NO-CH4-SCR Over Core-Shell MnH-Zeolite Composites
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
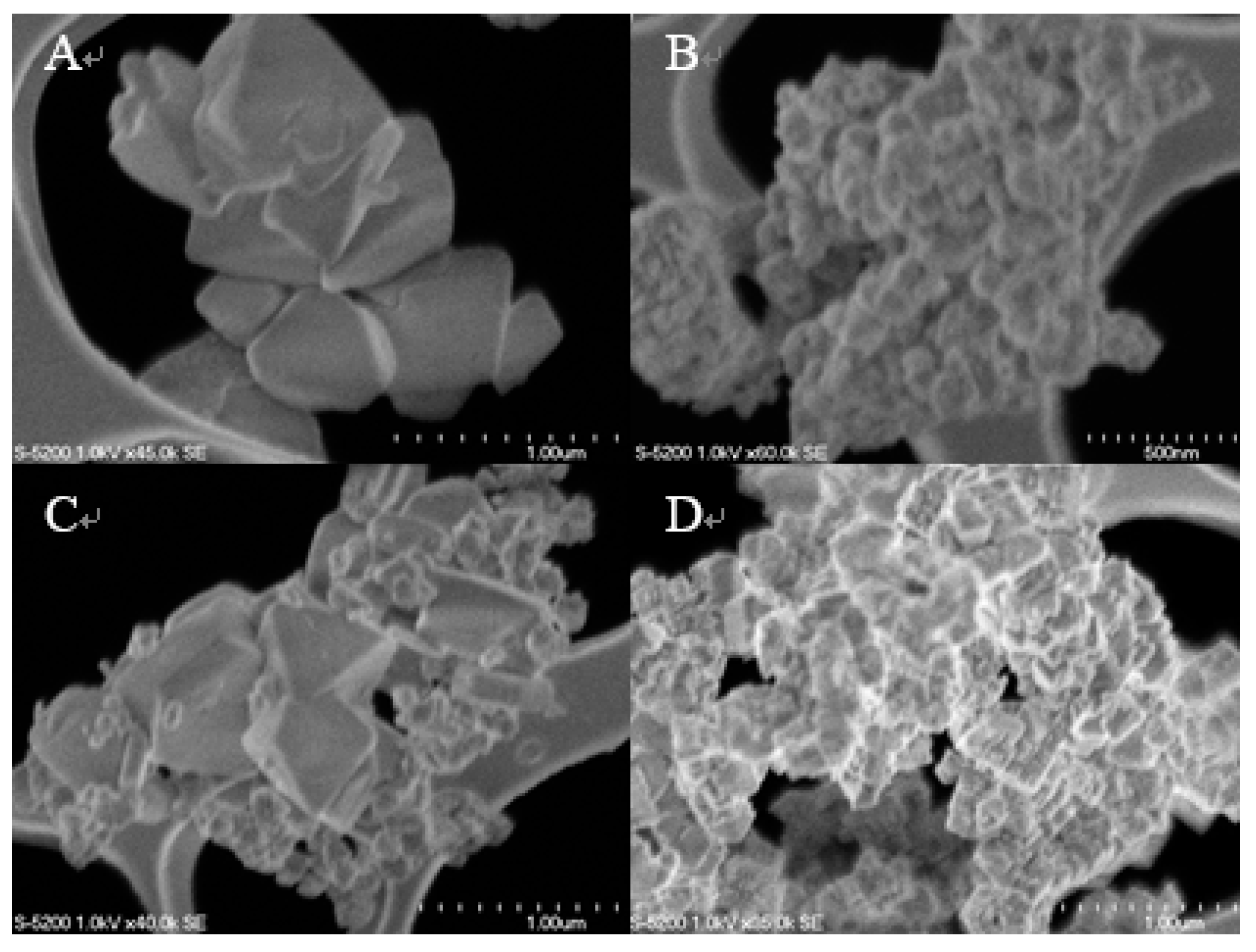
2.2. Catalyst Characterization
2.3. Catalytic Activity Measurements
3. Results and Discussion
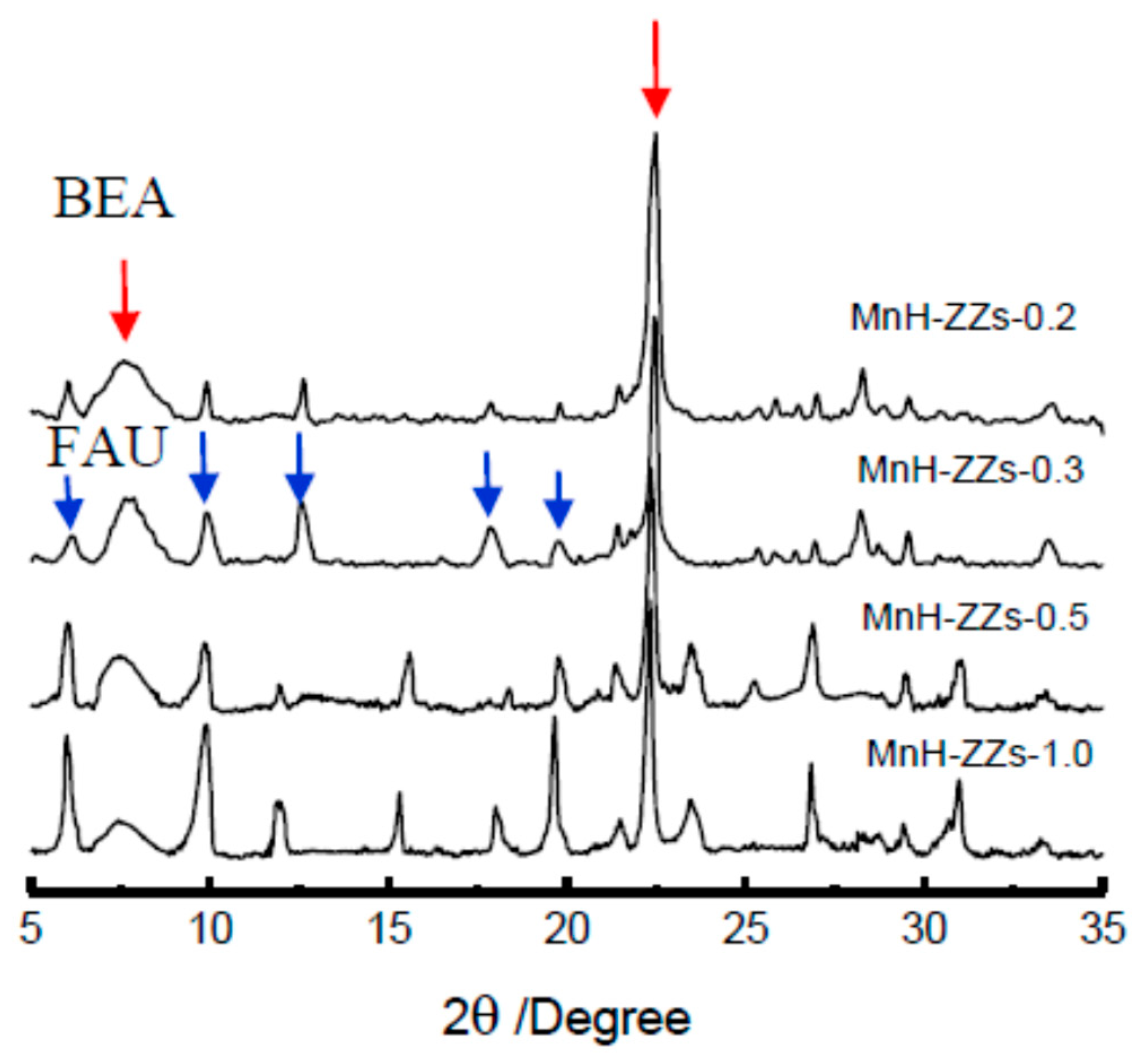
3.1. XRD Patterns of the Catalysts
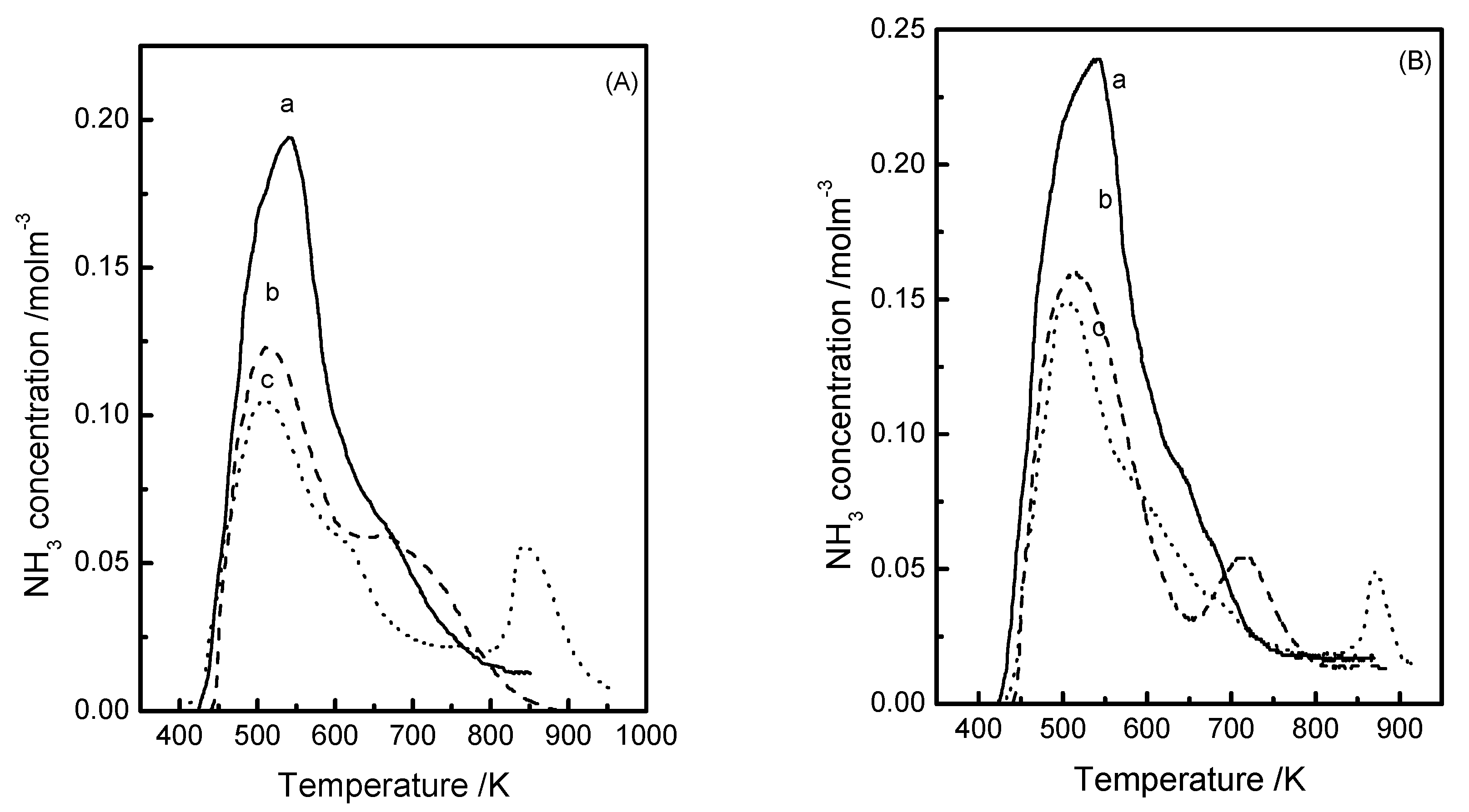
3.2. NH3-TPD Results
3.3. Catalytic Activity Studies
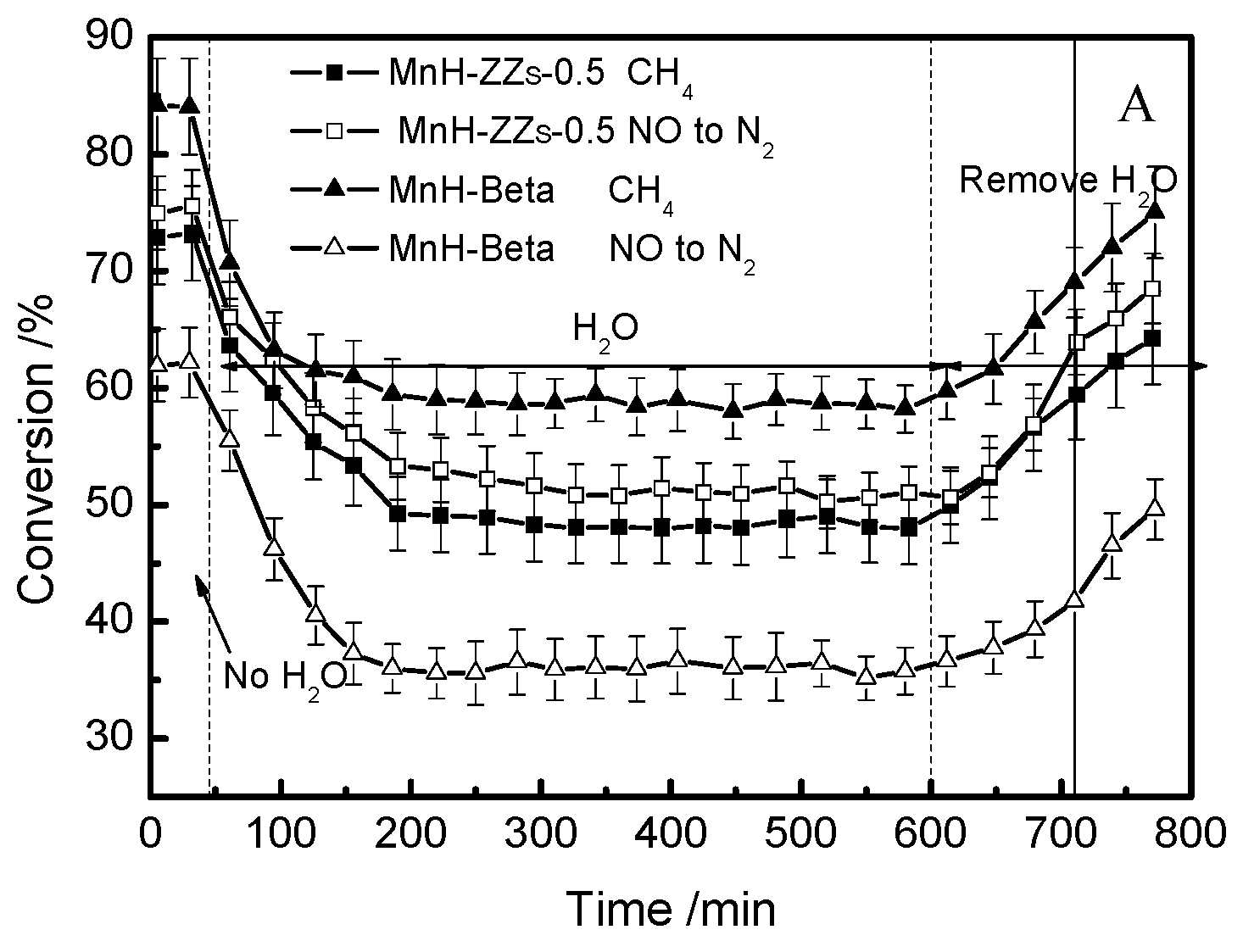
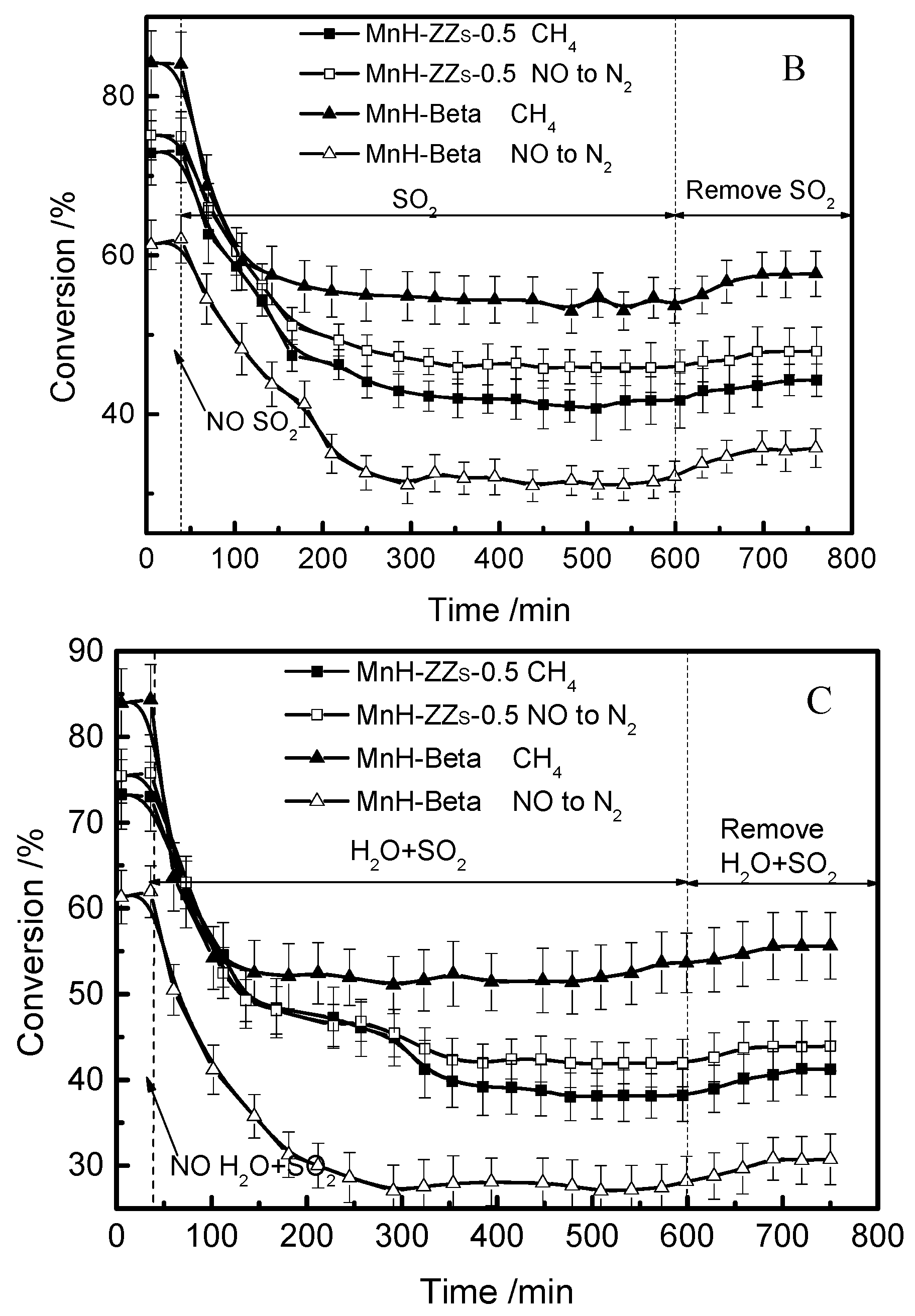
3.4. The Effects of H2O and SO2 on NO-CH4-SCR Activity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Teng, Z.; Zhang, H.; Huang, S.; Li, N.; Zhou, Q. Experimental study on reduction of NO by CH4 over La0.8 Sr0.2 MnO3/α-Al2O3 in excess of O2. J. Taiwan Inst. Chem. Eng. 2018, 87, 204–210. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Rottländer, C.; Andorf, R.; Plog, C.; Krutzsch, B.; Baerns, M. Selective NO reduction by propane and propene over a Pt/ZSM-5 catalyst: A transient study of the reaction mechanism. Appl. Catal. B Environ. 1996, 11, 49–63. [Google Scholar] [CrossRef]
- Mendes, A.N.; Zholobenko, V.L.; Thibault-Starzyk, F.; Da Costa, P.; Henriques, C. On the enhancing effect of Ce in Pd-MOR catalysts for NOx CH4-SCR: A structure-reactivity study. Appl. Catal. B Environ. 2016, 195, 121–131. [Google Scholar] [CrossRef]
- Lónyi, F.; Solt, H.E.; Valyon, J.; Boix, A.; Gutierrez, L.B. The activation of NO and CH4 for NO-SCR reaction over In- and Co-containing H-ZSM-5 catalysts. J. Mol. Catal. A Chem. 2011, 345, 75–80. [Google Scholar] [CrossRef]
