.K.; Makris, N. Automated Brainstem Co-registration (ABC) for MRI. NeuroImage 2006, 32, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Beissner, F.; Schumann, A.; Brunn, F.; Eisenträger, D.; Bär, K.-J. Advances in functional magnetic resonance imaging of the human brainstem. NeuroImage 2014, 86, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, D.E.; Wong, D.; Jevsevar, D.; Riley, P.O.; Hodge, W.A. Trunk Kinematics During Locomotor Activities. Phys. Ther. 1992, 72, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.T.; Lin, X.; Aboushoushah, S.; White, T.P.; Phillips, M.; Bowtell, R.; Constantinescu, C. fMRI analysis of active, passive and electrically stimulated ankle dorsiflexion. NeuroImage 2009, 44, 469–479. [Google Scholar] [CrossRef]
- Ashburner, J. SPM: A history. NeuroImage 2012, 62, 791–800. [Google Scholar] [CrossRef] [Green Version]
- Hennigan, K.; D’Ardenne, K.; McClure, S.M. Distinct Midbrain and Habenula Pathways Are Involved in Processing Aversive Events in Humans. J. Neurosci. 2015, 35, 198–208. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Stephan, K.E.; Mohlberg, H.; Grefkes, C.; Fink, G.R.; Amunts, K.; Zilles, K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 2005, 25, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Huang, X.F. Atlas of the Human Brainstem, 1st ed.; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Brain Connectivity Toolbox. Available online: https://www.nitrc.org/projects/bct (accessed on 20 May 2019).
- Newman, M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef] [Green Version]
- Bullmore, E.T.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Capelli, P.; Pivetta, C.; Esposito, M.S.; Arber, S. Locomotor speed control circuits in the caudal brainstem. Nat. Cell Biol. 2017, 551, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.S.; Capelli, P.; Arber, S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nat. Cell