Perspectives on Human Hearing Loss, Cochlear Regeneration, and the Potential for Hearing Restoration Therapies
Abstract
:1. Introduction: The Prevalence, Cost and Impact of Hearing Loss
2. Cochlear Function
3. Causes of Sensorineural Hearing Loss
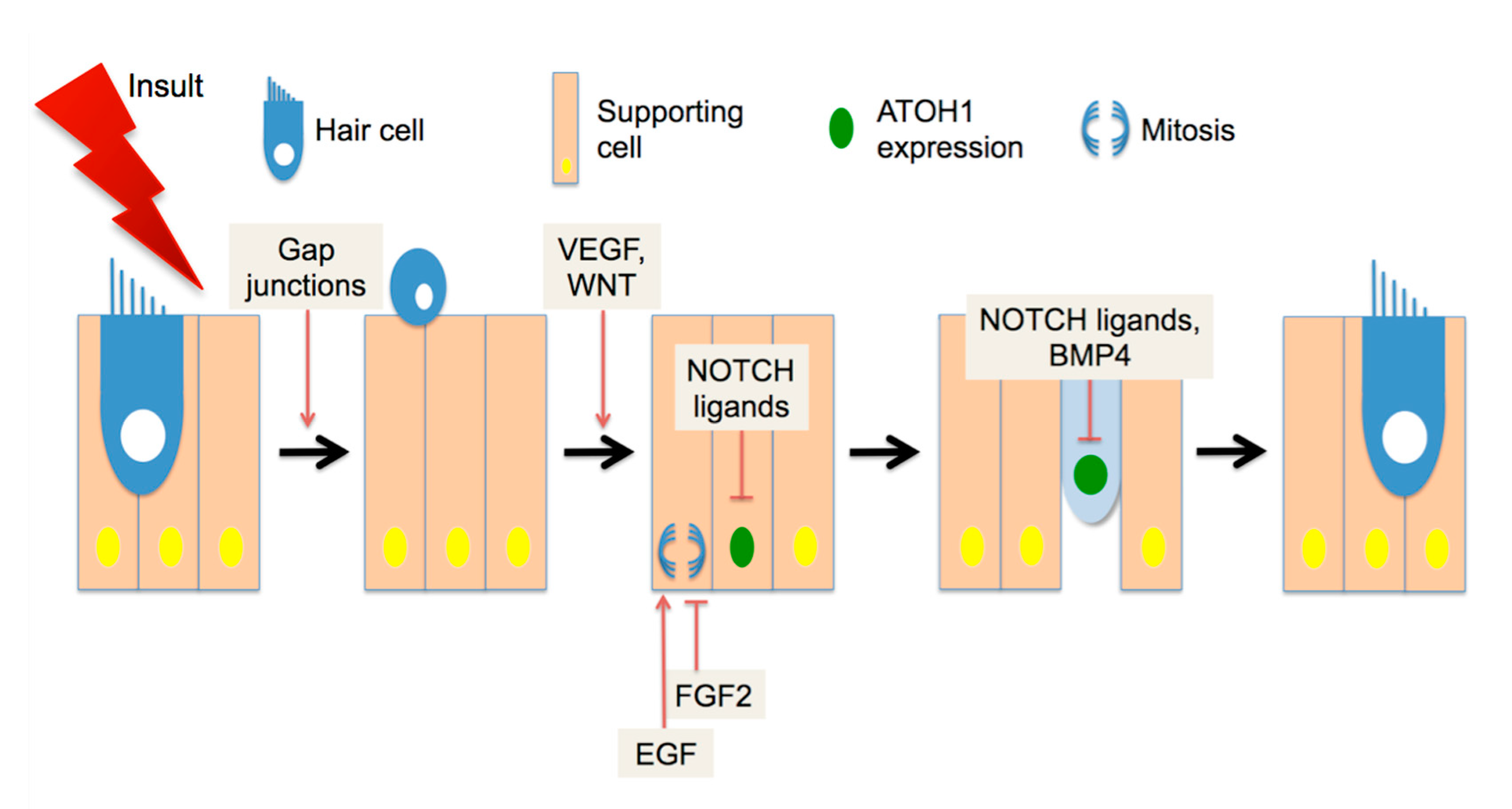
4. Spontaneous Cochlear Regeneration
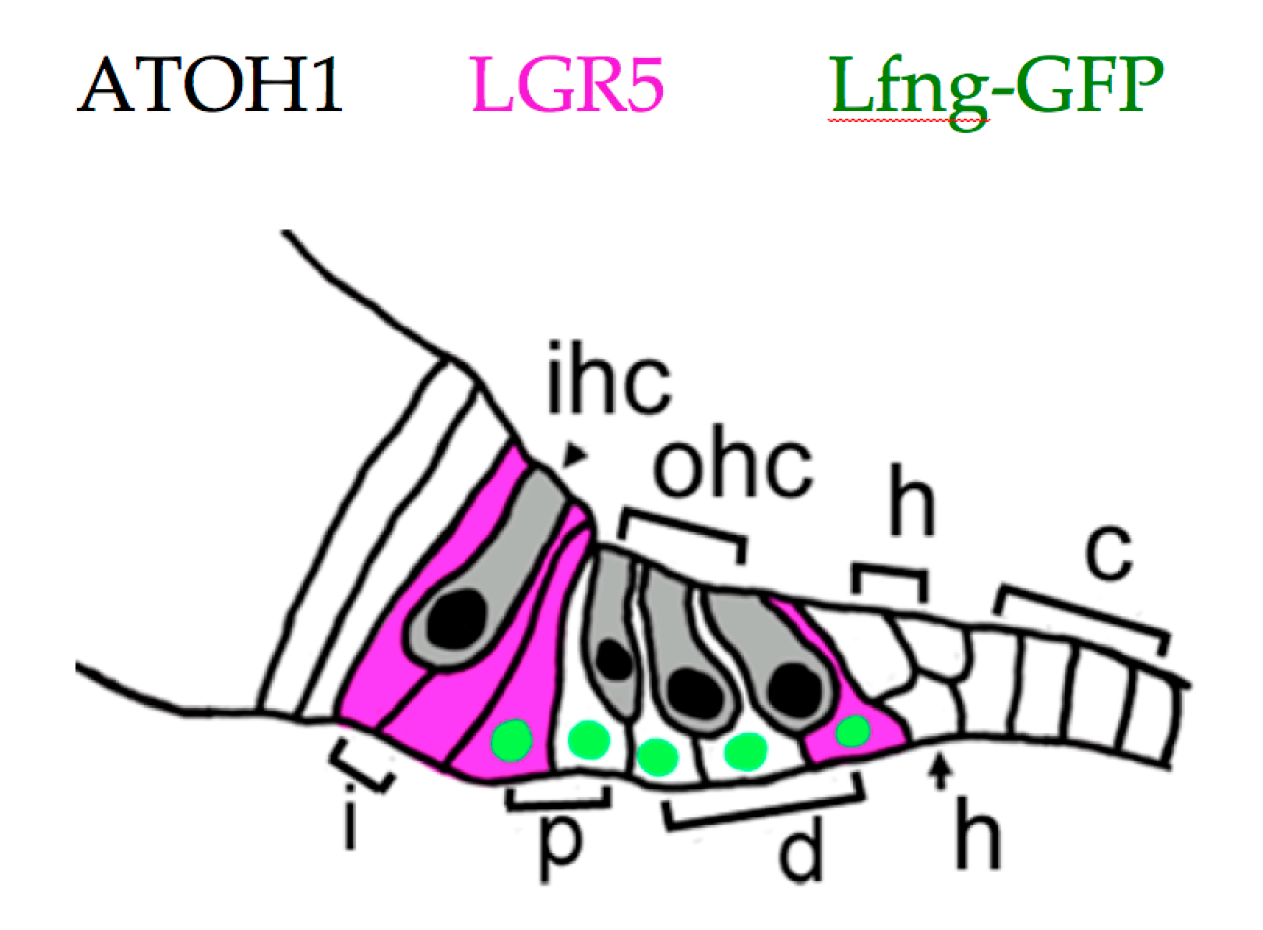
5. Recent Developments in Mammalian HC Regeneration
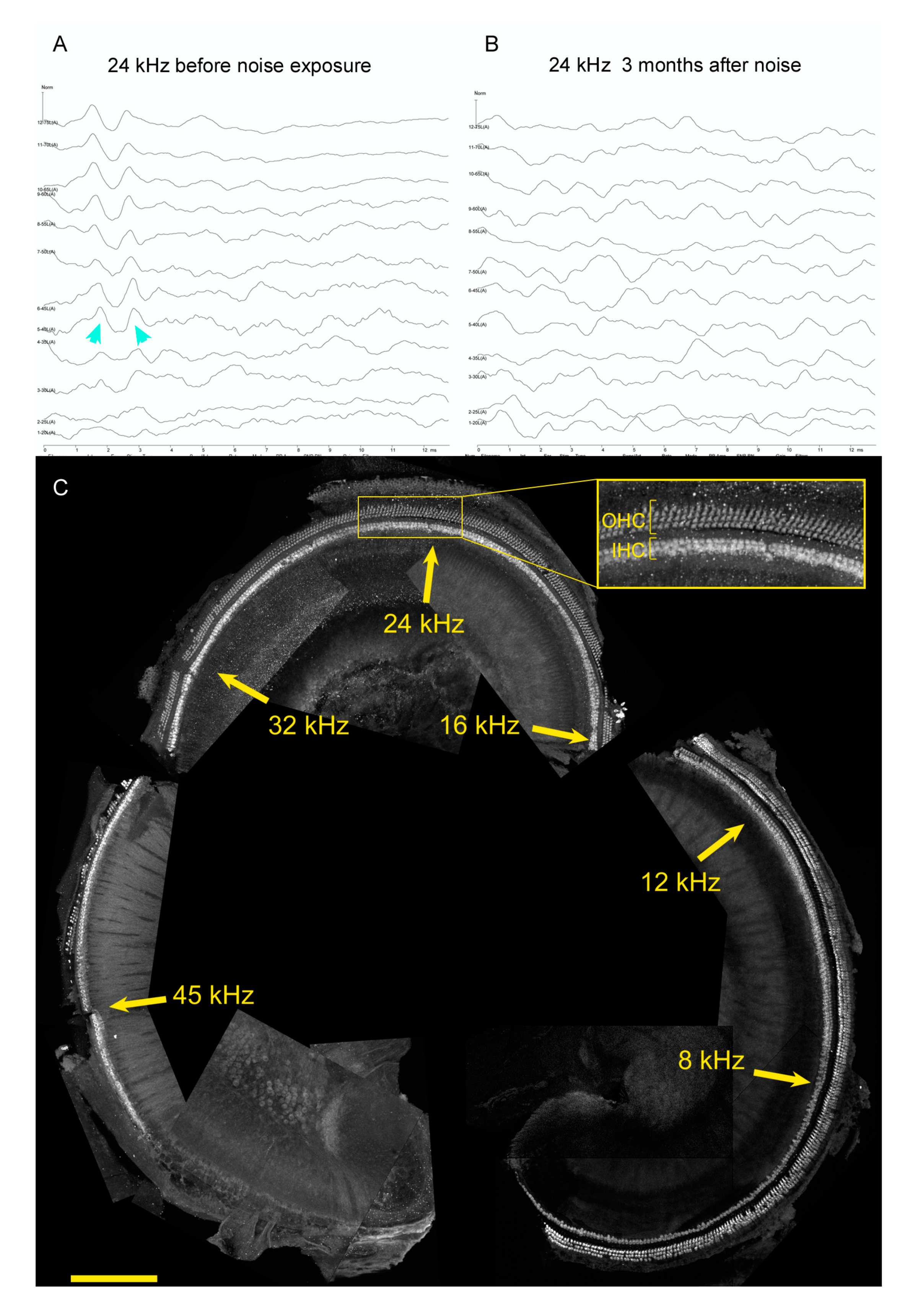
6. Hearing Restoration after Noise Damage in Adult Mammals
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- World-Health-Organization. Deafness and Hearing Loss. 2020. Available online: http://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed on 10 September 2020).
- NIDCD. Quick Statistics on Hearing Loss. 2016. Available online: http://www.nidcd.nih.gov/health/statistics/Pages/quick.aspx (accessed on 15 December 2016).
- Bowl, M.R.; Dawson, S.J. Age-Related Hearing Loss. Cold Spring Harb. Perspect. Med. 2018, 9, a033217. [Google Scholar] [CrossRef] [Green Version]
- Yankaskas, K. Prelude: Noise-Induced Tinnitus and Hearing Loss in the Military. Hear. Res. 2013, 295, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Department of Veterans Affairs, Veteran Administration. Annual Benefit Report FY 2019; Veterans Benefits Administration: Washington, DC, USA, 2019.
