Investigation of Functional Connectivity Differences between Voluntary Respirations via Mouth and Nose Using Resting State fMRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Data Acquisition
2.2. Functional Connectivity Processing
2.3. Laterality of Functional Connectivity
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Asymmetric Functional Connectivity Pattern
4.2. Sensorimotor Area
4.3. Central Opercular Cortex
4.4. Lateral Parietal Cortex in DMN
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elad, D.; Wolf, M.; Keck, T. Air-conditioning in the human nasal cavity. Respir. Physiol. Neurobiol. 2008, 163, 121–127. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.A. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur. Respir. J. 2005, 25, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Furukawa, T.; Sugimoto, A.; Katada, K.; Kotani, R.; Yoshizawa, T. Relationship between Oral Flow Patterns, Nasal Obstruction, and Respiratory Events during Sleep. J. Clin. Sleep Med. 2015, 11, 855–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abreu, R.R.; Rocha, R.L.; Lamounier, J.A.; Guerra, A.F.M. Etiology, clinical manifestations and concurrent findings in mouth-breathing children. J. Pediatr. 2008, 84, 529–535. [Google Scholar] [CrossRef]
- Izuhara, Y.; Matsumoto, H.; Nagasaki, T.; Kanemitsu, Y.; Murase, K.; Ito, I.; Oguma, T.; Muro, S.; Asai, K.; Tabara, Y.; et al. Mouth breathing, another risk factor for asthma: The Nagahama Study. Allergy 2016, 71, 1031–1036. [Google Scholar] [CrossRef]
- De Menezes, V.A.; Leal, R.B.; Pessoa, R.S.; Pontes, R.M.E.S. Prevalence and factors related to mouth breathing in school children at the Santo Amaro project-Recife, 2005. Braz. J. Otorhinolaryngol. 2006, 72, 394–398. [Google Scholar] [CrossRef]
- Hallani, M.; Wheatley, J.R.; Amis, T.C. Enforced mouth breathing decreases lung function in mild asthmatics. Respirology 2008, 13, 553–558. [Google Scholar] [CrossRef]
