Effect of Age in Auditory Go/No-Go Tasks: A Magnetoencephalographic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MEG Paradigm and Experimental Design
2.3. Behavioral Assessments
2.4. MEG Recordings and Pre-Processing
2.5. Data Analysis and Statistics
2.5.1. Behavioral Data
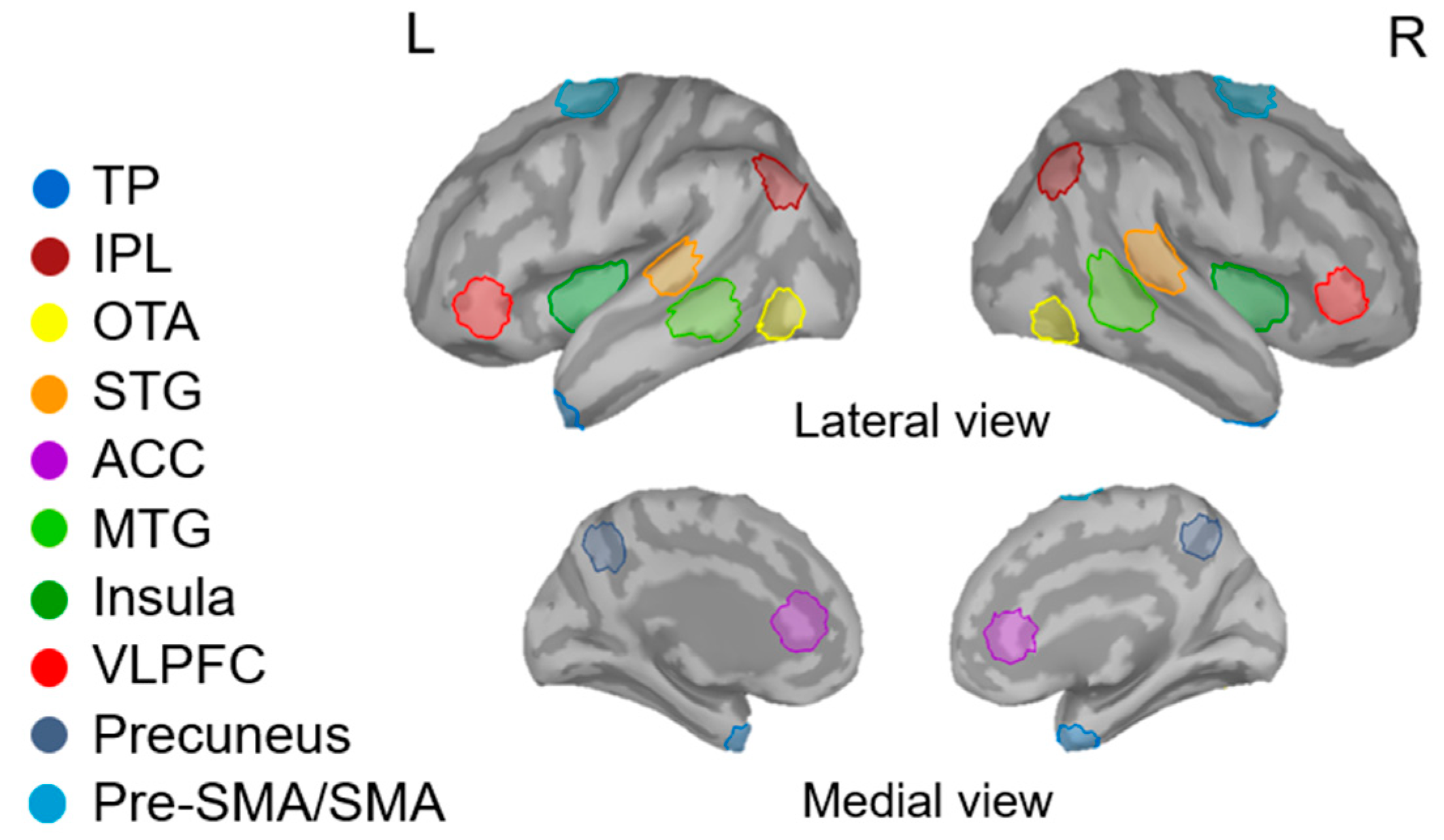
2.5.2. MEG Data
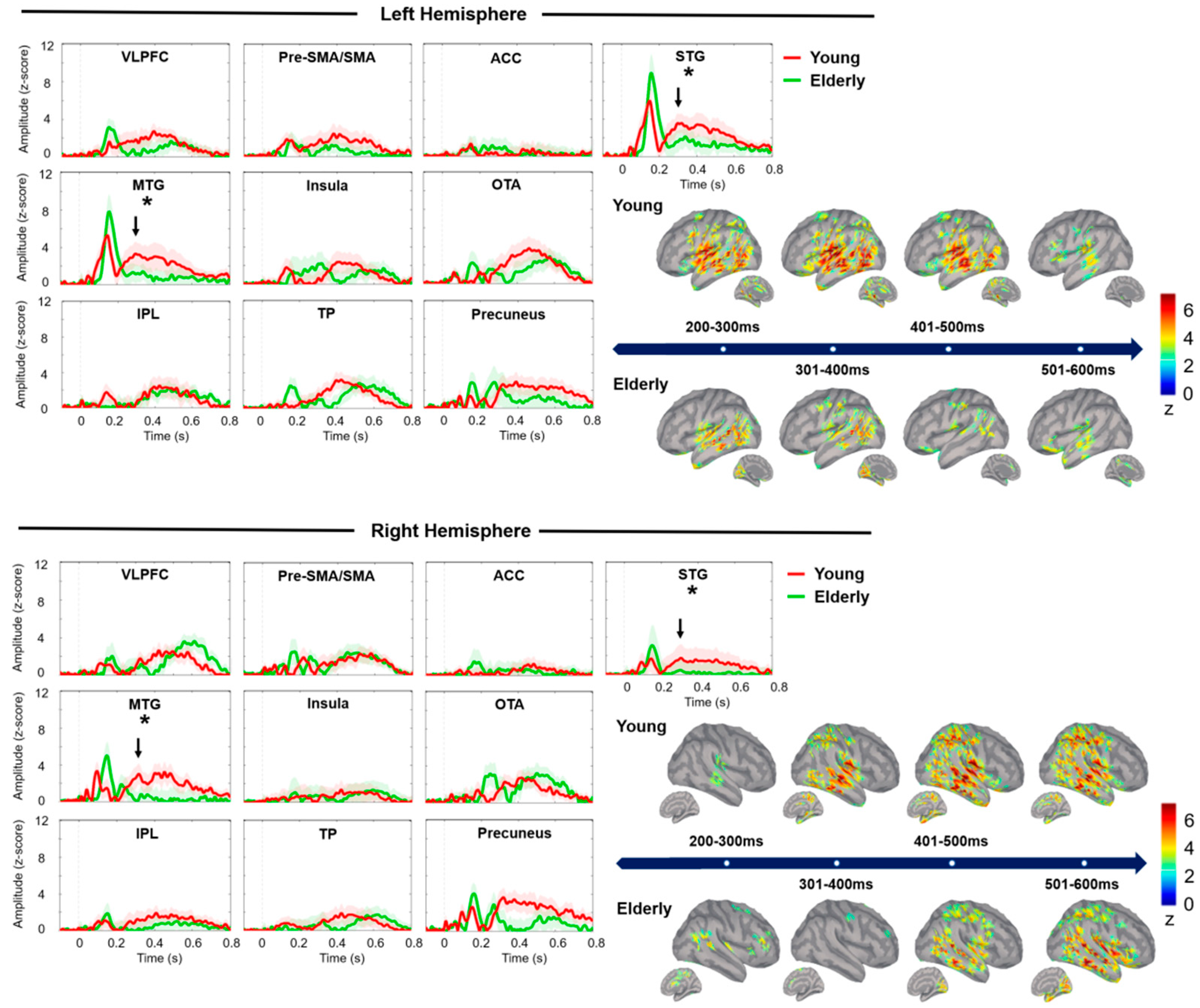
3. Results
3.1. Comparisons between Younger and Older Adult Groups
3.2. Comparisons between High- and Low-Performing Older Adults
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015.