- Uhlmann, R.F.; Larson, E.B.; Rees, T.S.; Koepsell, T.D.; Duckert, L.G. Relationship of Hearing Impairment to Dementia and Cognitive Dysfunction in Older Adults. JAMA 1989, 261, 1916–1919. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, R.K.; Ward, P.D.; Schwartz, S.; Norton, M.C.; Foster, N.L.; Tschanz, J.T. Relationship of Hearing Loss and Dementia. Otol. Neurotol. 2014, 35, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Deal, J.A.; Betz, J.; Yaffe, K.; Harris, T.; Purchase-Helzner, E.; Satterfield, S.; Pratt, S.; Govil, N.; Simonsick, E.M.; Lin, F.R.; et al. Hearing Impairment and Incident Dementia and Cognitive Decline in Older Adults: The Health ABC Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 72, 703–709. [Google Scholar] [CrossRef]
- Loughrey, D.G.; Kelly, M.E.; Kelley, G.A.; Brennan, S.; Lawlor, B.A. Association of Age-Related Hearing Loss with Cognitive Function, Cognitive Impairment, and Dementia. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 115–126. [Google Scholar] [CrossRef]
- Heine, C.; Browning, C.J. Communication and Psychosocial Consequences of Sensory Loss in Older Adults: Overview and Rehabilitation Directions. Disabil. Rehabil. 2002, 24, 763–773. [Google Scholar] [CrossRef]
- Walling, A.D.; Dickson, G.M. Hearing Loss in Older Adults. Am. Fam. Physician 2012, 307, 1147–1148. [Google Scholar] [CrossRef]
- Altieri, N.; Pisoni, D.B.; Townsend, J.T. Some Normative Data on Lip-Reading Skills. J. Acoust. Soc. Am. 2011, 130, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Auer, E.T.; Bernstein, L.E. Enhanced Visual Speech Perception in Individuals with Early-Onset Hearing Impairment. J. Speech Lang. Hear. Res. 2007, 50, 1157–1165. [Google Scholar] [CrossRef]
- Salonen, J.; Johansson, R.; Karjalainen, S.; Vahlberg, T.; Jero, J.P.; Isoaho, R. Hearing Aid Compliance in the Elderly. B-ENT 2013, 9, 23–28. [Google Scholar] [PubMed]
- Shrestha, B.R.; Chia, C.; Wu, L.; Kujawa, S.G.; Liberman, M.C.; Goodrich, L.V. Sensory Neuron Diversity in the Inner Ear Is Shaped by Activity. Cell 2018, 174, 1229–1246.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maison, S.F.; Luebke, A.E.; Liberman, M.C.; Zuo, J. Efferent Protection from Acoustic Injury Is Mediated via α9 Nicotinic Acetylcholine Receptors on Outer Hair Cells. J. Neurosci. 2002, 22, 10838–10846. [Google Scholar] [CrossRef]
- Wang, J.; Yin, S.; Chen, H.; Shi, L. Noise-Induced Cochlear Synaptopathy and Ribbon Synapse Regeneration: Repair Process and Therapeutic Target. Adv. Exp. Med. Biol. 2019, 1130, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Van Camp, G.; Smith, R. Hereditary Hearing Loss Homepage. 2018. Available online: http://hereditaryhearingloss.org (accessed on 20 September 2018).
- Neyroud, N.; Tesson, F.; Denjoy, I.; Leibovici, M.; Donger, C.; Barhanin, J.; Fauré, S.; Gary, F.; Coumel, P.; Petit, C.; et al. A Novel Mutation in the Potassium Channel Gene KVLQT1 Causes the Jervell and Lange-Nielsen Cardioauditory Syndrome. Nat. Genet. 1997, 15, 186–189. [Google Scholar] [CrossRef]
- Yang, T.; Vidarsson, H.; Rodrigo-Blomqvist, S.; Rosengren, S.S.; Enerback, S.; Smith, R.J. Ranscriptional Control of SLC26A4 Is Involved in Pendred Syndrome and Nonsyndromic Enlargement of Vestibular Aqueduct (DFNB4). Am. J. Hum. Genet. 2007, 80, 1055–1063. [Google Scholar]
- Koenekoop, R.K.; Arriaga, M.A.; Trzupek, K.M.; Lentz, J.J.; Adam, M.P.; Ardinger, H.H.; Pagon, R.A.; Wallace, S.E.; Bean, L.J.H.; Stephens, A.A. Usher Syndrome Type I. In GeneReviews; University of Washington: Seattle, WA, USA, 1999. [Google Scholar]
- White, P.M. Genetic Susceptibility to Hearing Loss from Noise Exposure. Hear. J. 2019, 72, 8. [Google Scholar] [CrossRef]
- Someya, S.; Prolla, T.A. Mitochondrial Oxidative Damage and Apoptosis in Age-Related Hearing Loss. Mech. Ageing Dev. 2010, 131, 480–486. [Google Scholar] [CrossRef] [Green Version]
- Wells, H.R.R.; Newman, T.A.; Williams, F.M. Genetics of Age-Related Hearing Loss. J. Neurosci. Res. 2020, 98, 1698–1704. [Google Scholar] [CrossRef]
- Ohl, C.; Dornier, L.; Czajka, C.; Chobaut, J.-C.; Tavernier, L. Newborn Hearing Screening on Infants at Risk. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1691–1695. [Google Scholar] [CrossRef]
- Smith, G.A.; Gussen, R. Inner Ear Pathologic Features Following Mumps Infection: Report of a Case in an Adult. Arch. Otolaryngol. Head Neck Surg. 1976, 102, 108–111. [Google Scholar] [CrossRef]
- Kohan, D.; Hammerschlag, P.E.; Holliday, R.A. Otologic Disease in AIDS Patients. Laryngoscope 1990, 100, 1326. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, K.J.; Müller, D.; Schumacher, S.; Beck, E.; Meszaros, K.; Koerber, F. Systematic Review of Invasive Meningococcal Disease: Sequelae and Quality of Life Impact on Patients and Their Caregivers. Infect. Dis. Ther. 2018, 7, 421–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmiedt, R.A.; Lang, H.; Okamura, H.-O.; Schulte, B.A. Effects of Furosemide Applied Chronically to the Round Window: A Model of Metabolic Presbyacusis. J. Neurosci. 2002, 22, 9643–9650. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss. Otolaryngol. Head Neck Surg. 2019, 161, S1–S45. [Google Scholar]
- Ahmadzai, N.; Kilty, S.; Cheng, W.; Esmaeilisaraji, L.; Wolfe, D.; Bonaparte, J.P.; Schramm, D.; Fitzpatrick, E.; Lin, V.; Skidmore, B.; et al. A Systematic Review and Network Meta-Analysis of Existing Pharmacologic Therapies in Patients with Idiopathic Sudden Sensorineural Hearing Loss. PLoS ONE 2019, 14, e0221713. [Google Scholar] [CrossRef]
- Alexiou, C.; Arnold, W.; Fauser, C.; Schratzenstaller, B.; Gloddek, B.; Fuhrmann, S.; Lamm, K. Sudden Sensorineural Hearing Loss: Does Application of Glucocorticoids Make Sense? Arch. Otolaryngol. Head Neck Surg. 2001, 127, 253–258. [Google Scholar]
- DiGiovanni, J.J.; Nair, P. Spontaneous Recovery of Sudden Sensorineural Hearing Loss: Possible Association with Autoimmune Disorders. J. Am. Acad. Audiol. 2006, 17, 498–505. [Google Scholar] [CrossRef]
- DiSogra, R.M. Common Aminoglycosides and Platinum-Based Ototoxic Drugs: Cochlear/Vestibular Side Effects and Incidence. Semin. Hear. 2019, 40, 104–107. [Google Scholar] [CrossRef]
- Guo, J.; Chai, R.; Li, H.; Sun, S. Protection of Hair Cells from Ototoxic Drug-Induced Hearing Loss. Hear. Loss Mech. Prev. Cure 2019, 1130, 17–36. [Google Scholar] [CrossRef]
- Friedman, R.; House, J.; Luxford, W.; Gherini, S.; Mills, D. Profound Hearing Loss Associated with Hydrocodone/Acetaminophen Abuse. Am. J. Otolaryngol. 2000, 21, 188–191. [Google Scholar] [CrossRef]
- NIDCD. Noise Induced Hearing Loss. 2019. Available online: https://www.nidcd.nih.gov/health/noise-induced-hearing-loss (accessed on 31 May 2019).
