Biomechanical Evaluation of the Effect of Mesenchymal Stem Cells on Cartilage Regeneration in Knee Joint Osteoarthritis
Abstract
:1. Introduction
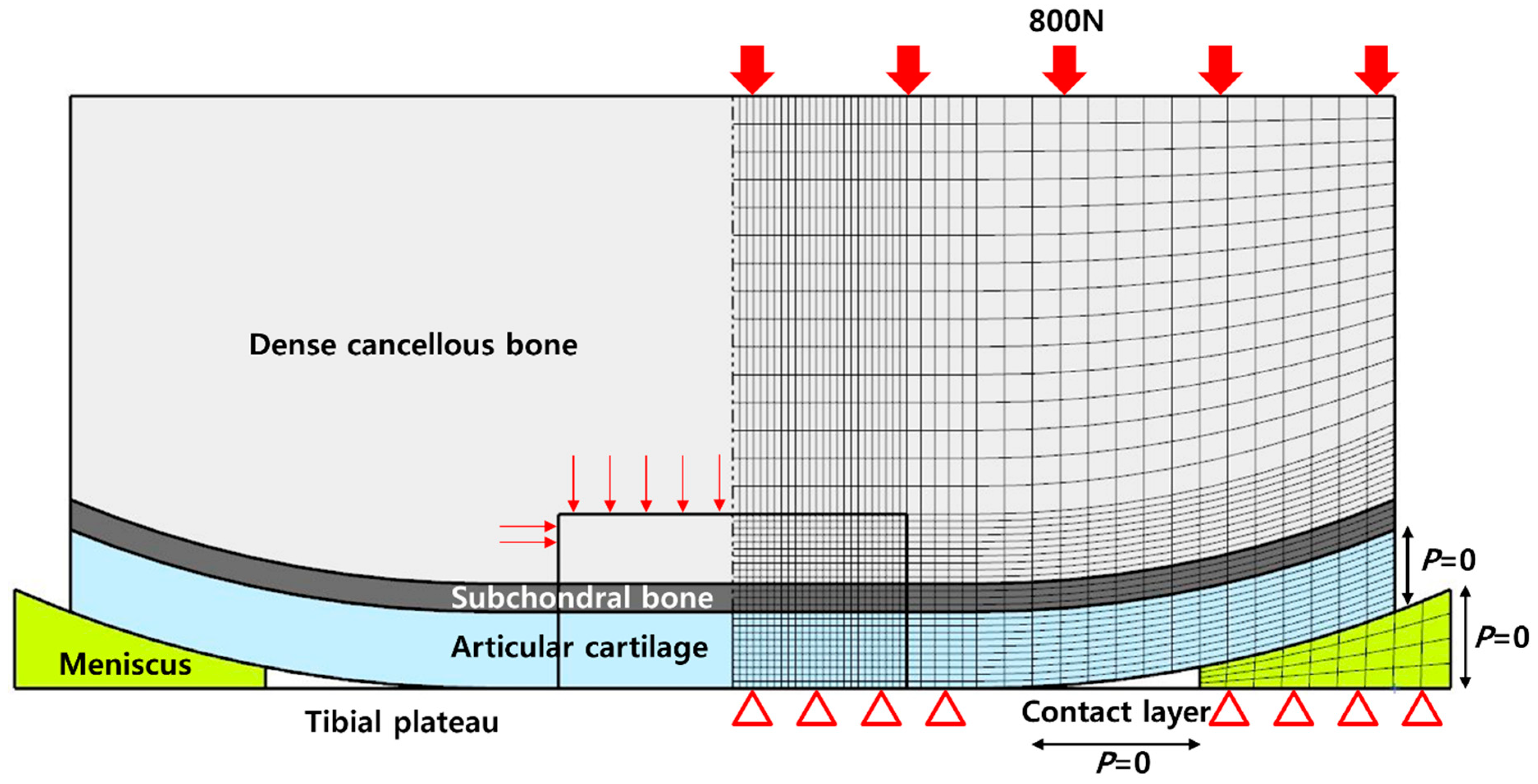
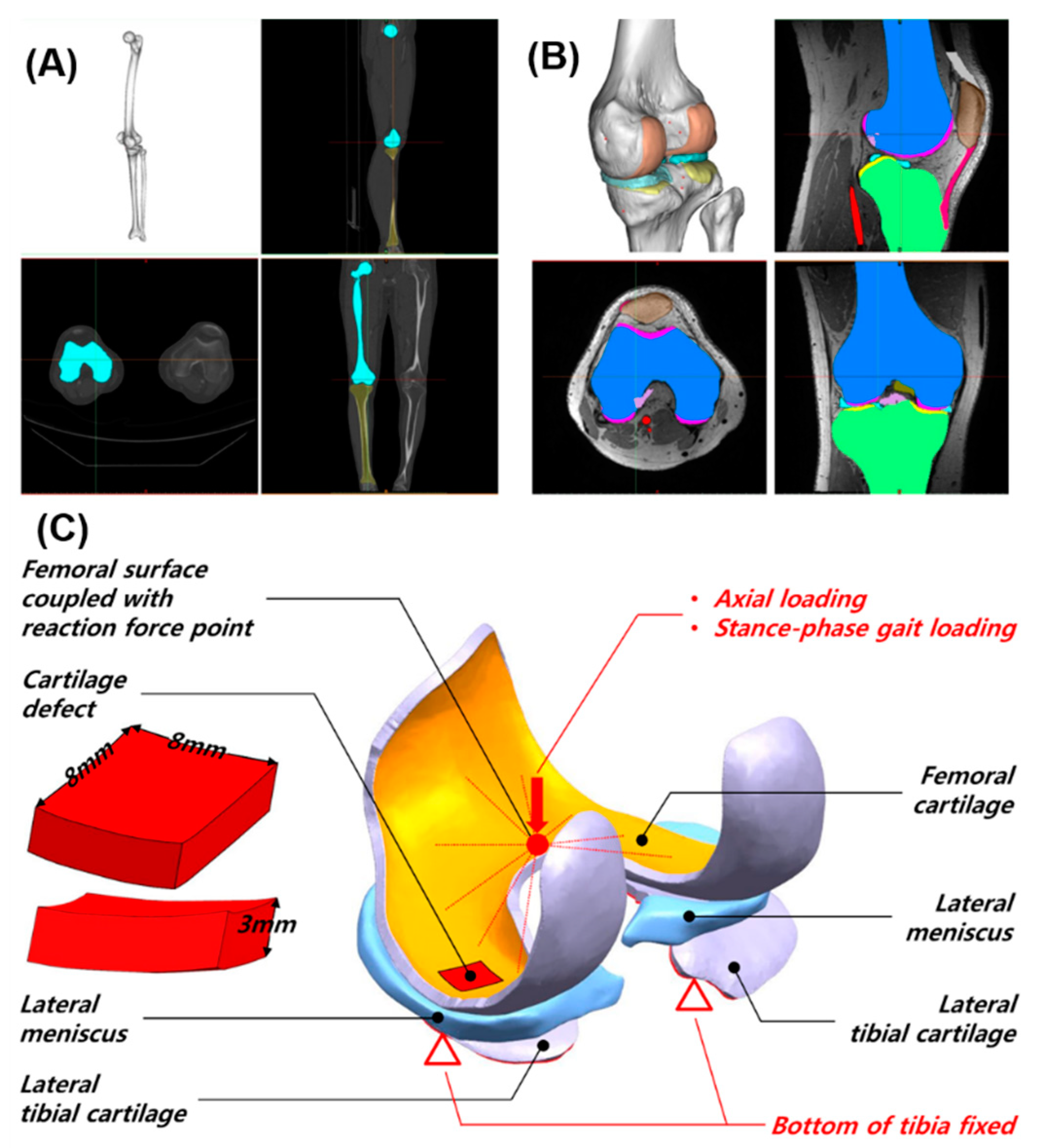
2. Materials and Methods
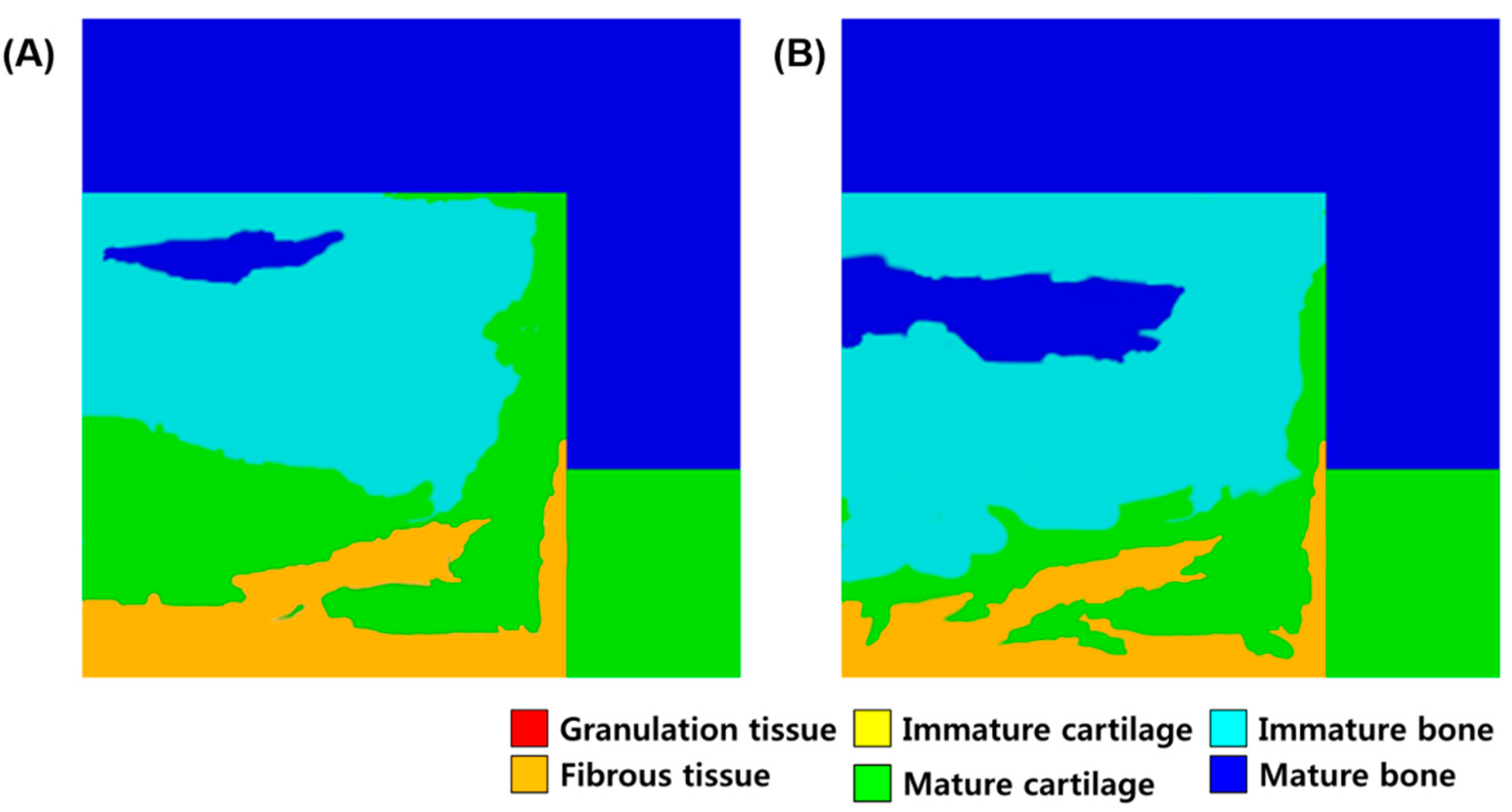
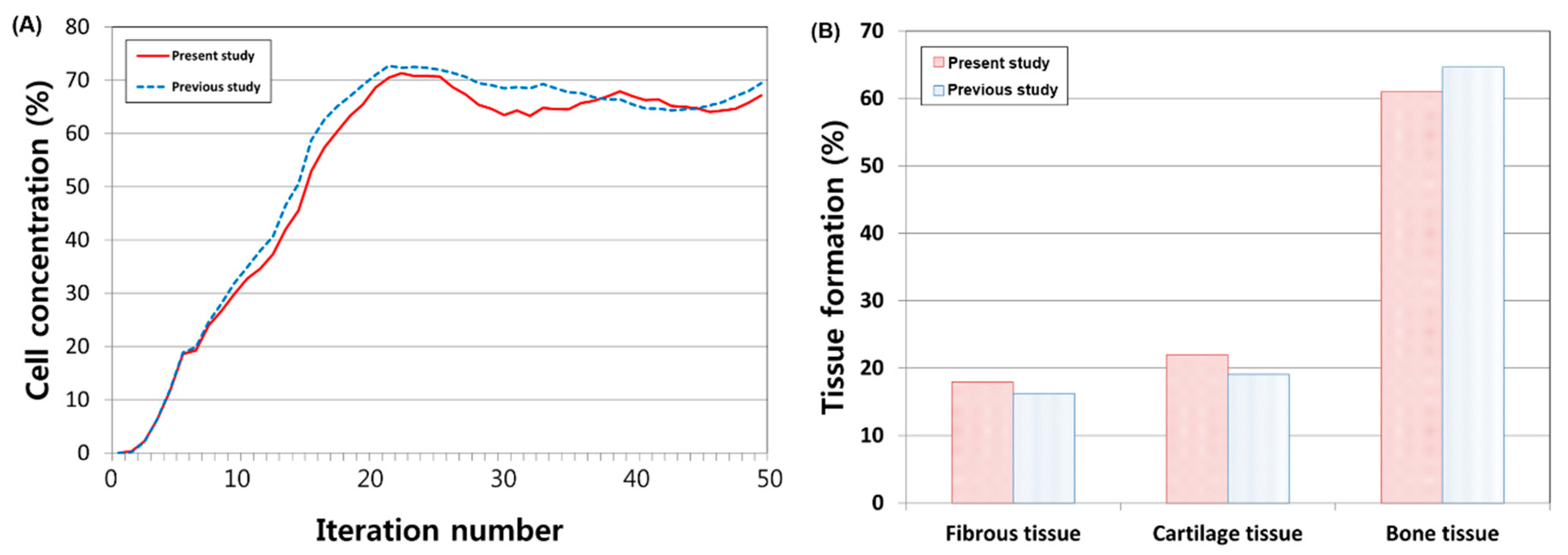
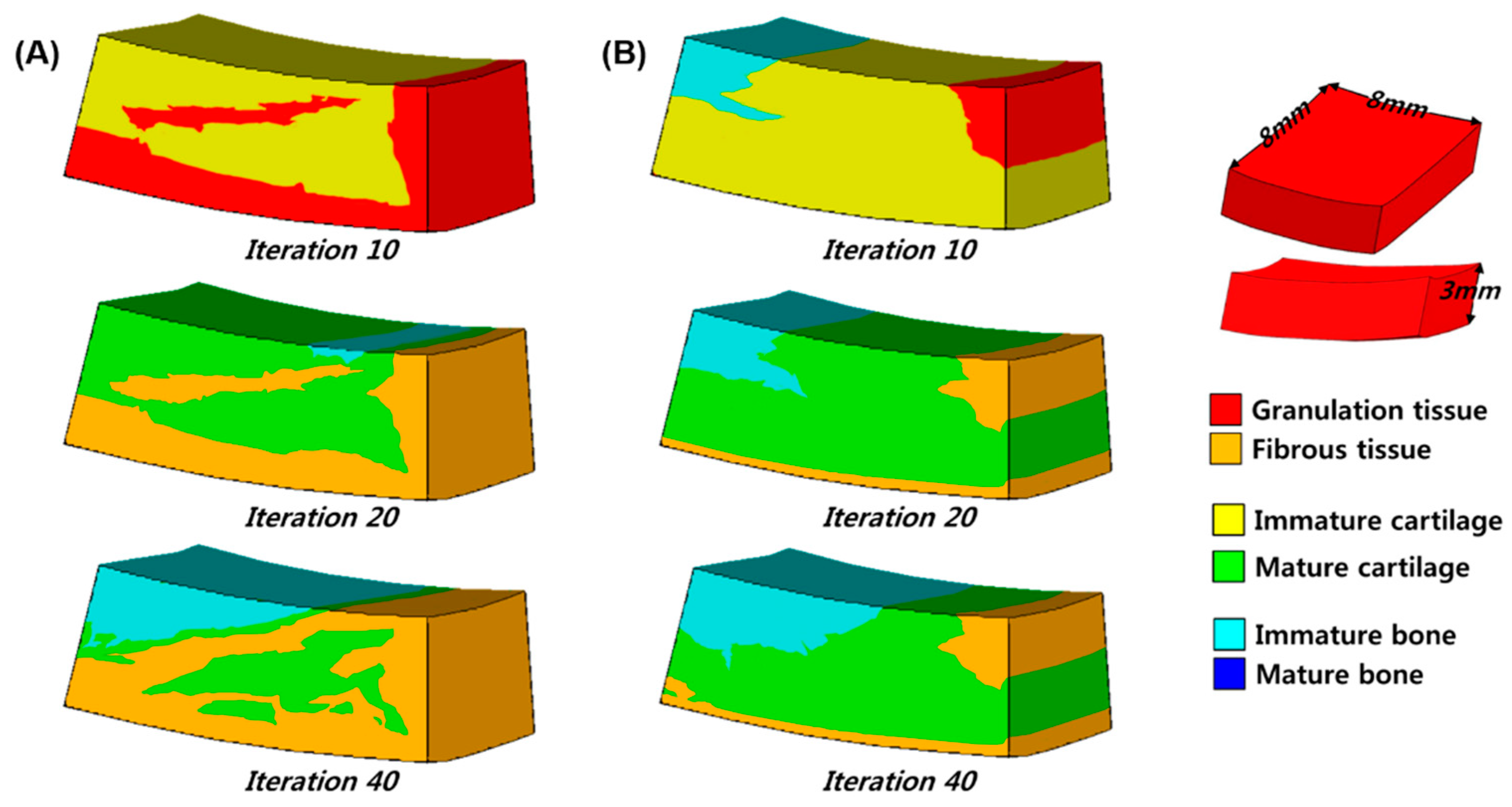
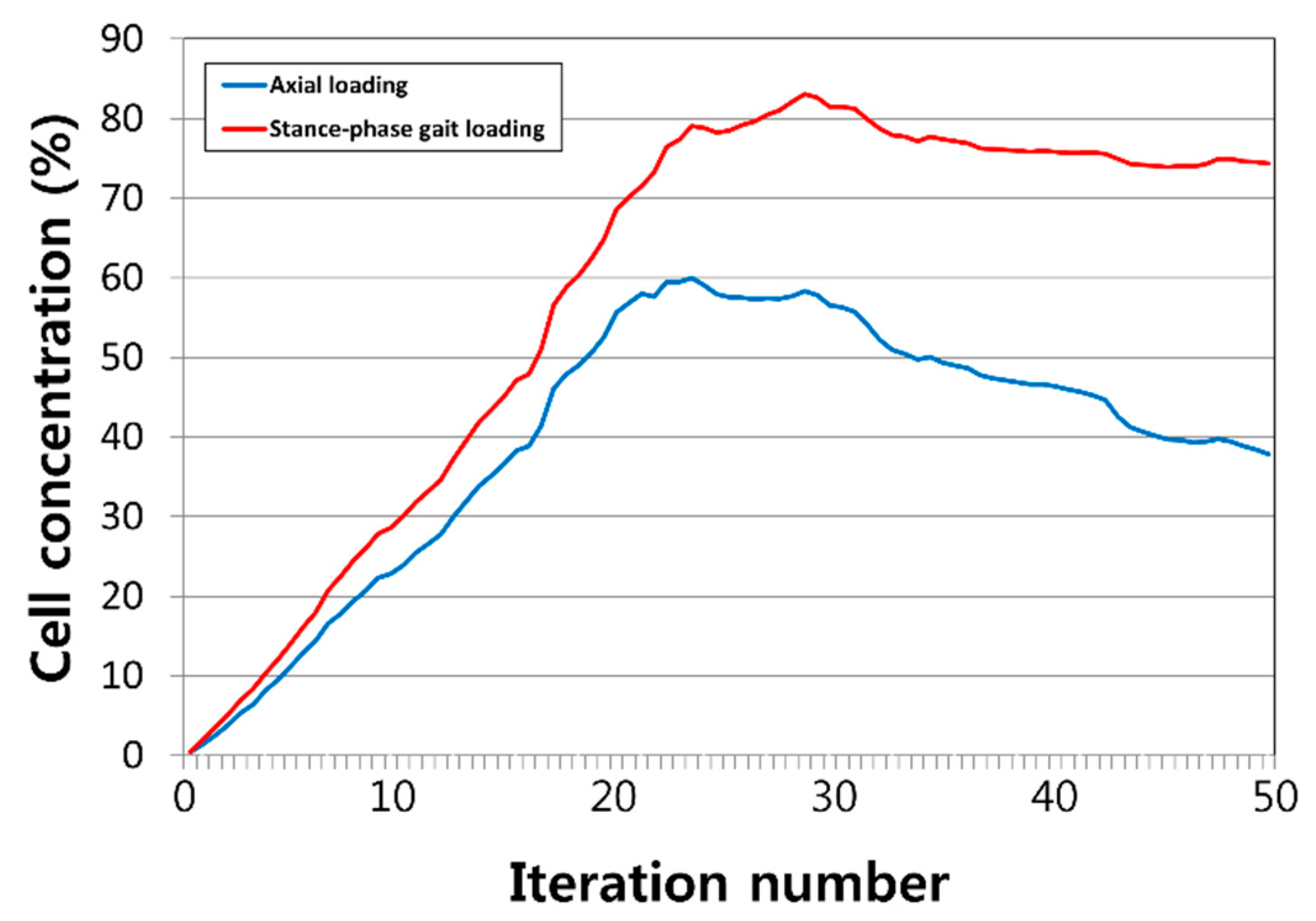
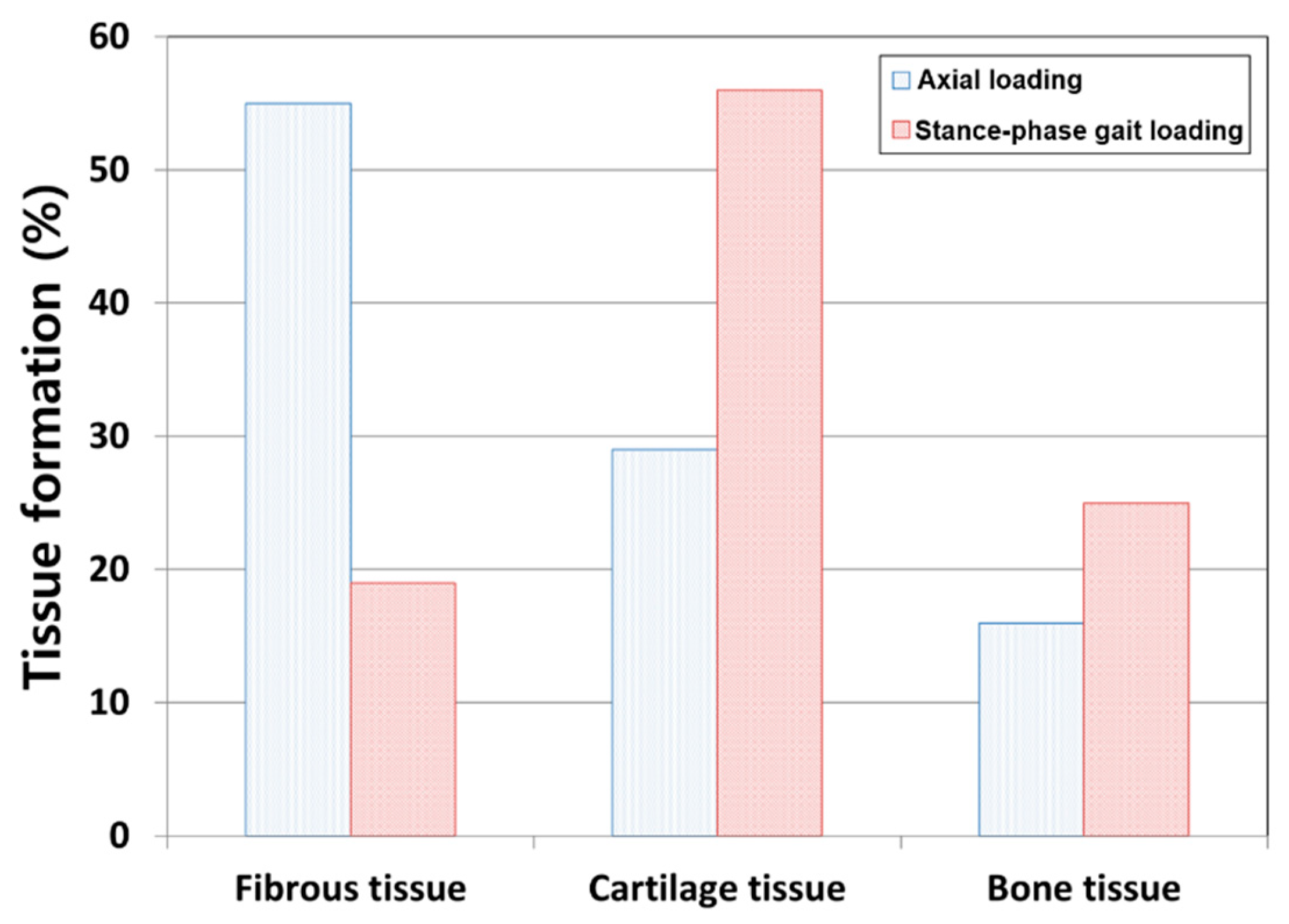
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. Part ii. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef]
- Dillon, C.F.; Rasch, E.K.; Gu, Q.; Hirsch, R. Prevalence of knee osteoarthritis in the united states: Arthritis data from the third national health and nutrition examination survey 1991–94. J. Rheumatol. 2006, 33, 2271–2279. [Google Scholar]