- Martin, U.; Bryden, K.; Devoy, M.; Howarth, P. Increased levels of exhaled nitric oxide during nasal and oral breathing in subjects with seasonal rhinitis. J. Allergy Clin. Immunol. 1996, 97, 768–772. [Google Scholar] [CrossRef]
- Flanell, M. The Athlete’s Secret Ingredient: The Power of Nasal Breathing. EC Pulmonol. Respir. Med. 2019, 8, 471–475. [Google Scholar]
- Bakor, S.F.; Enlow, D.H.; Pontes, P.; Biase, N.G.D. Craniofacial growth variations in nasal-breathing, oral-breathing, and tracheotomized children. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 486–492. [Google Scholar] [CrossRef]
- Cuccia, A.M.; Lotti, M.; Caradonna, D. Oral Breathing and Head Posture. Angle Orthod. 2008, 78, 77–82. [Google Scholar] [CrossRef]
- Kuroishi, R.C.S.; Garcia, R.B.; Valera, F.C.P.; Anselmo-Lima, W.T.; Fukuda, M.T.H. Deficits in working memory, reading comprehension and arithmetic skills in children with mouth breathing syndrome: Analytical cross-sectional study. Sao Paulo Med. J. 2014, 133, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ucar, F.I.; Ekizer, A.; Uysal, T. Comparison of craniofacial morphology, head posture and hyoid bone position with different breathing patterns. Saudi Dent. J. 2012, 24, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zicari, A.M.; Albani, F.; Ntrekou, P.; Rugiano, A.; Duse, M.; Mattei, A.; Marzo, G. Oral breathing and dental malocclusions. Eur. J. Paediatr. Dent 2009, 10, 59–64. [Google Scholar]
- Jefferson, Y. Mouth breathing: Adverse effects on facial growth, health, academics, and behavior. Gen. Dent. 2010, 58, 18–25, quiz 26–27, 79–80. [Google Scholar]
- Sano, M.; Sano, S.; Oka, N.; Yoshino, K.; Kato, T. Increased oxygen load in the prefrontal cortex from mouth breathing: A vector-based near-infrared spectroscopy study. NeuroReport 2013, 24, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-A.; Kang, C.-K. Sensing the effects of mouth breathing by using 3-tesla MRI. J. Korean Phys. Soc. 2017, 70, 1070–1076. [Google Scholar] [CrossRef]