Biol. 2014, 508, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, J.; Caggiano, V.; Leiras, R.; Caldeira, V.; Bellardita, C.; Balueva, K.; Fuchs, A.; Kiehn, O. Descending Command Neurons in the Brainstem that Halt Locomotion. Cell 2015, 163, 1191–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.J.; Reid, J.A.; Chakravorti, S.; Juttukonda, M.R.; Franco, G.; Trujillo, P.; Stark, A.J.; Dawant, B.M.; Donahue, M.J.; Claassen, D.O. Structural and functional connectivity of the nondecussating dentato-rubro-thalamic tract. NeuroImage 2018, 176, 364–371. [Google Scholar] [CrossRef]
- Harms, I.; Van Dijken, J.H.; Brookhuis, K.A.; De Waard, D. Walking without Awareness. Front. Psychol. 2019, 10, 1846. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.J. Automaticity of walking: Functional significance, mechanisms, measurement and rehabilitation strategies. Front. Hum. Neurosci. 2015, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Sacheli, L.M.; Zapparoli, L.; Bonandrini, R.; Preti, M.; Pelosi, C.; Sconfienza, L.M.; Banfi, G.; Paulesu, E. How aging affects the premotor control of lower limb movements in simulated gait. Hum. Brain Mapp. 2020, 41, 1889–1903. [Google Scholar] [CrossRef] [Green Version]
- Minassian, K.; Hofstoetter, U.S.; Dzeladini, F.; Guertin, P.A.; Ijspeert, A. The Human Central Pattern Generator for Locomotion: Does It Exist and Contribute to Walking? Neuroscientist 2017, 23, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Kannape, O.A.; Blanke, O. Self in motion: Sensorimotor and cognitive mechanisms in gait agency. J. Neurophysiol. 2013, 110, 1837–1847. [Google Scholar] [CrossRef] [Green Version]