- Traa, Y.; Burger, B.; Weitkamp, J. Zeolite-based materials for the selective catalytic reduction of NOx with hydrocarbons. Microporous Mesoporous Mater. 1999, 30, 3–41. [Google Scholar] [CrossRef]
- Děděcek, J.; Čapek, L.; Kaucký, D.; Sobalík, Z.; Wichterlová, B. Siting and Distribution of the Co Ions in Beta zeolite: A UV–Vis–NIR and FTIR study. J. Catal. 2002, 211, 198–207. [Google Scholar] [CrossRef]
- Resini, C.; Montanari, T.; Nappi, L.; Bagnasco, G.; Turco, M.; Busca, G.; Bregani, F.; Notaro, M.; Rocchini, G. Selective catalytic reduction of NOx by methane over Co-H-MFI and Co-H-FER zeolite catalysts: Characterisation and catalytic activity. J. Catal. 2003, 214, 179–190. [Google Scholar] [CrossRef]
- Aylor, A.W.; Lobree, L.J.; Reimer, J.A.; Bell, A.J. NO Adsorption, desorption, and reduction by CH4 over Mn-ZSM-5. J. Catal. 1997, 170, 390–401. [Google Scholar] [CrossRef]
- Campa, M.C.; Pietrogiacomi, D.; Tuti, S.; Ferraris, G.; Indovina, V. The selective catalytic reduction of NOx with CH4 on Mn-ZSM5: A comparison with Co-ZSM5 and Cu-ZSM5. Appl. Catal. B Environ. 1998, 18, 151–162. [Google Scholar] [CrossRef]
- Sun, Q.; Sachtler, W.M.H. Mn/MFI catalyzed reduction of NOx with alkanes. Appl. Catal. B Environ. 2003, 42, 393–401. [Google Scholar] [CrossRef]
- Costilla, I.O.; Sanchez, M.D.; Volpe, M.A.; Gigolaa, C.E. Ce effect on the selective catalytic reduction of NO with CH4 on Pd-mordenite in the presence of O2 and H2O. Catal. Today 2011, 172, 84–89. [Google Scholar] [CrossRef]
- Campa, M.C.; Pietrogiacomi, D.; Occhiuzzi, M. The simultaneous selective catalytic reduction of N2O and NOX with CH4 on Co- and Ni-exchanged mordenite. Appl. Catal. B Environ. 2015, 168, 293–302. [Google Scholar] [CrossRef]