- Koh, Y.G.; Choi, Y.J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J. The response of articular cartilage to mechanical injury. J. Bone Jt. Surg. 1982, 64, 460–466. [Google Scholar] [CrossRef]
- Kim, H.K.; Moran, M.E.; Salter, R.B. The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion. An experimental investigation in rabbits. J. Bone Jt. Surg. Am. Vol. 1991, 73, 1301–1315. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Roos, E.M. Clinical update: Treating osteoarthritis. Lancet (Lond. Engl.) 2007, 370, 2082–2084. [Google Scholar] [CrossRef]
- Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Chowaniec, D.; Falcone, L.M.; Falcone, J.A.; Dugdale, E.M.; Deberardino, T.M.; Arciero, R.A.; Mazzocca, A.D. Rapid isolation of human stem cells (connective progenitor cells) from the distal femur during arthroscopic knee surgery. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2012, 28, 74–84. [Google Scholar] [CrossRef]
- Raghunath, J.; Salacinski, H.J.; Sales, K.M.; Butler, P.E.; Seifalian, A.M. Advancing cartilage tissue engineering: The application of stem cell technology. Curr. Opin. Biotechnol. 2005, 16, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Prendergast, P.J. Mechano-regulation of stem cell differentiation and tissue regeneration in osteochondral defects. J. Biomech. 2005, 38, 1413–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ament, C.; Hofer, E.P. A fuzzy logic model of fracture healing. J. Biomech. 2000, 33, 961–968. [Google Scholar] [CrossRef]
- Prendergast, P.J.; Huiskes, R.; Søballe, K. Biophysical stimuli on cells during tissue differentiation at implant interfaces. J. Biomech. 1997, 30, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Isaksson, H.; van Donkelaar, C.C.; Huiskes, R.; Ito, K. A mechano-regulatory bone-healing model incorporating cell-phenotype specific activity. J. Theor. Biol. 2008, 252, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Nagel, T.; Kelly, D.J. Mechano-regulation of mesenchymal stem cell differentiation and collagen organisation during skeletal tissue repair. Biomech. Model. Mechanobiol. 2010, 9, 359–372. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, A.; Kelly, D.J. Role of oxygen as a regulator of stem cell fate during the spontaneous repair of osteochondral defects. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016, 34, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, A.; Kelly, D.J. A computational model of osteochondral defect repair following implantation of stem cell-laden multiphase scaffolds. Tissue Eng. Part A 2017, 23, 30–42. [Google Scholar] [CrossRef]
- Koh, Y.G.; Lee, J.A.; Kim, Y.S.; Lee, H.Y.; Kim, H.J.; Kang, K.T. Optimal mechanical properties of a scaffold for cartilage regeneration using finite element analysis. J. Tissue Eng. 2019, 10, 2041731419832133. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, D.; Prendergast, P.J.; Li, G.; Marsh, D. Biomechanical model to simulate tissue differentiation and bone regeneration: Application to fracture healing. Med. Biol. Eng. Comput. 2002, 40, 14–21. [Google Scholar] [CrossRef]
- Hayward, L.N.; Morgan, E.F. Assessment of a mechano-regulation theory of skeletal tissue differentiation in an in vivo model of mechanically induced cartilage formation. Biomech. Model. Mechanobiol. 2009, 8, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Kim, Y.I.; Ryu, J.S.; Koh, Y.G. Mesenchymal stem cell implantation in osteoarthritic knees: Is fibrin glue effective as a scaffold? Am. J. Sports Med. 2015, 43, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Choi, Y.J.; Kwon, O.R.; Kim, Y.S. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am. J. Sports Med. 2014, 42, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, Y.J.; Koh, Y.G. Mesenchymal