- Damoiseaux, J.S.; Rombouts, S.A.R.B.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef]
- Smitha, K.; Akhil Raja, K.; Arun, K.; Rajesh, P.; Thomas, B.; Kapilamoorthy, T.; Kesavadas, C. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol. J. 2017, 30, 305–317. [Google Scholar] [CrossRef]
- Yu, L.; De Mazancourt, M.; Hess, A.; Ashadi, F.R.; Klein, I.; Mal, H.; Courbage, M.; Mangin, L. Functional connectivity and information flow of the respiratory neural network in chronic obstructive pulmonary disease. Hum. Brain Mapp. 2016, 37, 2736–2754. [Google Scholar] [CrossRef]
- Power, J.D.; Lynch, C.J.; Silver, B.M.; Dubin, M.J.; Martin, A.; Jones, R.M. Distinctions among real and apparent respiratory motions in human fMRI data. NeuroImage 2019, 201, 116041. [Google Scholar] [CrossRef] [PubMed]
- Brosch, J.R.; Talavage, T.M.; Ulmer, J.L.; Nyenhuis, J.A. Simulation of human respiration in fMRI with a mechanical model. IEEE Trans. Biomed. Eng 2002, 49, 700–707. [Google Scholar] [CrossRef] [PubMed]
- NITRC: CONN: Functional Connectivity Toolbox: Tool/Resource Filelist. Available online: https://www.nitrc.org/frs/?group_id=279 (accessed on 2 October 2020).
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A Component Based Noise Correction Method (CompCor) for BOLD and Perfusion Based fMRI. NeuroImage 2007, 37, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Bernal, B.; Guillen, M.; Korman, B. Nontask-Related Brain Lateralization Biomarkers in Children: The Asymmetry of Language Areas on Functional Connectivity Functional Magnetic Resonance Imaging. Brain Connect. 2018, 8, 321–332. [Google Scholar] [CrossRef]
- Van den Heuvel, M.P.; Hulshoff Pol, H.E. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef]