- Pan, H.; Jian, Y.; Yu, Y.; He, C.; Shen, Z.; Liu, H. Regeneration and sulfur poisoning behavior of In/H-BEA catalyst for NOx reduction by CH4. Appl. Surf. Sci. 2017, 401, 120–126. [Google Scholar] [CrossRef]
- Guo, W.; Huang, L.; Deng, P.; Xue, Z.; Li, Q. Characterization of Beta/MCM-41 composite molecular sieve compared with the mechanical mixture. Microporous Mesoporous Mater. 2001, 44, 427–434. [Google Scholar] [CrossRef]
- Liu, H.; Bao, X.; Wei, W.; Shi, G. Synthesis and characterization of kaolin/NaY/MCM-41 composites. Microporous Mesoporous Mater. 2003, 66, 117–125. [Google Scholar] [CrossRef]
- Ooi, Y.S.; Zakaria, R.; Mohamed, A.R.; Bhatia, S. Synthesis of composite material MCM-41/Beta and its catalytic performance in waste used palm oil cracking. Appl. Catal. A Gen. 2004, 274, 15–23. [Google Scholar] [CrossRef]
- Mendes, A.N.; Matynia, A.; Toullec, A.; Capela, S.; Ribeiro, M.F.; Henriques, C.; Costa, P.D. Potential synergic effect between MOR and BEA zeolites in NOx SCRwith methane: A dual bed design approach. App. Catal. A Gen. 2015, 506, 246–253. [Google Scholar] [CrossRef]
- Li, Y.; Battavio, P.J.; Armor, J.N. Effect of water vapor on the selective reduction of NO by methane over cobalt-exchanged ZSM-5. J. Catal. 1993, 142, 561–571. [Google Scholar] [CrossRef]
- Boningari, T.; Pavani, S.M.; Ettireddy, P.R.; Chuang, S.S.C.; Smirniotis, P.G. Mechanistic investigations on NO reduction with CO over Mn/TiO2 catalyst at low temperatures. Mol. Catal. 2018, 451, 33–42. [Google Scholar] [CrossRef]