- Hasher, L.; Zacks, R.T. Working memory, comprehension, and aging: A review and a new view. In Psychology of Learning and Motivation; Elsevier: Amsterdam, The Netherlands, 1988; Volume 22, pp. 193–225. [Google Scholar]
- Lustig, C.; Hasher, L.; Zacks, R.T. Inhibitory deficit theory: Recent developments in a “new view”. Am. Psychol. Assoc. 2007, 145–162. [Google Scholar] [CrossRef]
- Hasher, L. Inhibitory deficit hypothesis. Encycl. Adulthood Aging 2015, 1–5. [Google Scholar] [CrossRef]
- Rey-Mermet, A.; Gade, M. Inhibition in aging: What is preserved? What declines? A meta-analysis. Psychon. Bull. Rev. 2018, 25, 1695–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrés, P.; Guerrini, C.; Phillips, L.H.; Perfect, T.J. Differential effects of aging on executive and automatic inhibition. Dev. Neuropsychol. 2008, 33, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Collette, F.; Schmidt, C.; Scherrer, C.; Adam, S.; Salmon, E. Specificity of inhibitory deficits in normal aging and Alzheimer’s disease. Neurobiol. Aging 2009, 30, 875–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Hippel, W. Aging, executive functioning, and social control. Curr. Dir. Psychol. Sci. 2007, 16, 240–244. [Google Scholar] [CrossRef]
- Shafto, M.A.; Tyler, L.K. Language in the aging brain: The network dynamics of cognitive decline and preservation. Science 2014, 346, 583–587. [Google Scholar] [CrossRef]
- Neumann, Y.; Vogel-Eyny, A.; Cahana-Amitay, D.; Spiro, A.; Hyun, J.; Albert, M.L.; Obler, L.K. Effects of inhibition on naming in aging. Let. Hoje 2018, 53, 13–23. [Google Scholar] [CrossRef]
- Schnitzspahn, K.M.; Stahl, C.; Zeintl, M.; Kaller, C.P.; Kliegel, M. The role of shifting, updating, and inhibition in prospective memory performance in young and older adults. Dev. Psychol. 2013, 49, 1544. [Google Scholar] [CrossRef] [Green Version]
- Wagster, M.V.; King, J.W.; Resnick, S.M.; Rapp, P.R. The 87%: Guest Editorial. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2012, 67, 739–740. [Google Scholar] [CrossRef]
- Chambers, C.D.; Garavan, H.; Bellgrove, M.A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 2009, 33, 631–646. [Google Scholar] [CrossRef]
- Vara, A.S.; Pang, E.W.; Vidal, J.; Anagnostou, E.; Taylor, M.J. Neural mechanisms of inhibitory control continue to mature in adolescence. Dev. Cogn. Neurosci. 2014, 10, 129–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiefer, M.; Marzinzik, F.; Weisbrod, M.; Scherg, M.; Spitzer, M. The time course of brain activations during response inhibition: Evidence from event-related potentials in a go/no go task. Neuroreport 1998, 9, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Pires, L.; Leitão, J.; Guerrini, C.; Simões, M.R. Event-related brain potentials in the study of inhibition: Cognitive control, source localization and age-related modulations. Neuropsychol. Rev. 2014, 24, 461–490. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Sakamoto, K.; Otsuka, A.; Yumoto, M.; Kakigi, R. Cortical rhythm of No-go processing in humans: An MEG study. Clin. Neurophysiol. 2013, 124, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, M.; Hoormann, J.; Hohnsbein, J. Inhibition-related ERP components: Variation with modality, age, and time-on-task. J. Psychophysiol. 2002, 16, 167. [Google Scholar] [CrossRef]
- Hsieh, S.; Wu, M.; Tang, C.-H. Adaptive strategies for the elderly in inhibiting irrelevant and conflict no-go trials while performing the go/no-go task. Front. Aging Neurosci. 2015, 7, 243. [Google Scholar] [CrossRef]
- Mudar, R.A.; Chiang, H.-S.; Maguire, M.J.; Spence, J.S.; Eroh, J.; Kraut, M.A.; Hart, J. Effects of age on cognitive control during semantic categorization. Behav. Brain Res. 2015, 287, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Staub, B.; Doignon-Camus, N.; Marques-Carneiro, J.E.; Bacon, E.; Bonnefond, A. Age-related differences in the use of automatic and controlled processes in a situation of sustained attention. Neuropsychologia 2015, 75, 607–616. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Tseng, Y.-J.; Cheng, C.-H. Age effects on spatiotemporal dynamics of response inhibition: An MEG study. Front. Aging Neurosci. 2018, 10, 386. [Google Scholar] [CrossRef]