- Moulin-Frier, C.; Nguyen, S.M.; Oudeyer, P.-Y. Self-organization of early vocal development in infants and machines: The role of intrinsic motivation. Front. Psychol. 2014, 4. [Google Scholar] [CrossRef][Green Version]
- Knecht, S.; Dräger, B.; Deppe, M.; Bobe, L.; Lohmann, H.; Flöel, A.; Ringelstein, E.-B.; Henningsen, H. Handedness and hemispheric language dominance in healthy humans. Brain 2000, 123, 2512–2518. [Google Scholar] [CrossRef]
- Marc, J.; Tzourio-Mazoyer, N.; Mazoyer, B. Intra-hemispheric intrinsic connectivity asymmetry and its relationships with handedness and language Lateralization. Neuropsychologia 2016, 93. [Google Scholar] [CrossRef]
- Rodd, J.M.; Davis, M.H.; Johnsrude, I.S. The neural mechanisms of speech comprehension: FMRI studies of semantic ambiguity. Cereb. Cortex 2005, 15, 1261–1269. [Google Scholar] [CrossRef]
- Davenport, P.W.; Vovk, A. Cortical and subcortical central neural pathways in respiratory sensations. Respir. Physiol. Neurobiol. 2009, 167, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.B. Resting state fMRI: A personal history. NeuroImage 2012, 62, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, C.; Aydogdu, I. Neurophysiology of swallowing. Clin. Neurophysiol. 2003, 114, 2226–2244. [Google Scholar] [CrossRef]
- Martin, R.E.; MacIntosh, B.J.; Smith, R.C.; Barr, A.M.; Stevens, T.K.; Gati, J.S.; Menon, R.S. Cerebral Areas Processing Swallowing and Tongue Movement Are Overlapping but Distinct: A Functional Magnetic Resonance Imaging Study. J. Neurophysiol. 2004, 92, 2428–2443. [Google Scholar] [CrossRef] [PubMed]
- Adrián-Ventura, J.; Costumero, V.; Parcet, M.A.; Ávila, C. Reward network connectivity “at rest” is associated with reward sensitivity in healthy adults: A resting-state fMRI study. Cogn. Affect. Behav. Neurosci 2019, 19, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Chenji, S.; Jha, S.; Lee, D.; Brown, M.; Seres, P.; Mah, D.; Kalra, S. Investigating Default Mode and Sensorimotor Network Connectivity in Amyotrophic Lateral Sclerosis. PLoS ONE 2016, 11, e0157443. [Google Scholar] [CrossRef]