- Damma, D.; Boningari, T.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. Direct Decomposition of NOx over TiO2 Supported transition metal oxides at low temperatures. Ind. Eng. Chem. Res. 2018, 57, 16615–16621. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Smirniotis, P.G. Metal oxide-confined interweaved titania nanotubes M/TNT (M = Mn, Cu, Ce, Fe, V, Cr, and Co) for the selective catalytic reduction of NOx in the presence of excess oxygen. J. Catal. 2018, 365, 320–333. [Google Scholar] [CrossRef]
- Eapen, M.J.; Reddy, K.S.N.; Shiralkar, V.P. Hydrothermal crystallization of zeolite beta using tetraethylammonium bromide. Zeolites 1994, 14, 295–302. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Katada, N.; Niwa, M. Acidity of β zeolite with different Si/Al2 ratio as measured by temperature programmed desorption of ammonia. Microporous Mesoporous Mater. 2000, 40, 271–281. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, W.; Liu, Y.; Li, R. Synthesis and catalytic property of a Co2+-exchanged Beta/Y composite for the selective catalytic reduction of NO by CH4 in the presence of excess oxygen. Appl. Catal. B Environ. 2007, 76, 174–184. [Google Scholar] [CrossRef]
- Primo, A.; Garcia, H. Zeolites as catalysts in oil refining. Chem. Soc. Rev. 2014, 43, 7548–7561. [Google Scholar] [CrossRef]
- Kaucký, D.; Vondrovà, A.; Děděcek, J.; Wichterlová, B. Activity of Co ion sites in ZSM-5, Ferrierite, and Mordenite in selective catalytic reduction of NO with methane. J. Catal. 2000, 194, 318–329. [Google Scholar] [CrossRef]
- Armor, J.N. Catalytic reduction of nitrogen oxides with methane in the presence of excess oxygen: A review. Catal. Today 1995, 26, 147–158. [Google Scholar] [CrossRef]