- McBride, D. Audiometric Notch as a Sign of Noise Induced Hearing Loss. Occup. Environ. Med. 2001, 58, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, J.E.; Johnsson, L.-G.; Stebbins, W.C.; Moody, D.B.; Coombs, S.L. Hearing Loss and Cochlear Pathology in Monkeys After Noise Exposure. Acta Oto-Laryngol. 1976, 81, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.J. Ultrastructural Cochlear Changes following Acoustic Hyperstimulation and Ototoxicity. Ann. Otol. Rhinol. Laryngol. 1976, 85, 740–751. [Google Scholar] [CrossRef]
- Crowe, S.J.; Guild, S.R.; Polvogt, L.M. Observations on the Pathology of High-Tone Deafness. Bull. Johns Hopkins Hosp. 1934, 80, 480. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after Temporary Noise-Induced Hearing Loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.W.; Furman, A.C.; Kujawa, S.G.; Liberman, M.C. Primary Neural Degeneration in the Guinea Pig Cochlea After Reversible Noise-Induced Threshold Shift. J. Assoc. Res. Otolaryngol. 2011, 12, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.X.; Payne, S.; Yang-Hood, A.; Li, S.-Z.; Davis, B.; Carlquist, J.; V-Ghaffari, B.; Gantz, J.A.; Kallogjeri, D.; Fitzpatrick, J.A.J.; et al. Vesicular Glutamatergic Transmission in Noise-Induced Loss and Repair of Cochlear Ribbon Synapses. J. Neurosci. 2019, 39, 4434–4447. [Google Scholar] [CrossRef] [Green Version]
- Furman, A.C.; Kujawa, S.G.; Liberman, M.C. Noise-Induced Cochlear Neuropathy Is Selective for Fibers with Low Spontaneous Rates. J. Neurophysiol. 2013, 110, 577–586. [Google Scholar] [CrossRef]
- Olsen, W.O.; Noffsinger, D.; Kurdziel, S. Speech Discrimination in Quiet and in White Noise by Patients with Peripheral and Central Lesions. Acta Oto-Laryngol. 1975, 80, 375–382. [Google Scholar] [CrossRef]
- Wu, J.S.; Manca, E.; Yi, M.; Javaid, H.; Lauer, A.M.; Glowatzki, E. Sound Exposure Dynamically Induces Dopamine Synthesis in Cholinergic Loc Efferents for Feedback to Auditory Nerve Fibers. eLife 2020, 9, e52419. [Google Scholar] [CrossRef] [PubMed]
- Corwin, J.; Cotanche, D.; Cotanche, D.A. Regeneration of Sensory Hair Cells After Acoustic Trauma. Science 1988, 240, 1772–1774. [Google Scholar] [CrossRef]
- Ryals, B.; Rubel, E. Hair Cell Regeneration After Acoustic Trauma in Adult Coturnix Quail. Science 1988, 240, 1774–1776. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.M.; Lambert, P.R.; Rubel, E.W. Light Microscopic Evidence of Hair Cell Regeneration After Gentamicin Toxicity in Chick Cochlea. Arch. Otolaryngol. Head Neck Surg. 1987, 113, 1058–1062. [Google Scholar] [CrossRef]
- Cotanche, D.A. Regeneration of Hair Cell Stereociliary Bundles in the Chick Cochlea Following Severe Acoustic Trauma. Hear. Res. 1987, 30, 181–195. [Google Scholar] [CrossRef]
- Raphael, Y. Evidence for Supporting Cell Mitosis in Response to Acoustic Trauma in the Avian Inner Ear. J. Neurocytol. 1992, 21, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Girod, D.A.; Tucci, D.L.; Rubel, E.W. Anatomical Correlates of Functional Recovery in the Avian Inner Ear Following Aminoglycoside Ototoxicity. Laryngoscope 1991, 101, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Ryals, B.M.; Dent, M.L.; Dooling, R. Return of Function After Hair Cell Regeneration. Hear. Res. 2013, 297, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagger, D.J.; Nickel, R.; Forge, A. Gap Junctional Coupling Is Essential for Epithelial Repair in the Avian Cochlea. J. Neurosci. 2014, 34, 15851–15860. [Google Scholar] [CrossRef] [Green Version]
- Jacques, B.E.; Montgomery, W.H.; Uribe, P.M.; Yatteau, A.; Asuncion, J.D.; Resendiz, G.; Matsui, J.I.; Dabdoub, A. The Role of Wnt/β-Catenin Signaling in Proliferation and Regeneration of the Developing Basilar Papilla and Lateral Line. Dev. Neurobiol. 2013, 74, 438–456. [Google Scholar] [CrossRef]
- Hawkins, R.D.; Bashiardes, S.; Powder, K.; Sajan, S.A.; Bhonagiri, V.; Alvarado, D.M.; Speck, J.; Warchol, M.E.; Lovett, M. Large Scale Gene Expression Profiles of Regenerating Inner Ear Sensory Epithelia. PLoS ONE 2007, 2, e525. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.-C.; Lovett, M.; Warchol, M.E.; Stone, J.S. Vascular Endothelial Growth Factor Is Required for Regeneration of Auditory Hair Cells in the Avian Inner Ear. Hear. Res. 2020, 385, 107839. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, J.; Lee, G.S.; Stone, J.S. Atoh1 Expression Defines Activated Progenitors and Differentiating Hair Cells During Avian Hair Cell Regeneration. Dev. Dyn. 2007, 236, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, N.A. Math1: An Essential Gene for the Generation of Inner Ear Hair Cells. Science 1999, 284, 1837–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.L.; Gao, W.-Q. Overexpression of math1 Induces Robust Production of Extra Hair Cells in Postnatal Rat Inner Ears. Nat. Neurosci. 2000, 3, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, J.; Dearman, J.; Zhang, L.; Zuo, J. In Vivo Generation of Immature Inner Hair Cells in Neonatal Mouse Cochleae by Ectopic Atoh1 Expression. PLoS ONE 2014, 9, e89377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helms, A.W.; Abney, A.L.; Ben-Arie, N.; Zoghbi, H.Y.; Johnson, J.E. Autoregulation and Multiple Enhancers Control math1 Expression in the Developing Nervous System. Development 2000, 127, 1185–1196. [Google Scholar]