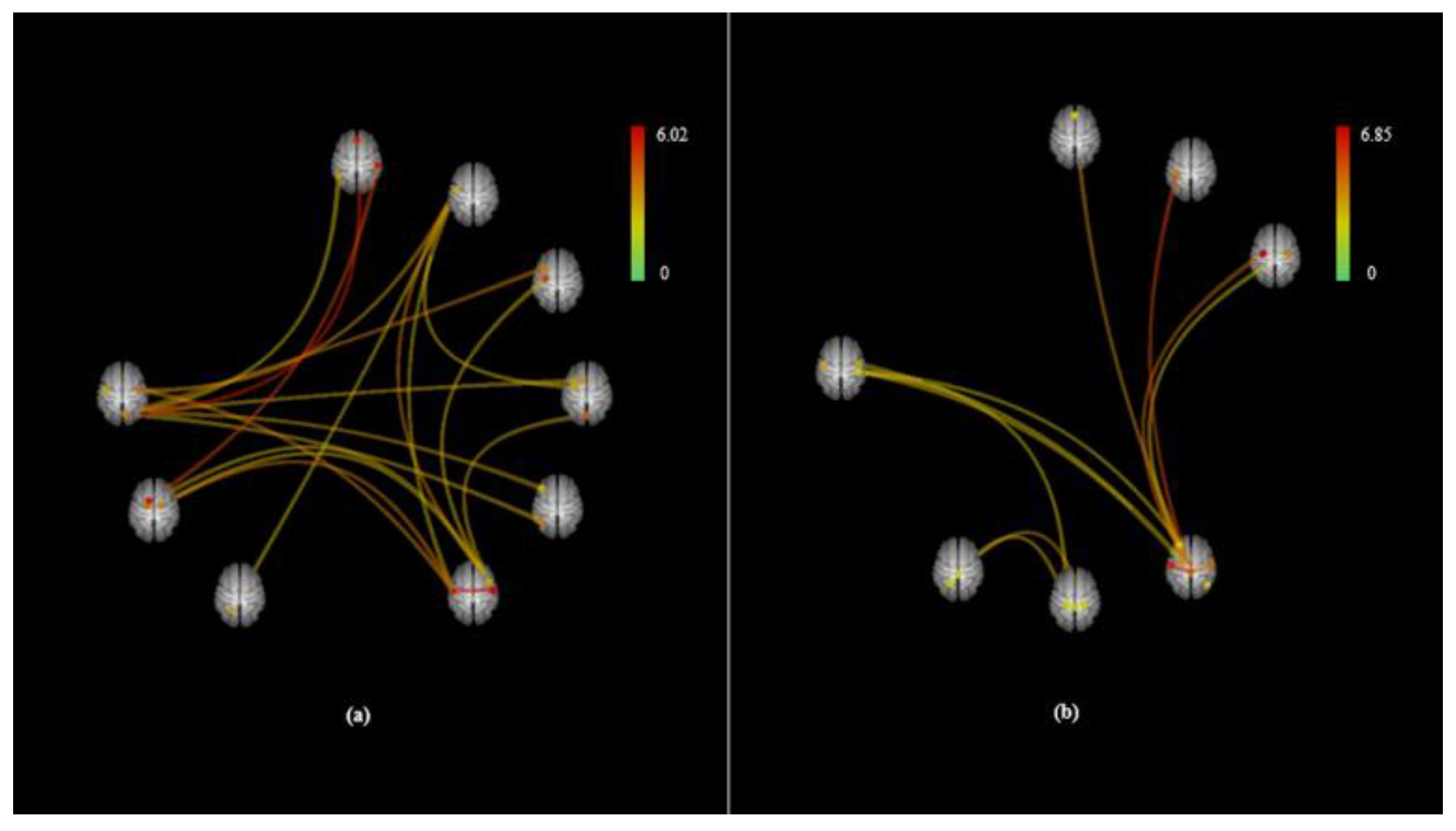
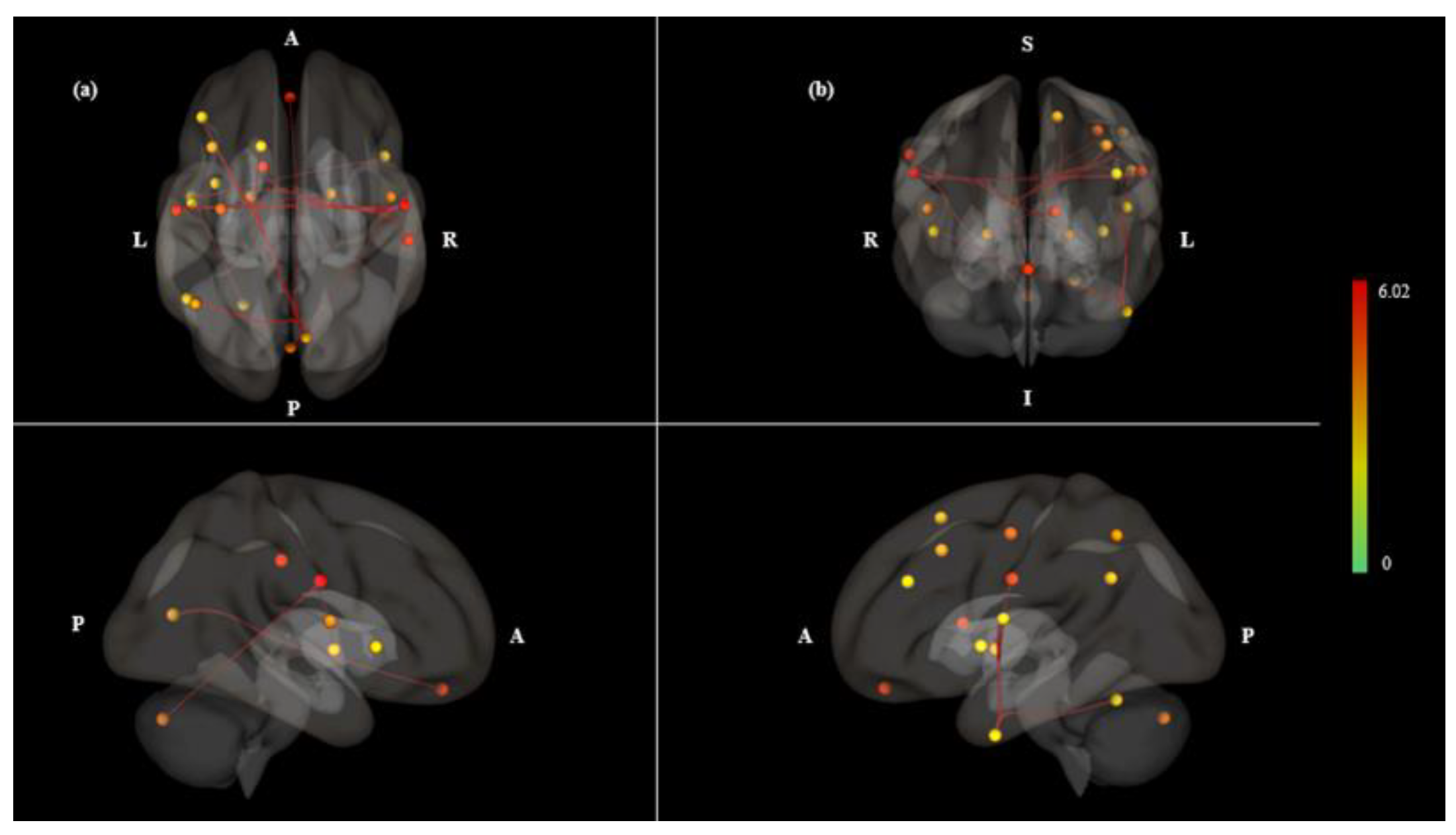
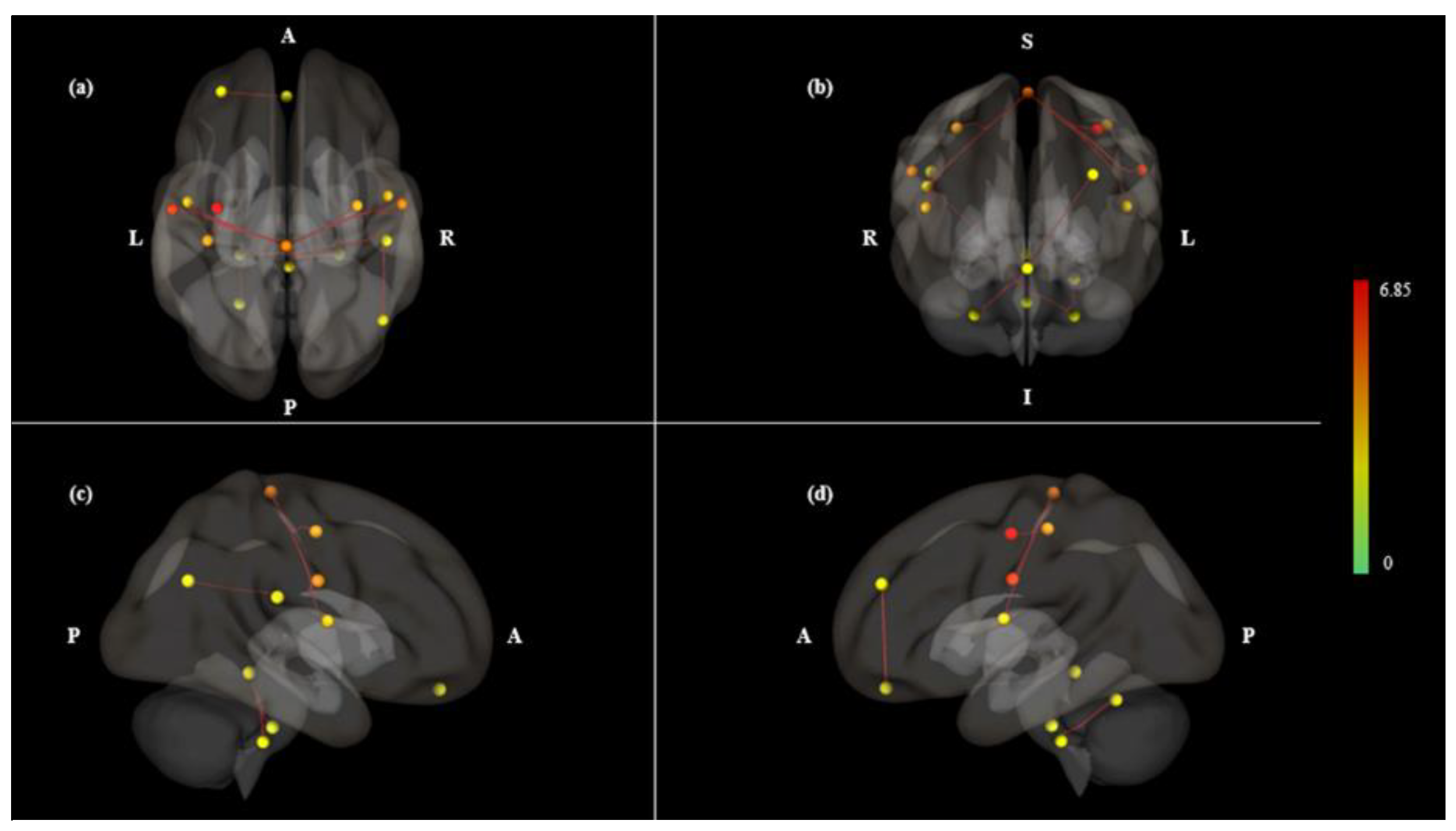
| Contrast | Pair Connection | Statistics (T) | P Value | |
|---|---|---|---|---|
| Uncorrected | FDR | |||
| Mouth > Nose | net.SM.Lateral (L)-net.SM.Lateral (R) | 6.02 | 0.0000 | 0.0006 |
| net.SM.Lateral (R)-networks.SM.Lateral (L) | 6.02 | 0.0000 | 0.0006 | |
| SCC (R)-MedFC | 5.49 | 0.0000 | 0.0018 | |
| aSMG (R)-Caudate (L) | 5.02 | 0.0000 | 0.0054 | |
| aITG (L)-net.Salience.AInsula (R) | 4.36 | 0.0002 | 0.0248 | |
| net.SM.Lateral (L)-Pallidum (R) | 4.34 | 0.0002 | 0.0113 | |
| SCC (R)-MidFG (L) | 4.3 | 0.0002 | 0.0142 | |
| net.SM.Lateral (L)-CO (R) | 4.22 | 0.0002 | 0.0113 | |
| CO (R)-net.SM.Lateral (L) | 4.22 | 0.0002 | 0.0339 | |
| aITG (L)-CO (L) | 3.87 | 0.0005 | 0.0389 | |
| SCC (R)-net.FrontoParietal.LPFC (L) | 3.85 | 0.0005 | 0.0269 | |
| net.SM.Lateral (R)-net.Cerebellar.Posterior | 3.78 | 0.0006 | 0.0425 | |
| SCC (R)-SFG (L) | 3.65 | 0.0008 | 0.0302 | |
| SCC (R)-net.FrontoParietal.PPC (L) | 3.58 | 0.0009 | 0.0302 | |
| aITG (L)-IC (L) | 3.56 | 0.001 | 0.0437 | |
| net.SM.Lateral (L)-aITG (L) | 3.52 | 0.0011 | 0.0437 | |
| aITG (L)-net.SM.Lateral (L) | 3.52 | 0.0011 | 0.0437 | |
| net.SM.Lateral (R)-Pallidum (L) | 3.51 | 0.0011 | 0.0425 | |
| net.SM.Lateral (R)-PreCG (L) | 3.46 | 0.0012 | 0.0425 | |
| net.SM.Lateral (R)-Pallidum (R) | 3.44 | 0.0013 | 0.0425 | |
| aITG (L)-Cereb6 (L) | 3.41 | 0.0014 | 0.0451 | |
| SCC (R)-AG (L) | 3.3 | 0.0018 | 0.0485 | |
| Area | Statistics | P Value |
|---|---|---|
| Uncorrected | ||
| CO (R) | F(5)(16) = 6.73 | 0.0015 |
| Intensity = 4.22 | 0.0389 | |
| Size = 1 | 0.0448 | |
| SM.Lateral (L) | F(5)(16) = 6.68 | 0.0015 |
| Intensity = 18.11 | 0.0037 | |
| Size = 4 | 0.0055 | |