- Armaroli, T.; Trombetta, M.; Gutierrez, A.A.; Ramirez, S.J.; Busca, G. FTIR study of the interaction of some branched aliphatic molecules with the external and internal sites of H-ZSM5 zeolite. Phys. Chem. Chem. Phys. 2000, 2, 3341–3348. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Boningari, T.; Ettireddy, P.R.; Somogyvari, A.; Liu, Y.; Vorontsov, A.; McDonald, C.A.; Smirniotis, P.G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. J. Catal. 2015, 325, 145–155. [Google Scholar] [CrossRef]
- Ciambelli, P.; Sannino, D.; Palo, E.; Ruggiero, A. Improved stability of Co-Ferrierite catalyst by Mn in dry–wet cycles of lean CH4-SCR of NOx. Top. Catal. 2007, 42, 177–181. [Google Scholar] [CrossRef]
| Catalyst | Si/Al | Ion-Exchange Condition a | Mn wt. % | Mn/Al | SBET (m2∙g−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| T (K) | C (mol∙L−1) | L/S | t (h) | N | |||||
| MnH-Y | 2.0 | 353 | 0.01 | 20 | 24 | 3 | 2.55 | 0.12 | 576 |
| MnH-Beta | 15.0 | 353 | 0.01 | 20 | 24 | 3 | 2.12 | 0.38 | 405 |
| H-ZZs-0.5 | 12.2 | 353 | — | — | — | — | 0 | 0 | 420 |
| MnNa-ZZs-0.5 | 12.2 | 353 | 0.01 | 20 | 24 | 3 | 2.07 | 0.37 | 428 |
| MnH-ZZs-1.0 | 10.8 | 353 | 0.01 | 20 | 24 | 3 | 2.27 | 0.31 | 379 |
| MnH-ZZs-0.5 | 12.2 | 353 | 0.01 | 20 | 24 | 3 | 2.15 | 0.38 | 359 |
| MnH-ZZs-0.3 | 13.1 | 353 | 0.01 | 20 | 24 | 3 | 2.16 | 0.38 | 365 |
| MnH-ZZs-0.2 | 14.5 | 353 | 0.01 | 20 | 24 | 3 | 2.23 | 0.39 | 363 |
| MnH-ZZs-0.5–1 | 12.2 | 353 | 0.01 | 20 | 24 | 2 | 1.82 | 0.32 | 359 |
| MnH-ZZs-0.5–2 | 12.2 | 353 | 0.01 | 20 | 24 | 1 | 1.27 | 0.25 | 356 |