- Yamamoto, N.; Tanigaki, K.; Tsuji, M.; Yabe, D.; Ito, J.; Honjo, T. Inhibition of Notch/RBP-J Signaling Induces Hair Cell Formation in Neonate Mouse Cochleas. J. Mol. Med. 2005, 84, 37–45. [Google Scholar] [CrossRef]
- Jones, J.M.; Montcouquiol, M.; Dabdoub, A.; Woods, C.; Kelley, M.W. Nhibitors of Differentiation and DNA Binding (Ids) Regulate Math1 and Hair Cell Formation During the Development of the Organ of Corti. J. Neurosci. 2006, 26, 550–558. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.L.; Shou, J.; Guillemot, F.; Kageyama, R.; Gao, W.-Q. Hes1 Is a Negative Regulator of Inner Ear Hair Cell Differentiation. Development 2000, 127, 4551–4560. [Google Scholar]
- Stone, J.S.; Rubel, E.W. Delta1 Expression During Avian Hair Cell Regeneration. Development 1999, 126, 961–973. [Google Scholar] [PubMed]
- Adam, J.; Myat, A.; Le Roux, I.; Eddison, M.; Henrique, D.; Ish-Horowicz, D.; Lewis, J. Cell Fate Choices and the Expression of Notch, Delta and Serrate Homologues in the Chick Inner Ear: Parallels with Drosophila Sense-Organ Development. Development 1998, 125, 4645–4654. [Google Scholar] [PubMed]
- Daudet, N.; Gibson, R.; Shang, J.; Bernard, A.; Lewis, J.; Stone, J.S. Notch Regulation of Progenitor Cell Behavior in Quiescent and Regenerating Auditory Epithelium of Mature Birds. Dev. Biol. 2008, 326, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.M.; Keller, J.; Wan, L.; Stone, J.S. Bone Morphogenetic Protein 4 Antagonizes Hair Cell Regeneration in the Avian Auditory Epithelium. Hear. Res. 2018, 364, 1–11. [Google Scholar] [CrossRef]
- Stone, J.S.; Leaño, S.G.; Baker, L.P.; Rubel, E.W. Hair Cell Differentiation in Chick Cochlear Epithelium after Aminoglycoside Toxicity: In Vivoand In Vitro Observations. J. Neurosci. 1996, 16, 6157–6174. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.S.; Cotanche, D.A. Identification of the Timing of S Phase and the Patterns of Cell Proliferation During Hair Cell Regeneration in the Chick Cochlea. J. Comp. Neurol. 1994, 341, 50–67. [Google Scholar] [CrossRef]
- White, P.M.; Stone, J.S.; Groves, A.K.; Segil, N. EGFR Signaling Is Required for Regenerative Proliferation in the Cochlea: Conservation in Birds and Mammals. Dev. Biol. 2012, 363, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Oesterle, E.C.; Bhave, S.A.; Coltrera, M.D. Basic Fibroblast Growth Factor Inhibits Cell Proliferation in Cultured Avian Inner Ear Sensory Epithelia. J. Comp. Neurol. 2000, 424, 307–326. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, J.; Jin, R.; Bai, H.; Zhang, M.; Yang, S.; Zhang, X.; Zhang, X.; Han, Z.; Zeng, S. Transcriptomic Analysis of Chicken Cochleae After Gentamicin Damage and the Involvement of Four Signaling Pathways (Notch, FGF, Wnt and BMP) in Hair Cell Regeneration. Hear. Res. 2018, 361, 66–79. [Google Scholar] [CrossRef]
- Jones, J.; Corwin, J. Regeneration of Sensory Cells After Laser Ablation in the Lateral Line System: Hair Cell Lineage and Macrophage Behavior Revealed by Time- Lapse Video Microscopy. J. Neurosci. 1996, 16, 649–662. [Google Scholar] [CrossRef] [Green Version]
- Ma, E.Y.; Raible, D.W. Signaling Pathways Regulating Zebrafish Lateral Line Development. Curr. Biol. 2009, 19, R381–R386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.A.; Cheng, A.G.; Cunningham, L.L.; Macdonald, G.; Raible, D.W.; Rubel, E.W. Neomycin-Induced Hair Cell Death and Rapid Regeneration in the Lateral Line of Zebrafish. J. Assoc. Res. Otolaryngol. 2003, 4, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Romero-Carvajal, A.; Acedo, J.N.; Jiang, L.; Kozlovskaja-Gumbrienė, A.; Alexander, R.; Li, H.; Piotrowski, T. Regeneration of Sensory Hair Cells Requires Localized Interactions between the Notch and Wnt Pathways. Dev. Cell 2015, 34, 267–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seleit, A.; Krämer, I.; Riebesehl, B.F.; Ambrosio, E.M.; Stolper, J.S.; Lischik, C.; Dross, N.; Centanin, L. Neural Stem Cells Induce the Formation of Their Physical Niche During Organogenesis. eLife 2017, 6, 29173. [Google Scholar] [CrossRef] [PubMed]
- Lush, M.E.; Diaz, D.C.; Koenecke, N.; Baek, S.; Boldt, H.; Peter, M.K.S.; Gaitan-Escudero, T.; Romero-Carvajal, A.; Busch-Nentwich, E.M.; Perera, A.G.; et al. scRNA-Seq Reveals Distinct Stem Cell Populations That Drive Hair Cell Regeneration After Loss of FGF and Notch Signaling. eLife 2019, 8, 44431. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Romero-Carvajal, A.; Haug, J.S.; Seidel, C.W.; Piotrowski, T. Gene-Expression Analysis of Hair Cell Regeneration in the Zebrafish Lateral Line. Proc. Natl. Acad. Sci. USA 2014, 111, E1383–E1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, A.B.; Kim, T.; Cabot, V.; Hudspeth, A.J. Dynamic Gene Expression by Putative Hair-Cell Progenitors During Regeneration in the Zebrafish Lateral Line. Proc. Natl. Acad. Sci. USA 2014, 111, E1393–E1401. [Google Scholar] [CrossRef] [Green Version]