- Vallesi, A. Targets and non-targets in the aging brain: A go/nogo event-related potential study. Neurosci. Lett. 2011, 487, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Smucny, J.; Olincy, A.; Eichman, L.C.; Lyons, E.; Tregellas, J.R. Early sensory processing deficits predict sensitivity to distraction in schizophrenia. Schizophr. Res. 2013, 147, 196–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trezise, K.L.; Gray, K.M.; Sheppard, D.M. Attention and vigilance in children with Down syndrome. J. Appl. Res. Intellect. Disabil. 2008, 21, 502–508. [Google Scholar] [CrossRef]
- Fisher, T.; Aharon-Peretz, J.; Pratt, H. Dis-regulation of response inhibition in adult Attention Deficit Hyperactivity Disorder (ADHD): An ERP study. Clin. Neurophysiol. 2011, 122, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Rentrop, M.; Backenstrass, M.; Jaentsch, B.; Kaiser, S.; Roth, A.; Unger, J.; Weisbrod, M.; Renneberg, B. Response inhibition in borderline personality disorder: Performance in a Go/Nogo task. Psychopathology 2008, 41, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Horváth, J.; Czigler, I.; Birkás, E.; Winkler, I.; Gervai, J. Age-related differences in distraction and reorientation in an auditory task. Neurobiol. Aging 2009, 30, 1157–1172. [Google Scholar] [CrossRef]
- Barry, R.J.; De Blasio, F.M.; Cave, A.E. Sequential processing in young and older adults in the equiprobable auditory Go/NoGo task. Clin. Neurophysiol. 2016, 127, 2273–2285. [Google Scholar] [CrossRef] [Green Version]
- Vallesi, A.; McIntosh, A.R.; Stuss, D.T. Overrecruitment in the aging brain as a function of task demands: Evidence for a compensatory view. J. Cogn. Neurosci. 2011, 23, 801–815. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Sun, J.; Bengson, J.J.; Tong, S. Age-related spatiotemporal reorganization during response inhibition. Int. J. Psychophysiol. 2014, 93, 371–380. [Google Scholar] [CrossRef]
- Staub, B.; Doignon-Camus, N.; Bacon, É.; Bonnefond, A. The effects of aging on sustained attention ability: An ERP study. Psychol. Aging 2014, 29, 684. [Google Scholar] [CrossRef]
- Walther, S.; Goya-Maldonado, R.; Stippich, C.; Weisbrod, M.; Kaiser, S. A supramodal network for response inhibition. Neuroreport 2010, 21, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Seli, P.; Cheyne, J.A.; Barton, K.R.; Smilek, D. Consistency of sustained attention across modalities: Comparing visual and auditory versions of the SART. Can. J. Exp. Psychol. Rev. Can. Psychol. Expérimentale 2012, 66, 44. [Google Scholar] [CrossRef] [PubMed]
- Gross, J. Magnetoencephalography in cognitive neuroscience: A primer. Neuron 2019, 104, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.R.; Hillebrand, A.; Fawcett, I.P.; Singh, K.D. Realistic spatial sampling for MEG beamformer images. Hum. Brain Mapp. 2004, 23, 120–127. [Google Scholar] [CrossRef]
- Miltner, W.H.; Lemke, U.; Weiss, T.; Holroyd, C.; Scheffers, M.K.; Coles, M.G. Implementation of error-processing in the human anterior cingulate cortex: A source analysis of the magnetic equivalent of the error-related negativity. Biol. Psychol. 2003, 64, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Hultsch, D.F.; MacDonald, S.W.; Dixon, R.A. Variability in reaction time performance of younger and older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2002, 57, P101–P115. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Yin, S.; Zhu, X.; Ren, W.; Yu, J.; Wang, P.; Zheng, Z.; Niu, Y.-N.; Huang, X.; Li, J. Linking inter-individual variability in functional brain connectivity to cognitive ability in elderly individuals. Front. Aging Neurosci. 2017, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-C.; Lindenberger, U. Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. In Cognitive Neuroscience of Memory; Hogrefe & Huber: Cambridge, MA, USA, 1999; pp. 103–146. [Google Scholar]