| MnH-ZZs-0.5–3 | 12.2 | 353 | 0.008 | 20 | 24 | 1 | 0.92 | 0.17 | 354 |
| Sample | NO Conversion to N2 (CH4 Conversion to CO2) (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 573 K | 623 K | 673 K | 723 K | 773 K | 823 K | 873 K | 923 K | |
| MnH-ZZs-1.0 | 3.4 ± 0.14 | 5.6 ± 0.23 | 20.5 ± 1.01 | 47.6 ± 2.00 | 69.0 ± 3.01 | 74.6 ± 3.56 | 71.8 ± 3.20 | 66.5 ± 3.01 |
| (1.3 ± 0.11) | (3.2 ± 0.21) | (15.5 ± 0.78) | (39.8 ± 1.99) | (66.4 ± 3.60) | (84.4 ± 5.49) | (98.4 ± 6.00) | (100.0 ± 0.36) | |
| MnH-ZZs-0.5 | 2.8 ± 0.13 | 5.4 ± 0.26 | 21.6 ± 1.00 | 54.8 ± 2.61 | 75.5 ± 3.11 | 77.3 ± 3.66 | 72.8 ± 2.90 | 68.7 ± 3.00 |
| (1.6 ± 0.11) | (2.8 ± 0.18) | (14.8 ± 0.74) | (42.4 ± 2.10) | (73.2 ± 4.06) | (85.4 ± 5.25) | (99.4 ± 6.01) | (100.0 ± 0.50) | |
| MnH-ZZs-0.3 | 2.5 ± 0.11 | 4.8 ± 0.22 | 17.5 ± 0.81 | 36.5 ± 1.62 | 57.8 ± 2.25 | 65.8 ± 3.29 | 61.6 ± 2.10 | 54.4 ± 2.45 |
| (1.4 ± 0.09) | (3.1 ± 0.20) | (11.4 ± 0.56) | (26.2 ± 1.29) | (58.5 ± 0.76) | (80.2 ± 5.10) | (98.2 ± 5.95) | (100.0 ± 0.42) | |
| MnH-ZZs-0.2 | 2.1 ± 0.10 | 3.6 ± 0.16 | 10.5 ± 0.49 | 25.5 ± 1.05 | 41.8 ± 1.80 | 46.8 ± 2.36 | 47.4 ± 1.01 | 43.4 ± 1.98 |
| (1.2 ± 0.08) | (3.0 ± 0.19) | (8.7 ± 0.43) | (22.6 ± 1.10) | (50.7 ± 2.82) | (73.6 ± 4.66) | (93.2 ± 7.50) | (100.0 ± 0.36) | |
| H-ZZs-0.5 | 0.9 ± 0.09 | 1.5 ± 0.09 | 3.7 ± 0.15 | 10.8 ± 0.45 | 17.3 ± 0.71 | 20.5 ± 1.03 | 22.1 ± 0.53 | 23.9 ± 1.01 |
| (0.1 ± 0.01) | (0.2 ± 0.02) | (1.6 ± 0.10) | (6.2 ± 0.70) | (14.2 ± 0.80) | (25.1 ± 1.93) | (30.2 ± 2.51) | (34.8 ± 1.50) | |
| MnH-Y | 2.5 ± 0.11 | 3.9 ± 0.18 | 4.7 ± 0.19 | 5.7 ± 0.25 | 6.4 ± 0.30 | 6.7 ± 0.33 | 10.6 ± 2.21 | 11.9 ± 0.53 |