stem cell implantation in knee osteoarthritis: An assessment of the factors influencing clinical outcomes. Am. J. Sports Med. 2015, 43, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, H.; Van Donkelaar, C.C.; Huiskes, R.; Yao, J.; Ita, K. Determining the most important cellular characteristics for fracture healing using design of experiments methods. J. Theor. Biol. 2008, 255, 26–39. [Google Scholar] [CrossRef]
- Lacroix, D.; Prendergast, P.J. A homogenization procedure to prevent numerical instabilities in poroelastic tissue differentiation models. In Proceedings of the Eighth Annual Symposium: Computational Methods in Orthopaedic Biomechanics, Lake Buena Vista, FL, USA, 11 March 2000. [Google Scholar]
- Kang, K.T.; Kim, S.H.; Son, J.; Lee, Y.H.; Chun, H.J. Computational model-based probabilistic analysis of in vivo material properties for ligament stiffness using the laxity test and computed tomography. J. Mater. Sci. Mater. Med. 2016, 27, 183. [Google Scholar] [CrossRef]
- Koh, Y.G.; Son, J.; Kwon, S.K.; Kim, H.J.; Kwon, O.R.; Kang, K.T. Preservation of kinematics with posterior cruciate-, bicruciate- and patient-specific bicruciate-retaining prostheses in total knee arthroplasty by using computational simulation with normal knee model. Bone Jt. Res. 2017, 6, 557–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.T.; Koh, Y.G.; Son, J.; Kim, S.J.; Choi, S.; Jung, M.; Kim, S.H. Finite element analysis of the biomechanical effects of 3 posterolateral corner reconstruction techniques for the knee joint. J. Arthrosc. 2017, 33, 1537–1550. [Google Scholar] [CrossRef]
- 14243-1, I. Implants for surgery—wear of total knee-joint prostheses—part 1: Loading and displacement parameters for wear-testing machines with load control and corresponding environmental conditions for test. 2009.