| SM.Lateral (R) | F(5)(16) = 6.05 | 0.0025 |
| Intensity = 20.21 | 0.0029 | |
| Size = 5 | 0.0037 | |
| aITG (L) | F(5)(16) = 5.88 | 0.0029 |
| Intensity = 18.72 | 0.0035 | |
| Size = 5 | 0.0037 | |
| SCC (R) | F(5)(17) = 4.83 | 0.007 |
| Intensity = 24.18 | 0.0021 | |
| Size = 6 | 0.0026 | |
| aSMG (R) | F(5)(17) = 3.28 | 0.0315 |
| Intensity = 5.02 | 0.0194 | |
| Size = 1 | 0.0448 |
| Contrast | Pair Connection | Statistics (T) | P Value | |
|---|---|---|---|---|
| Uncorrected | FDR | |||
| Mouth < Nose | net.SM.Lateral (L)-net.SM.Superior | 6.85 | 0.0000 | 0.0001 |
| net.SM.Superior-net.SM.Lateral (L) | 6.85 | 0.0000 | 0.0001 | |
| net.SM.Superior–PostCG (L) | 5.74 | 0.0000 | 0.0005 | |
| net.SM.Superior–PreCG (L) | 5.13 | 0.0000 | 0.0012 | |
| net.SM.Superior-net.SM.Lateral (R) | 5.06 | 0.0000 | 0.0012 | |
| net.SM.Lateral (R)-net.SM.Superior | 5.06 | 0.0000 | 0.0049 | |
| net.Salience.RPFC (L)-MedFC | 4.69 | 0.0001 | 0.0114 | |
| Cereb10 (R)-Brain-Stem | 4.67 | 0.0001 | 0.0121 | |
| Cereb10 (R)-Vermis3 | 4.28 | 0.0002 | 0.0149 | |
| Cereb10 (L)-Cereb6 (L) | 4.13 | 0.0003 | 0.0421 | |
| net.SM.Superior–CO (L) | 3.97 | 0.0004 | 0.0123 | |
| PO (R)-net.DefaultMode.LP (R) | 3.93 | 0.0004 | 0.0465 | |
| PO (R)-Cereb10 (L) | 3.79 | 0.0006 | 0.0465 | |
| Cereb10 (L)-PO (R) | 3.79 | 0.0006 | 0.0465 | |
| net.SM.Superior–CO (R) | 3.77 | 0.0006 | 0.0163 | |
| net.SM.Superior–PreCG (R) | 3.7 | 0.0007 | 0.0166 | |
| Area | Statistics | P Value |
|---|---|---|
| Uncorrected | ||
| SM.Lateral (L) | F(5)(16) = 6.68 | 0.0015 |
| Intensity = 6.85 | 0.0168 | |
| Size = 1 | 0.0458 | |
| SM.Lateral (R) | F(5)(16) = 6.05 | 0.0025 |
| Intensity = 5.06 | 0.0191 | |
| Size = 1 | 0.0458 | |
| SM.Superior | F(5)(16) = 5.63 | 0.0035 |
| Intensity = 34.21 | 0.0009 | |
| Size = 7 | 0.0019 | |
| PO (R) | F(5)(16) = 4.25 | 0.012 |
| Intensity = 7.72 | 0.0165 | |
| Size = 2 | 0.0167 | |
| Cereb10 (L) | F(5)(16) = 3.7 | 0.0204 |