| (2.7 ± 0.19) | (3.1 ± 0.20) | (2.8 ± 0.13) | (2.9 ± 0.32) | (7.3 ± 0.45) | (11.4 ± 1.71) | (17.6 ± 1.47) | (23.9 ± 1.00) | |
| MnH-Beta | 3.6 ± 0.15 | 7.0 ± 0.20 | 19.2 ± 0.82 | 47.4 ± 2.01 | 61.1 ± 3.00 | 56.3 ± 2.56 | 49.7 ± 2.30 | - |
| (2.4 ± 0.17) | (4.4 ± 0.27) | (14.7 ± 0.70) | (46.2 ± 2.15) | (84.2 ± 4.10) | (100.0 ± 0.60) | (100.0 ± 0.34) | ||
| MnNa-ZZs-0.5 | 2.8 ± 0.12 | 4.3 ± 0.20 | 21.1 ± 0.76 | 49.9 ± 2.12 | 60.1 ± 2.90 | 55.4 ± 2.61 | 41.2 ± 1.98 | - |
| (3.1 ± 0.21) | (4.6 ± 0.28) | (18.6 ± 0.90) | (44.7 ± 2.20) | (86.4 ± 0.10) | (99.8 ± 5.99) | (100.0 ± 0.46) | ||
| MnH-ZZm-1.0 | 4.0 ± 0.14 | 5.8 ± 0.19 | 16.8 ± 0.75 | 46.2 ± 2.00 | 56.8 ± 2.52 | 56.1 ± 2.56 | 56.4 ± 2.41 | - |
| (1.3 ± 0.09) | (4.6 ± 0.27) | (17.5 ± 0.87) | (36.4 ± 1.65) | (70.8 ± 2.85) | (98.5 ± 5.85) | (100.0 ± 0.35) | ||
| MnH-ZZm-0.5 | 3.1 ± 0.14 | 4.9 ± 0.24 | 12.7 ± 0.59 | 35.1 ± 1.56 | 55.6 ± 2.45 | 56.6 ± 2.57 | 51.7 ± 2.36 | - |
| (1.3 ± 0.09) | (2.2 ± 0.14) | (16.6 ± 0.82) | (28.6 ± 1.39) | (67.9 ± 2.80) | (93.2 ± 6.56) | (100.0 ± 0.42) | ||
| MnH-ZZm-0.3 | 2.4 ± 0.14 | 3.6 ± 0.22 | 11.7 ± 0.50 | 26.1 ± 1.10 | 43.7 ± 1.95 | 51.1 ± 2.42 | 50.4 ± 2.35 | 41.0 ± 1.90 |
| (1.2 ± 0.10) | (2.1 ± 0.13) | (12.1 ± 0.61) | (2.35 ± 0.10) | (45.3 ± 2.25) | (81.5 ± 5.25) | (95.8 ± 5.10) | (100.0 ± 0.45) | |
| MnH-ZZm-0.2 | 2.4 ± 0.14 | 3.8 ± 0.12 | 5.9 ± 0.21 | 17.7 ± 0.71 | 33.2 ± 1.59 | 43.7 ± 2.19 | 49.1 ± 2.32 | 47.4 ± 2.10 |
| (1.2 ± 0.09) | (1.9 ± 0.12) | (6.4 ± 0.32) | (14.8 ± 1.35) | (29.5 ± 1.60) | (58.6 ± 4.20) | (86.0 ± 4.98) | (99.5 ± 0.14) | |