- Langhanns, C.; Muller, H. Empirical Support for ‘Hastening-Through-Re-Automatization’ by Contrasting Two Motor-Cognitive Dual Tasks. Front. Psychol. 2018, 9, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matyas, F.; Sreenivasan, V.; Marbach, F.; Wacongne, C.; Barsy, B.; Mateo, C.; Aronoff, R.; Petersen, C.C.H. Motor Control by Sensory Cortex. Science 2010, 330, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Grabher, P.; Blaiotta, C.; Ashburner, J.; Freund, P. Relationship between brainstem neurodegeneration and clinical impairment in traumatic spinal cord injury. NeuroImage Clin. 2017, 15, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Lutti, A.; Helms, G.; Frackowiak, R.; Ashburner, J. Multiparametric brainstem segmentation using a modified multivariate mixture of Gaussians. NeuroImage Clin. 2013, 2, 684–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friese, S.; Hamhaber, U.; Erb, M.; Klose, U. B-Waves in Cerebral and Spinal Cerebrospinal Fluid Pulsation Measurement by Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2004, 28, 255–262. [Google Scholar] [CrossRef]
- Wei, P.; Li, J.; Gao, F.; Ye, D.; Zhong, Q.; Liu, S. Resting state networks in human cervical spinal cord observed with fMRI. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 108, 265–271. [Google Scholar] [CrossRef]
- Weiskopf, N.; Hutton, C.; Josephs, O.; Deichmann, R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: A whole-brain analysis at 3 T and 1.5 T. NeuroImage 2006, 33, 493–504. [Google Scholar] [CrossRef]
- Beissner, F.; Deichmann, R.; Baudrexel, S. fMRI of the brainstem using dual-echo EPI. NeuroImage 2011, 55, 1593–1599. [Google Scholar] [CrossRef]
- Bürki, C.N.; Bridenbaugh, S.A.; Reinhardt, J.; Stippich, C.; Kressig, R.W.; Blatow, M. Imaging gait analysis: An fMRI dual task study. Brain Behav. 2017, 7, e00724. [Google Scholar] [CrossRef]
- Trinastic, J.P.; Kautz, S.A.; McGregor, K.M.; Gregory, C.; Bowden, M.; Benjamin, M.B.; Kurtzman, M.; Chang, Y.-L.; Conway, T.; Crosson, B. An fMRI Study of the Differences in Brain Activity during Active Ankle Dorsiflexion and Plantarflexion. Brain Imaging Behav. 