- White, P.M.; Doetzlhofer, A.; Lee, Y.S.; Groves, A.K.; Segil, N. Mammalian Cochlear Supporting Cells Can Divide and Trans-Differentiate into Hair Cells. Nat. Cell Biol. 2006, 441, 984–987. [Google Scholar] [CrossRef]
- Cox, B.C.; Chai, R.; Lenoir, A.; Liu, Z.; Zhang, L.; Nguyen, D.-H.; Chalasani, K.; Steigelman, K.A.; Fang, J.; Cheng, A.G.; et al. Spontaneous Hair Cell Regeneration in the Neonatal Mouse Cochlea in Vivo. Development 2014, 141, 816–829. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.C.; Stone, J.S. Development and Regeneration of Vestibular Hair Cells in Mammals. Semin. Cell Dev. Biol. 2017, 65, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.; Zuo, J. Cochlear Hair Cell Regeneration After Noise-Induced Hearing Loss: Does Regeneration Follow Development? Hear. Res. 2016, 349, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Kuo, B.; Wang, T.; Liaw, E.J.; Xia, A.; Jan, T.A.; Liu, Z.; Taketo, M.M.; Oghalai, J.S.; Nusse, R.; et al. Wnt Signaling Induces Proliferation of Sensory Precursors in the Postnatal Mouse Cochlea. Proc. Natl. Acad. Sci. USA 2012, 109, 8167–8172. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Kempfle, J.S.; Edge, A.S.B. Wnt- Responsive Lgr5-Expressing Stem Cells Are Hair Cell Progenitors in the Cochlea. J. Neurosci. 2012, 32, 9639–9648. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, X.; Guo, L.; Ni, W.; Zhang, Y.; Zhao, L.; Wu, L.; Sun, S.; Zhang, S.; Tang, M.; et al. Hedgehog Signaling Promotes the Proliferation and Subsequent Hair Cell Formation of Progenitor Cells in the Neonatal Mouse Cochlea. Front. Mol. Neurosci. 2017, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, Y.; Guo, L.; Lu, X.; Zhu, W.; Muhammad, W.; Zhang, L.; Lu, L.; Gao, J.; Tang, M.; et al. Age-Related Transcriptome Changes in Sox2+ Supporting Cells in the Mouse Cochlea. Stem Cell Res. Ther. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, W.J.; Yin, X.; Lu, L.; Lenz, D.R.; McLean, D.; Langer, R.; Karp, J.M.; Edge, A.S.B. Clonal Expansion of Lgr5-Positive Cells from Mammalian Cochlea and High-Purity Generation of Sensory Hair Cells. Cell Rep. 2017, 18, 1917–1929. [Google Scholar] [CrossRef] [Green Version]
- Senn, P.; Mina, A.; Volkenstein, S.; Kranebitter, V.; Oshima, K.; Heller, S. Progenitor Cells from the Adult Human Inner Ear. Anat. Rec. 2019, 303, 461–470. [Google Scholar] [CrossRef]
- McGovern, M.M.; Randle, M.R.; Cuppini, C.L.; Graves, K.A.; Cox, B.C. Multiple Supporting Cell Subtypes Are Capable of Spontaneous Hair Cell Regeneration in the Neonatal Mouse Cochlea. Development 2019, 146, dev171009. [Google Scholar] [CrossRef] [Green Version]
- Samarajeewa, A.; Jacques, B.E.; Dabdoub, A. Therapeutic Potential of Wnt and Notch Signaling and Epigenetic Regulation in Mammalian Sensory Hair Cell Regeneration. Mol. Ther. 2019, 27, 904–911. [Google Scholar] [CrossRef] [Green Version]
- Doetzlhofer, A.; Basch, M.L.; Ohyama, T.; Gessler, M.; Groves, A.K.; Segil, N. Hey2 Regulation by FGF Provides a Notch-Independent Mechanism for Maintaining Pillar Cell Fate in the Organ of Corti. Dev. Cell 2009, 16, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Maass, J.C.; Gu, R.; Basch, M.L.; Waldhaus, J.; Martín-López, E.; Xia, A.; Oghalai, J.S.; Heller, S.; Groves, A.K. Changes in the Regulation of the Notch Signaling Pathway Are Temporally Correlated with Regenerative Failure in the Mouse Cochlea. Front. Cell. Neurosci. 2015, 9, 110. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, A.E.; Xu, J.; Gridley, T. The Notch Ligand JAG1 Is Required for Sensory Progenitor Development in the Mammalian Inner Ear. PLoS Genet. 2006, 2, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steevens, A.R.; Glatzer, J.C.; Kellogg, C.C.; Low, W.C.; Santi, P.A.; Kiernan, A.E. SOX2 is Required for Inner Ear Growth and Cochlear Nonsensory Formation Before Sensory Development. Development 2019, 146, dev170522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, J.; Uchikawa, M.; Bigas, A.; Giraldez, F. The Prosensory Function of Sox2 in the Chicken Inner Ear Relies on the Direct Regulation of Atoh. PLoS ONE 2012, 7, e30871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, J.; Vachkov, I.; Giraldez, F. Sox2 Regulation of Hair Cell Development: Incoherence Makes Sense. Hear. Res. 2013, 297, 20–29. [Google Scholar] [CrossRef]
- Alon, U. Network Motifs: Theory and Experimental Approaches. Nat. Rev. Genet. 2007, 8, 450–461. [Google Scholar] [CrossRef]
- Atkinson, P.J.; Dong, Y.; Gu, S.; Liu, W.; Najarro, E.H.; Udagawa, T.; Cheng, A.G. Sox2 Haploinsufficiency Primes Regeneration and Wnt Responsiveness in the Mouse Cochlea. J. Clin. Investig. 2018, 128, 1641–1656. [Google Scholar] [CrossRef] [Green Version]