- Curl, W.W.; Krome, J.; Gordon, E.S.; Rushing, J.; Smith, B.P.; Poehling, G.G. Cartilage injuries: A review of 31,516 knee arthroscopies. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 1997, 13, 456–460. [Google Scholar] [CrossRef]
- Aroen, A.; Loken, S.; Heir, S.; Alvik, E.; Ekeland, A.; Granlund, O.G.; Engebretsen, L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am. J. Sports Med. 2004, 32, 211–215. [Google Scholar] [CrossRef]
- Mithoefer, K.; Williams, R.J., 3rd; Warren, R.F.; Potter, H.G.; Spock, C.R.; Jones, E.C.; Wickiewicz, T.L.; Marx, R.G. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J. Bone Jt. Surg. Am. Vol. 2005, 87, 1911–1920. [Google Scholar] [CrossRef]
- Steadman, J.R.; Briggs, K.K.; Rodrigo, J.J.; Kocher, M.S.; Gill, T.J.; Rodkey, W.G. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2003, 19, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Rodkey, W.G.; Rodrigo, J.J. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res. 2001, S391, S362–S369. [Google Scholar] [CrossRef]
- Pareek, A.; Reardon, P.J.; Macalena, J.A.; Levy, B.A.; Stuart, M.J.; Williams, R.J., 3rd; Krych, A.J. Osteochondral autograft transfer versus microfracture in the knee: A meta-analysis of prospective comparative studies at midterm. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2016, 32, 2118–2130. [Google Scholar] [CrossRef]
- Shelbourne, K.D.; Jari, S.; Gray, T. Outcome of untreated traumatic articular cartilage defects of the knee: A natural history study. J. Bone Jt. Surg. Am. Vol. 2003, 85A (Suppl. 2), 8–16. [Google Scholar] [CrossRef]
- Ashton, B.A.; Allen, T.D.; Howlett, C.R.; Eaglesom, C.C.; Hattori, A.; Owen, M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin. Orthop. Relat. Res. 1980, 151, 294–307. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vivo chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwon, O.R.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Comparative matched-pair analysis of the injection versus implantation of mesenchymal stem cells for knee osteoarthritis. Am. J. Sports Med. 2015, 43, 2738–2746. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells (Dayt. Ohio) 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, D.; Prendergast, P.J. A mechano-regulation model for tissue differentiation during fracture healing: Analysis of gap size and loading. J. Biomech. 2002, 35, 1163–1171. [Google Scholar] [CrossRef]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Jt. Surg. Am. Vol. 1993, 75, 532–553. [Google Scholar] [CrossRef]
- Kelly, D.J.; Prendergast, P.J. Prediction of the optimal mechanical properties for a scaffold used in osteochondral defect repair. Tissue Eng. 2006, 12, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.L.; Woolridge, N.; Lumsden, C.J. In vitro, in vivo, in silico: Computational systems in tissue engineering and regenerative medicine. Tissue Eng. 2005, 11, 341–356. [Google Scholar] [CrossRef] [PubMed]
| Granulation Tissue | Fibrous Tissue | Cartilage | Immature Bone | Mature Bone | Cortical Bone | |
|---|---|---|---|---|---|---|
| Young’s modulus (MPa) | 0.2 | 2 | 10 | 1000 | 6000 | 17,000 |
| Poisson’s ratio (ν) | 0.167 | 0.167 | 0.167 | 0.3 | 0.3 | 0.3 |
| Permeability | 1 | 1 | 0.5 | 0.1 | 0.37 | 0.001 |
| Porosity | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.04 |
| Diffusion coefficient (mm2/iteration) | 0.8 | 0.1 | 0.05 | 0.01 | 0.01 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, Y.-G.; Lee, J.-A.; Lee, H.-Y.; Kim, H.-J.; Kang, K.-T. Biomechanical Evaluation of the Effect of Mesenchymal Stem Cells on Cartilage Regeneration in Knee Joint Osteoarthritis. Appl. Sci. 2019, 9, 1868. https://doi.org/10.3390/app9091868
Koh Y-G, Lee J-A, Lee H-Y, Kim H-J, Kang K-T. Biomechanical Evaluation of the Effect of Mesenchymal Stem Cells on Cartilage Regeneration in Knee Joint Osteoarthritis. Applied Sciences. 2019; 9(9):1868. https://doi.org/10.3390/app9091868
Chicago/Turabian StyleKoh, Yong-Gon, Jin-Ah Lee, Hwa-Yong Lee, Hyo-Jeong Kim, and Kyoung-Tak Kang. 2019. "Biomechanical Evaluation of the Effect of Mesenchymal Stem Cells on Cartilage Regeneration in Knee Joint Osteoarthritis" Applied Sciences 9, no. 9: 1868. https://doi.org/10.3390/app9091868