2010, 4, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ferraye, M.U.; Debu, B.; Heil, L.; Carpenter, M.; Bloem, B.R.; Toni, I. Using Motor Imagery to Study the Neural Substrates of Dynamic Balance. PLoS ONE 2014, 9, e91183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malonek, D.; Grinvald, A. Interactions between Electrical Activity and Cortical Microcirculation Revealed by Imaging Spectroscopy: Implications for Functional Brain Mapping. Science 1996, 272, 551–554. [Google Scholar] [CrossRef] [PubMed]
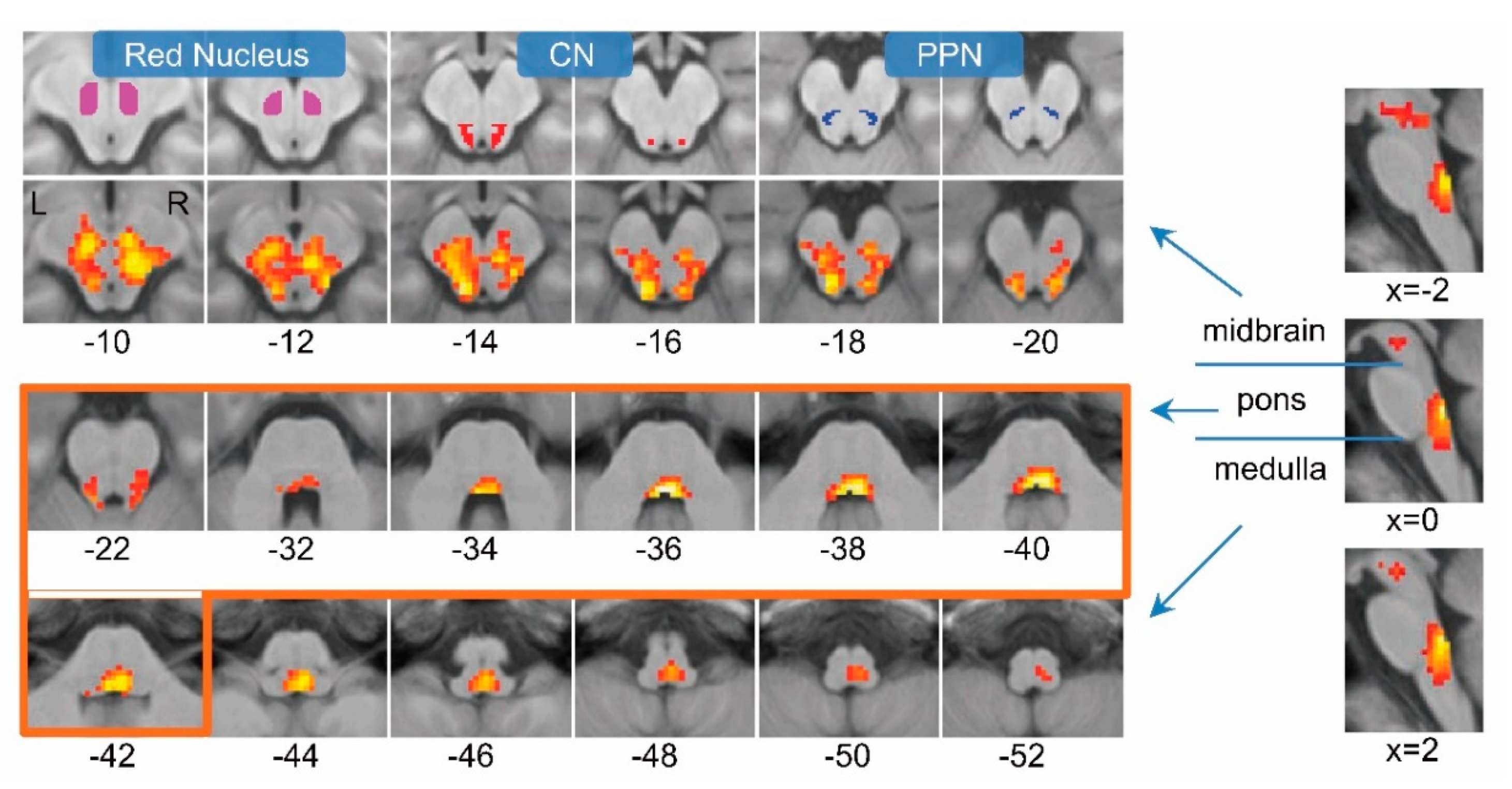

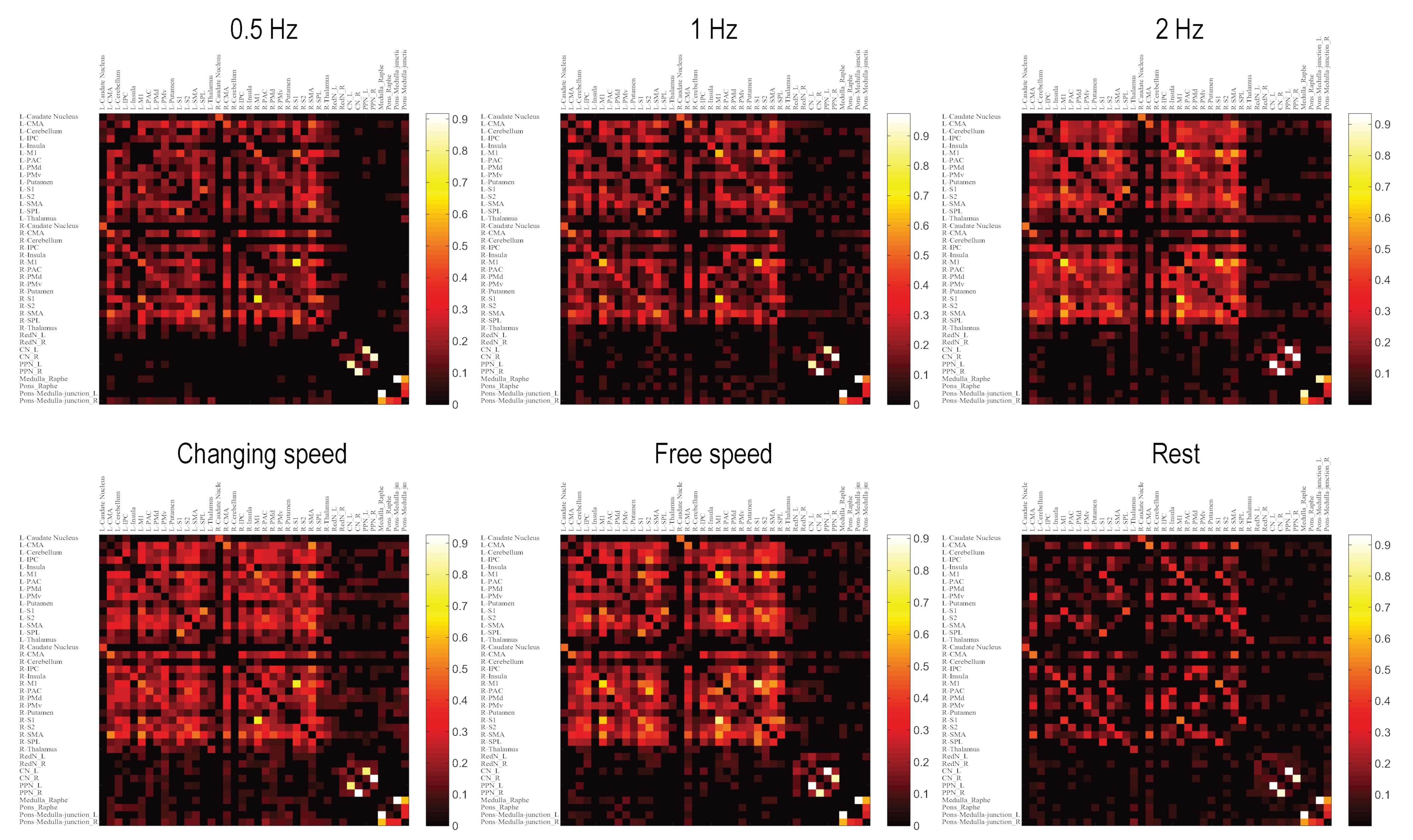
| Brain Areas | 0.5 Hz | 1 Hz | 2 Hz | Changing Speed | Free Speed | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI Coordinates | T Value | MNI Coordinates | T Value | MNI Coordinates | T Value | MNI Coordinates | T Value | MNI Coordinates | T Value | |||||||||||
| L_SMA | −4 | 8 | 45 | 9.25 | −8 | 6 | 53 | 12.73 | −6 | 4 | 56 | 11.05 | −4 | 6 | 49 | 11.06 | −2 | −18 | 73 | 11.65 |
| R_SMA | 4 | −10 | 64 | 10.81 | 2 | −10 | 67 | 11.74 | 2 | −10 | 64 | 11.00 | 6 | 8 | 64 | 9.70 | 4 | −4 | 62 | 11.35 |
| L_M1 | −2 | −28 | 64 | 5.42 | −2 | −28 | 62 | 7.16 | −2 | −28 | 64 | 10.28 | −2 | −30 | 62 | 9.22 | −6 | −42 | 69 | 12.16 |
| R_M1 | 6 | −26 | 71 | 5.79 | 6 | −26 | 69 | 5.51 | 6 | −38 | 75 | 8.35 | 6 | −22 | 69 | 7.65 | 8 | −36 | 75 | 11.16 |
| L_CMA | −4 | 4 | 45 | 7.65 | −4 | 4 | 45 | 7.15 | −6 | 10 | 42 | 6.91 | −8 | 10 | 42 | 7.88 | −10 | 8 | 36 | 9.03 |
| R_CMA | 12 | 4 | 40 | 7.51 | 12 | 8 | 45 | 6.91 | 14 | 16 | 36 | 7.04 | 4 | 14 | 45 | 8.32 | 4 | 14 | 45 | 7.85 |
| L_PMd | −14 | −18 | 73 | 6.52 | −52 | −2 | 51 | 5.11 | −50 | −2 | 45 | 7.85 | −50 | −4 | 47 | 8.94 | −48 | 0 | 51 | 6.75 |
| R_PMd | 50 | 4 | 51 | 6.47 | 50 | 2 | 51 | 8.53 | 48 | 6 | 53 | 7.80 | 40 | 6 | 51 | 8.21 | 52 | 10 | 49 | 8.90 |
| L_PMv | −50 | 0 | 7 | 8.77 | −54 | 2 | 5 | 6.90 | −48 | 0 | 7 | 9.69 | −54 | 2 | 5 | 8.10 | −52 | 2 | 7 | 9.06 |