- Sylvain-Roy, S.; Belleville, S. Interindividual differences in attentional control profiles among younger and older adults. Aging Neuropsychol. Cogn. 2015, 22, 259–279. [Google Scholar] [CrossRef]
- Lin, K.N.; Wang, P.N.; Liu, H.C.; Teng, E.L. Cognitive abilities screening instrument, Chinese version 2.0 (CASI C-2.0): Administration and clinical application. Acta Neurol Taiwan 2012, 21, 180–189. [Google Scholar]
- Falkenstein, M.; Hoormann, J.; Hohnsbein, J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. 1999, 101, 267–291. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Hari, R.; Ilmoniemi, R.J.; Knuutila, J.; Lounasmaa, O.V. Magnetoencephalography—Theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 1993, 65, 413. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Elorrieta, E.; Pylkkänen, L. Bilingual language control in perception versus action: MEG reveals comprehension control mechanisms in anterior cingulate cortex and domain-general control of production in dorsolateral prefrontal cortex. J. Neurosci. 2016, 36, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.; Baillet, S.; Barnes, G.R.; Henson, R.N.; Hillebrand, A.; Jensen, O.; Jerbi, K.; Litvak, V.; Maess, B.; Oostenveld, R. Good practice for conducting and reporting MEG research. Neuroimage 2013, 65, 349–363. [Google Scholar] [CrossRef]
- Uusitalo, M.A.; Ilmoniemi, R.J. Signal-space projection method for separating MEG or EEG into components. Med Biol. Eng. Comput. 1997, 35, 135–140. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Mosher, J.C.; Leahy, R. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys. Med. Biol. 1999, 44, 423. [Google Scholar] [CrossRef]
- Hämäläinen, M.S.; Ilmoniemi, R.J. Interpreting magnetic fields of the brain: Minimum norm estimates. Med. Biol. Eng. Comput. 1994, 32, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.; Lee, A.K. Potential use of MEG to understand abnormalities in auditory function in clinical populations. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonov, V.S.; Evans, A.C.; McKinstry, R.C.; Almli, C.; Collins, D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage 2009, 47, S102. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Baillet, S.; Lin, Y.-Y. Region-specific reduction of auditory sensory gating in older adults. Brain Cogn. 2015, 101, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.; Mills, T.; Pang, E.W.; Taylor, M.J. Response inhibition in adults and teenagers: Spatiotemporal differences in the prefrontal cortex. Brain Cogn. 2012, 79, 49–59. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Criaud, M.; Boulinguez, P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci. Biobehav. Rev. 2013, 37, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- O’Connell, R.G.; Bellgrove, M.A.; Dockree, P.M.; Lau, A.; Fitzgerald, M.; Robertson, I.H. Self-alert training: Volitional modulation of autonomic arousal improves sustained attention. Neuropsychologia 2008, 46, 1379–1390. [Google Scholar] [CrossRef]
- Cheyne, J.A.; Carriere, J.S.; Smilek, D. Absent minds and absent agents: Attention-lapse induced alienation of agency. Conscious. Cogn. 2009, 18, 481–493. [Google Scholar] [CrossRef]
- Bagur, S.; Averseng, M.; Elgueda, D.; David, S.; Fritz, J.; Yin, P.; Shamma, S.; Boubenec, Y.; Ostojic, S. Go/No-Go task engagement enhances population representation of target stimuli in primary auditory cortex. Nat. Commun. 2018, 9, 2529. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-H.; Niddam, D.M.; Hsu, S.-C.; Liu, C.-Y.; Tsai, S.-Y. Resting GABA concentration predicts inhibitory control during an auditory Go-Nogo task. Exp. Brain Res. 2017, 235, 3833–3841. [Google Scholar] [CrossRef]
- Balz, J.; Keil, J.; Romero, Y.R.; Mekle, R.; Schubert, F.; Aydin, S.; Ittermann, B.; Gallinat, J.; Senkowski, D. GABA concentration in superior temporal sulcus predicts gamma power and perception in the sound-induced flash illusion. Neuroimage 2016, 125, 724–730. [Google Scholar] [CrossRef]