| Intensity = 7.93 | 0.0155 | |
| Size = 2 | 0.0167 | |
| Cereb10 (R) | F(5)(16) = 2.98 | 0.0435 |
| Intensity = 8.95 | 0.0105 | |
| Size = 2 | 0.0167 | |
| Salience.RPFC | F(5)(16) = 3.53 | 0.0242 |
| Intensity = 4.69 | 0.0232 | |
| Size = 1 | 0.0458 |
| Left Pair Connection | Statistics (T) | Right Pair Connection | Statistics (T) | L + R | L-R | LI |
|---|---|---|---|---|---|---|
| aITG (L)-CO (L) | 3.87 | aITG (R)-CO (R) | 1.76 | 5.63 | 2.11 | 0.375 * |
| aITG (L)-IC (L) | 3.56 | aITG (R)-IC (R) | 0.92 | 4.48 | 2.64 | 0.589 * |
| net.SM.Lateral (L)-aITG (L) | 3.52 | net.SM.Lateral (R)–aITG (R) | 1.39 | 4.91 | 2.13 | 0.434 * |
| aITG (L)-Cereb6 (L) | 3.41 | aITG (R)-Cereb6 (R) | 0.75 | 4.16 | 2.66 | 0.639 * |
| net.SM.Lateral (L)-Pallidum (L) | 3.31 | net.SM.Lateral (R)-Pallidum (R) | 3.44 | 6.75 | −0.13 | −0.019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-Y.; Park, C.-A.; Lee, Y.-B.; Kang, C.-K. Investigation of Functional Connectivity Differences between Voluntary Respirations via Mouth and Nose Using Resting State fMRI. Brain Sci. 2020, 10, 704. https://doi.org/10.3390/brainsci10100704
Jung J-Y, Park C-A, Lee Y-B, Kang C-K. Investigation of Functional Connectivity Differences between Voluntary Respirations via Mouth and Nose Using Resting State fMRI. Brain Sciences. 2020; 10(10):704. https://doi.org/10.3390/brainsci10100704
Chicago/Turabian StyleJung, Ju-Yeon, Chan-A Park, Yeong-Bae Lee, and Chang-Ki Kang. 2020. "Investigation of Functional Connectivity Differences between Voluntary Respirations via Mouth and Nose Using Resting State fMRI" Brain Sciences 10, no. 10: 704. https://doi.org/10.3390/brainsci10100704
APA StyleJung, J.-Y., Park, C.-A., Lee, Y.-B., & Kang, C.-K. (2020). Investigation of Functional Connectivity Differences between Voluntary Respirations via Mouth and Nose Using Resting State fMRI. Brain Sciences, 10(10), 704. https://doi.org/10.3390/brainsci10100704