| R_PMv | 52 | 8 | 12 | 8.56 | 48 | 6 | 9 | 5.49 | 56 | 4 | 3 | 9.08 | 56 | 4 | 3 | 8.52 | 58 | 4 | 5 | 7.65 |
| L_S1 | −12 | −44 | 73 | 4.39 | −12 | −42 | 67 | 3.33 | −12 | −44 | 67 | 6.78 | −12 | −44 | 67 | 6.64 | −12 | −36 | 62 | 9.49 |
| R_S1 | 12 | −36 | 71 | 4.49 | 12 | −36 | 71 | 3.81 | 8 | −40 | 75 | 7.31 | 10 | −36 | 71 | 6.59 | 10 | −36 | 71 | 9.24 |
| L_SPL | −18 | −48 | 67 | 4.74 | −12 | −48 | 80 | 3.46 | −12 | −42 | 62 | 6.12 | −34 | −54 | 47 | 6.71 | −16 | −38 | 69 | 8.49 |
| R_SPL | 16 | −46 | 78 | 6.26 | 16 | −46 | 80 | 4.81 | 10 | −40 | 75 | 6.56 | 12 | −40 | 75 | 6.55 | 14 | −42 | 75 | 8.90 |
| L_IPC | −60 | −24 | 23 | 6.10 | −50 | −34 | 16 | 7.75 | −50 | −36 | 20 | 8.70 | −50 | −38 | 20 | 10.13 | −52 | −36 | 18 | 11.85 |
| R_ IPC | 60 | −34 | 25 | 6.24 | 58 | −30 | 23 | 6.52 | 50 | −28 | 29 | 10.96 | 62 | −46 | 36 | 10.24 | 68 | −30 | 20 | 8.59 |
| L_S2 | −50 | 0 | 7 | 8.77 | −60 | −28 | 14 | 8.41 | −50 | −12 | 7 | 11.57 | −56 | −28 | 14 | 10.83 | −38 | −24 | 14 | 12.27 |
| R_S2 | 32 | −24 | 16 | 6.32 | 44 | −22 | 12 | 7.14 | 46 | −22 | 12 | 11.55 | 66 | −18 | 14 | 14.62 | 44 | −20 | 12 | 15.96 |
| L_PAC | −40 | −22 | 3 | 5.77 | −40 | −28 | 12 | 4.96 | −40 | −28 | 12 | 10.23 | −48 | −10 | 3 | 10.59 | −40 | −28 | 12 | 15.45 |
| R_ PAC | 52 | −12 | 5 | 3.87 | 50 | −10 | 3 | 5.49 | 56 | −10 | −2 | 8.64 | 50 | −12 | 5 | 10.55 | 48 | −20 | 9 | 15.23 |
| L_mid-posterior insula | −42 | 2 | 5 | 9.45 | −44 | 0 | 5 | 5.56 | −46 | −10 | 3 | 7.22 | −46 | −10 | 3 | 7.22 | −34 | −24 | 16 | 12.01 |
| R_mid-posterior insula | 46 | 4 | 3 | 7.99 | 48 | −6 | 1 | 5.31 | 50 | −4 | 3 | 7.79 | 50 | −4 | 3 | 8.12 | 38 | −20 | 14 | 8.39 |
| L_anterior insula | −36 | 18 | 7 | 5.22 | −34 | −8 | 5 | 5.32 | −38 | 16 | 7 | 9.44 | −36 | 18 | 7 | 10.02 | −28 | 20 | 9 | 6.09 |
| R_anterior insula | 42 | 22 | 3 | 3.74 | 34 | 22 | 7 | 3.5 * | 34 | 18 | 9 | 7.62 | 32 | 26 | 3 | 7.75 | 34 | 26 | 5 | 5.66 |