- Gao, F.; Edden, R.A.; Li, M.; Puts, N.A.; Wang, G.; Liu, C.; Zhao, B.; Wang, H.; Bai, X.; Zhao, C. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 2013, 78, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauwels, L.; Maes, C.; Swinnen, S.P. Aging, inhibition and GABA. Aging 2018, 10, 3645. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.; Thompson, H.E.; Hallam, G.; Karapanagiotidis, T.; Murphy, C.; De Caso, I.; Krieger-Redwood, K.; Bernhardt, B.C.; Smallwood, J.; Jefferies, E. Exploring the role of the posterior middle temporal gyrus in semantic cognition: Integration of anterior temporal lobe with executive processes. NeuroImage 2016, 137, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehaene, S.; Cohen, L. Cerebral pathways for calculation: Double dissociation between rote verbal and quantitative knowledge of arithmetic. Cortex 1997, 33, 219–250. [Google Scholar] [CrossRef]
- Dehaene, S.; Akhavein, R. Attention, automaticity, and levels of representation in number processing. J. Exp. Psychol. Learn. Mem. Cogn. 1995, 21, 314. [Google Scholar] [CrossRef]
- Klein, E.; Suchan, J.; Moeller, K.; Karnath, H.-O.; Knops, A.; Wood, G.; Nuerk, H.-C.; Willmes, K. Considering structural connectivity in the triple code model of numerical cognition: Differential connectivity for magnitude processing and arithmetic facts. Brain Struct. Funct. 2014, 221, 979–995. [Google Scholar] [CrossRef]
- Noonan, K.A.; Jefferies, E.; Visser, M.; Lambon Ralph, M.A. Going beyond inferior prefrontal involvement in semantic control: Evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J. Cogn. Neurosci. 2013, 25, 1824–1850. [Google Scholar] [CrossRef]
- Butorina, A.V.; Pavlova, A.A.; Nikolaeva, A.Y.; Prokofyev, A.O.; Bondarev, D.P.; Stroganova, T.A. Simultaneous processing of noun cue and to-be-produced verb in verb generation task: Electromagnetic evidence. Front. Hum. Neurosci. 2017, 11, 11. [Google Scholar] [CrossRef]
- Jefferies, E. The neural basis of semantic cognition: Converging evidence from neuropsychology, neuroimaging and TMS. Cortex 2013, 49, 611–625. [Google Scholar] [CrossRef]
- Lehnert, G.; Zimmer, H.D. Modality and domain specific components in auditory and visual working memory tasks. Cogn. Process. 2008, 9, 53. [Google Scholar] [CrossRef]
- Bushara, K.O.; Weeks, R.A.; Ishii, K.; Catalan, M.-J.; Tian, B.; Rauschecker, J.P.; Hallett, M. Modality-specific frontal and parietal areas for auditory and visual spatial localization in humans. Nat. Neurosci. 1999, 2, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Keitel, C.; Maess, B.; Schröger, E.; Müller, M.M. Early visual and auditory processing rely on modality-specific attentional resources. Neuroimage 2013, 70, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Ü.; Vorwerk, J.; Dümpelmann, M.; Küpper, P.; Kugel, H.; Heers, M.; Wellmer, J.; Kellinghaus, C.; Haueisen, J.; Rampp, S. Combined EEG/MEG can outperform single modality EEG or MEG source reconstruction in presurgical epilepsy diagnosis. PLoS ONE 2015, 10, e0118753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hari, R.; Salmelin, R. Magnetoencephalography: From SQUIDs to neuroscience: Neuroimage 20th anniversary special edition. Neuroimage 2012, 61, 386–396. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-Y.; Cheng, C.-H. Effect of Age in Auditory Go/No-Go Tasks: A Magnetoencephalographic Study. Brain Sci. 2020, 10, 667. https://doi.org/10.3390/brainsci10100667
Lin M-Y, Cheng C-H. Effect of Age in Auditory Go/No-Go Tasks: A Magnetoencephalographic Study. Brain Sciences. 2020; 10(10):667. https://doi.org/10.3390/brainsci10100667
Chicago/Turabian StyleLin, Mei-Yin, and Chia-Hsiung Cheng. 2020. "Effect of Age in Auditory Go/No-Go Tasks: A Magnetoencephalographic Study" Brain Sciences 10, no. 10: 667. https://doi.org/10.3390/brainsci10100667
APA StyleLin, M.-Y., & Cheng, C.-H. (2020). Effect of Age in Auditory Go/No-Go Tasks: A Magnetoencephalographic Study. Brain Sciences, 10(10), 667. https://doi.org/10.3390/brainsci10100667