- McGovern, M.M.; Zhou, L.; Randle, M.R.; Cox, B.C. Spontaneous Hair Cell Regeneration Is Prevented by Increased Notch Signaling in Supporting Cells. Front. Cell. Neurosci. 2018, 12, 120. [Google Scholar] [CrossRef] [Green Version]
- Maass, J.C.; Gu, R.; Cai, T.; Wan, Y.-W.; Cantellano, S.C.; Asprer, J.S.T.; Zhang, H.; Jen, H.-I.; Edlund, R.K.; Liu, Z.; et al. Transcriptomic Analysis of Mouse Cochlear Supporting Cell Maturation Reveals Large-Scale Changes in Notch Responsiveness Prior to the Onset of Hearing. PLoS ONE 2016, 11, e0167286. [Google Scholar] [CrossRef]
- Hoa, M.; Olszewski, R.; Linthicum, F.H.; Taukulis, I.; Gu, S.; Detorres, A.; Lopez, I.A., Jr.; Linthicum, F.H.; Ishiyama, A.; Martin, D.; et al. Characterizing Adult Cochlear Supporting Cell Transcriptional Diversity Using Single-Cell RNA-Seq: Validation in the Adult Mouse and Translational Implications for the Adult Human Cochlea. Front. Mol. Neurosci. 2020, 13, 13. [Google Scholar] [CrossRef]
- Jayasena, C.S.; Ohyama, T.; Segil, N.; Groves, A.K. Notch Signaling Augments the Canonical Wnt Pathway to Specify the Size of the Otic Placode. Development 2008, 135, 2251–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohyama, T.; Mohamed, O.A.; Taketo, M.M.; Dufort, D.; Groves, A.K. Wnt Signals Mediate a Fate Decision Between Otic Placode and Epidermis. Development 2006, 133, 865–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, F.; Cheng, Y.F.; Wang, X.L.; Edge, A.S. Beta-Catenin up-Regulates Atoh1 Expression in Neural Progenitor Cells by Interaction with an Atoh1 3’ Enhancer. J. Biol. Chem. 2010, 285, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O.A. Regulation of Wnt Signaling during Adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar] [CrossRef] [Green Version]
- Kuo, B.R.; Baldwin, E.M.; Layman, W.S.; Taketo, M.M.; Zuo, J. In Vivo Cochlear Hair Cell Generation and Survival by Coactivation of Beta-Catenin and Atoh1. J. Neurosci. 2015, 35, 10786–10798. [Google Scholar] [CrossRef]
- Walters, B.J.; Coak, E.; Dearman, J.; Bailey, G.; Yamashita, T.; Kuo, B.; Zuo, J. In Vivo Interplay between p27 Kip1, GATA3, ATOH1, and POU4F3 Converts Non-sensory Cells to Hair Cells in Adult Mice. Cell Rep. 2017, 19, 307–320. [Google Scholar] [CrossRef]
- Hu, L.; Lu, J.; Chiang, H.; Wu, H.; Edge, A.S.B.; Shi, F. Diphtheria Toxin-Induced Cell Death Triggers Wnt-Dependent Hair Cell Regeneration in Neonatal Mice. J. Neurosci. 2016, 36, 9479–9489. [Google Scholar] [CrossRef] [Green Version]
- Samarajeewa, A.; Lenz, D.R.; Xie, L.; Chiang, H.; Kirchner, R.; Mulvaney, J.F.; Edge, A.S.B.; Dabdoub, A. Transcriptional Response to Wnt Activation Regulates the Regenerative Capacity of the Mammalian Cochlea. Development 2018, 145, dev166579. [Google Scholar] [CrossRef] [Green Version]
- Riccomagno, M.M.; Martinu, L.; Mulheisen, M.; Wu, D.K.; Epstein, D.J. Specification of the Mammalian Cochlea Is Dependent on Sonic Hedgehog. Genes Dev. 2002, 16, 2365–2378. [Google Scholar] [CrossRef] [Green Version]
- Driver, E.C.; Pryor, S.P.; Hill, P.; Turner, J.; Rüther, U.; Biesecker, L.G.; Griffith, A.J.; Kelley, M.W. Hedgehog Signaling Regulates Sensory Cell Formation and Auditory Function in Mice and Humans. J. Neurosci. 2008, 28, 7350–7358. [Google Scholar] [CrossRef]
- Bok, J.; Dolson, D.K.; Hill, P.; Rüther, U.; Epstein, D.J.; Wu, D.K. Opposing Gradients of Gli Repressor and Activators Mediate Shh Signaling Along the Dorsoventral Axis of the Inner Ear. Development 2007, 134, 1713–1722. [Google Scholar] [CrossRef] [Green Version]
- Hume, C.R.; Kirkegaard, M.; Oesterle, E.C. ErbB Expression: The Mouse Inner Ear and Maturation of the Mitogenic Response to Heregulin. J. Assoc. Res. Otolaryngol. 2003, 4, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.; Abdul-Aziz, D.; Mattiacio, J.; Edge, A.S.B.; White, P.M. ERBB 2 Signaling Drives Supporting Cell Proliferation in Vitro and Apparent Supernumerary Hair Cell Formation In Vivo in the Neonatal Mouse Cochlea. Eur. J. Neurosci. 2018, 48, 3299–3316. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Chow, L.T.; Paterson, A.J.; Chin, E.; Kudlow, J.E. Conditional Expression of the ErbB2 Oncogene Elicits Reversible Hyperplasia in Stratified Epithelia and up-Regulation of TGF Alpha Expression in Transgenic Mice. Oncogene 1999, 18, 3593–3607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumikawa, M.; Minoda, R.; Kawamoto, K.; Abrashkin, K.A.; Swiderski, D.L.; Dolan, D.F.; Brough, D.E.; Raphael, Y. Auditory Hair Cell Replacement and Hearing Improvement by atoh1 Gene Therapy in Deaf Mammals. Nat. Med. 2005, 11, 271–276. [Google Scholar] [CrossRef] [PubMed]
- U.S.-National-Library-Of-Medicine. Safety, Tolerability and Efficacy for CGF166 in Patients with Unilateral or Bilateral Severe-to-profound Hearing Loss. 2014. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02132130 (accessed on 12 October 2020).