| L_Caudate Nucleus | −14 | 4 | 12 | 6.14 | 10 | 2 | 12 | 6.09 | −18 | 0 | 20 | 5.79 | −14 | 0 | 14 | 7.59 | −10 | 4 | 7 | 7.01 |
| R_Caudate Nucleus | 10 | 8 | 14 | 6.92 | −16 | 4 | 14 | 7.06 | 16 | −4 | 18 | 6.24 | 18 | 0 | 18 | 7.08 | 14 | 4 | 12 | 6.21 |
| L_Putamen | −28 | −4 | 12 | 11.33 | −26 | 0 | 12 | 9.04 | −30 | −8 | 7 | 8.19 | −26 | −2 | 14 | 8.79 | −30 | −14 | 7 | 10.95 |
| R_Putamen | 28 | −4 | 14 | 10.00 | 28 | 0 | 9 | 9.63 | 30 | −4 | 7 | 9.6 | 30 | −6 | 9 | 10.03 | 30 | −4 | 12 | 10.05 |
| L_Thalamus | −8 | −20 | 3 | 9.72 | −6 | −18 | 3 | 8.28 | −14 | −16 | 14 | 8.48 | −14 | −14 | 9 | 10.85 | −16 | −18 | 14 | 11.22 |
| R_Thalamus | 14 | −8 | 7 | 9.45 | 16 | −14 | 9 | 9.39 | 20 | −16 | 14 | 10.58 | 18 | −18 | 14 | 11.14 | 22 | −22 | 3 | 8.09 |
| L_Cerebellum | −22 | −38 | −26 | 10.21 | −22 | −38 | −26 | 13.59 | −16 | −40 | −24 | 12.72 | −16 | −38 | −21 | 15.69 | −16 | −38 | −21 | 13.13 |
| R_Cerebellum | 26 | −38 | −30 | 8.41 | 20 | −38 | −24 | 13.23 | 18 | −38 | −24 | 11.56 | 22 | −38 | −24 | 14.68 | 18 | −52 | −54 | 6.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.; Zou, T.; Lv, Z.; Fan, Y. Functional MRI Reveals Locomotion-Control Neural Circuits in Human Brainstem. Brain Sci. 2020, 10, 757. https://doi.org/10.3390/brainsci10100757
Wei P, Zou T, Lv Z, Fan Y. Functional MRI Reveals Locomotion-Control Neural Circuits in Human Brainstem. Brain Sciences. 2020; 10(10):757. https://doi.org/10.3390/brainsci10100757
Chicago/Turabian StyleWei, Pengxu, Tong Zou, Zeping Lv, and Yubo Fan. 2020. "Functional MRI Reveals Locomotion-Control Neural Circuits in Human Brainstem" Brain Sciences 10, no. 10: 757. https://doi.org/10.3390/brainsci10100757
APA StyleWei, P., Zou, T., Lv, Z., & Fan, Y. (2020). Functional MRI Reveals Locomotion-Control Neural Circuits in Human Brainstem. Brain Sciences, 10(10), 757. https://doi.org/10.3390/brainsci10100757