- Mizutari, K.; Fujioka, M.; Hosoya, M.; Bramhall, N.; Okano, H.J.; Okano, H.; Edge, A.S. Notch Inhibition Induces Cochlear Hair Cell Regeneration and Recovery of Hearing After Acoustic Trauma. Neuron 2013, 77, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Tona, Y.; Hamaguchi, K.; Ishikawa, M.; Miyoshi, T.; Eyamamoto, N.; Yamahara, K.; Ito, J.; Nakagawa, T. Therapeutic Potential of a Gamma-Secretase Inhibitor for Hearing Restoration in a Guinea Pig Model with Noise-Induced Hearing Loss. BMC Neurosci. 2014, 15, 66. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Cai, Q.; West, M.B.; Youm, I.; Huang, X.; Li, W.; Cheng, W.; Nakmali, D.; Ewert, D.L.; Kopke, R.D. Regeneration of Cochlear Hair Cells and Hearing Recovery through Hes1 Modulation with siRNA Nanoparticles in Adult Guinea Pigs. Mol. Ther. 2018, 26, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Fetoni, A.R.; Piacentini, R.; Fiorita, A.; Paludetti, G.; Troiani, D. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL). Brain Res. 2009, 1257, 108–116. [Google Scholar] [CrossRef]
- Yamashita, T.; Zheng, F.; Finkelstein, D.; Kellard, Z.; Carter, R.; Rosencrance, C.; Sugino, K.; Easton, J.; Gawad, C.; Zuo, J. High-Resolution Transcriptional Dissection of In Vivo Atoh1-Mediated Hair Cell Conversion in Mature Cochleae Identifies Isl1 as a Co-Reprogramming Factor. PLoS Genet. 2018, 14, e1007552. [Google Scholar] [CrossRef]
- Stojanova, Z.P.; Kwan, T.; Segil, N. Epigenetic Regulation of Atoh1 Guides Hair Cell Development in the Mammalian Cochlea. Development 2015, 142, 3529–3536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VandenBosch, L.S.; Reh, T.A. Epigenetics in Neuronal Regeneration. Semin. Cell Dev. Biol. 2019, 97, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, M.M.M.; Cox, B.C.; Fang, J.; Taylor, R.; Forge, A.; Zuo, J. Selective Ablation of Pillar and Deiters’ Cells Severely Affects Cochlear Postnatal Development and Hearing in Mice. J. Neurosci. 2013, 33, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Li, W.; Huang, M.; Quan, Y.-Z.; Scheffer, D.; Tian, C.; Tao, Y.; Liu, X.; Hochedlinger, K.; Indzhykulian, A.A.; et al. Renewed Proliferation in Adult Mouse Cochlea and Regeneration of Hair Cells. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Tao, Y.; Lamas, V.; Huang, M.; Yeh, W.H.; Pan, B.; Hu, Y.J.; Hu, J.H.; Thompson, D.B.; Shu, Y.; et al. Treatment of Autosomal Dominant Hearing Loss by in Vivo Delivery of Genome Editing Agents. Nature 2018, 553, 217–221. [Google Scholar] [CrossRef]
- Crowson, M.G.; Hertzano, R.; Tucci, D.L. Emerging Therapies for Sensorineural Hearing Loss. Otol. Neurotol. 2017, 38, 792–803. [Google Scholar] [CrossRef]
- Schilder, A.G.M.; Su, M.P.; Blackshaw, H.; Lustig, L.; Staecker, H.; Lenarz, T.; Safieddine, S.; Gomes-Santos, C.S.; Holme, R.; Warnecke, A. Hearing Protection, Restoration, and Regeneration. Otol. Neurotol. 2019, 40, 559–570. [Google Scholar] [CrossRef]
- Zhang, J.; Na, D.; Dilts, M.; Henry, K.R.; White, P.M. Partial Hearing Restoration after ERBB2 Induction in Deafened Adult Mice. bioRxiv 2019, 11, 838649. [Google Scholar]
- Ohlemiller, K.K.; Wright, J.S.; Heidbreder, A.F. Vulnerability to Noise-Induced Hearing Loss in ‘Middle-Aged’ and Young Adult Mice: A Dose–Response Approach in CBA, C57BL, and BALB Inbred Strains. Hear. Res. 2000, 149, 239–247. [Google Scholar] [CrossRef]
- Harding, G.W.; Bohne, B.A.; Vos, J.D. The Effect of an Age-Related Hearing Loss Gene (Ahl) on Noise-Induced Hearing Loss and Cochlear Damage from Low-Frequency Noise. Hear. Res. 2005, 204, 90–100. [Google Scholar] [CrossRef]
- Lane, H. Ethnicity, Ethics, and the Deaf-World. J. Deaf Stud. Deaf Educ. 2005, 10, 291–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrow, R. Defending Deaf Culture: The Case of Cochlear Implants. J. Political Philos. 2005, 13, 135–152. [Google Scholar] [CrossRef]
- Finkl, T.; Hahne, A.; Friederici, A.D.; Gerber, J.; Mürbe, D.; Anwander, A. Language Without Speech: Segregating Distinct Circuits in the Human Brain. Cereb. Cortex 2019, 30, 812–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacSweeney, M.; Woll, B.; Campbell, R.; McGuire, P.K.; David, A.S.; Williams, S.C.R.; Suckling, J.; Calvert, G.A.; Brammer, M.J. Neural systems underlying British Sign Language and audio-visual English processing in native users. Brain 2002, 125, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Hauser, P.C.; O’Hearn, A.; McKee, M.; Steider, A.; Thew, D. Deaf epistemology: Deafhood and Deafness. Am. Ann. Deaf. 2010, 154, 486–492. [Google Scholar] [CrossRef]
- Ladd, P.; Lane, H. Deaf Ethnicity, Deafhood, and Their Relationship. Sign Lang. Stud. 2013, 13, 565–579. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, P.M. Perspectives on Human Hearing Loss, Cochlear Regeneration, and the Potential for Hearing Restoration Therapies. Brain Sci. 2020, 10, 756. https://doi.org/10.3390/brainsci10100756
White PM. Perspectives on Human Hearing Loss, Cochlear Regeneration, and the Potential for Hearing Restoration Therapies. Brain Sciences. 2020; 10(10):756. https://doi.org/10.3390/brainsci10100756
Chicago/Turabian StyleWhite, Patricia M. 2020. "Perspectives on Human Hearing Loss, Cochlear Regeneration, and the Potential for Hearing Restoration Therapies" Brain Sciences 10, no. 10: 756. https://doi.org/10.3390/brainsci10100756
APA StyleWhite, P. M. (2020). Perspectives on Human Hearing Loss, Cochlear Regeneration, and the Potential for Hearing Restoration Therapies. Brain Sciences, 10(10), 756. https://doi.org/10.3390/brainsci10100756