| Sample | NO Conversion to N2 (CH4 Conversion to CO2) (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 573 K | 623 K | 673 K | 723 K | 773 K | 823 K | 873 K | 923 K | |
| MnNa-ZZs-0.5 | 2.8 ± 0.12 | 4.3 ± 0.20 | 21.1 ± 0.76 | 49.9 ± 2.12 | 60.1 ± 2.90 | 55.4 ± 2.61 | 41.2 ± 1.98 | - |
| (3.1 ± 0.21) | (4.6 ± 0.28) | (18.6 ± 0.90) | (44.7 ± 2.20) | (86.4 ± 0.10) | (99.8 ± 5.99) | (100.0 ± 0.46) | ||
| MnH-ZZs-0.5 | 2.8 ± 0.13 | 5.4 ± 0.26 | 21.6 ± 1.00 | 54.8 ± 2.61 | 75.5 ± 3.11 | 77.3 ± 3.66 | 72.8 ± 2.90 | 68.7 ± 3.00 |
| (1.6 ± 0.11) | (2.8 ± 0.18) | (14.8 ± 0.74) | (42.4 ± 2.10) | (73.2 ± 4.06) | (85.4 ± 5.25) | (99.4 ± 6.01) | (100.0 ± 0.50) | |
| MnH-ZZs-0.5-1 | 2.4 ± 0.18 | 4.2 ± 1.13 | 16.9 ± 1.13 | 43.4 ± 2.71 | 55.1 ± 3.30 | 59.8 ± 3.60 | 62.6 ± 3.21 | 56.9 ± 3.01 |
| (1.2 ± 0.10) | (3.6 ± 0.80) | (11.6 ± 0.81) | (31.9 ± 2.01) | (63.3 ± 3.50) | (81.4 ± 5.00) | (99.8 ± 5.52) | (100.0 ± 0.51) | |
| MnH-ZZs-0.5-2 | 2.3 ± 0.16 | 4.5 ± 0.93 | 13.9 ± 0.95 | 33.2 ± 2.10 | 48.1 ± 2.90 | 53.9 ± 3.45 | 55.3 ± 2.95 | 50.6 ± 2.61 |
| (1.3 ± 0.11) | (3.5 ± 0.75) | (10.7 ± 0.75) | (26.5 ± 1.81) | (47.6 ± 2.81) | (79.7 ± 4.95) | (91.2 ± 5.01) | (100.0 ± 0.46) | |
| MnH-ZZs-0.5-3 | 2.5 ± 0.17 | 3.9 ± 0.75 | 10.9 ± 0.80 | 26.6 ± 1.72 | 38.1 ± 2.15 | 43.2 ± 2.81 | 45.6 ± 2.60 | 45.2 ± 2.41 |
| (1.1 ± 0.09) | (3.3 ± 0.65) | (8.3 ± 0.66) | (24.7 ± 1.71) | (36.2 ± 2.16) | (65.7 ± 4.01) | (85.4 ± 4.80) | (100.0 ± 0.50) | |
| H-ZZs-0.5 | 0.9 ± 0.09 | 1.5 ± 0.09 | 3.7 ± 0.15 | 10.8 ± 0.45 | 17.3 ± 0.71 | 20.5 ± 1.03 | 22.1 ± 0.53 | 23.9 ± 1.01 |
| (0.1 ± 0.01) | (0.2 ± 0.02) | (1.6 ± 0.10) | (6.2 ± 0.70) | (14.2 ± 0.80) | (25.1 ± 1.93) | (30.2 ± 2.51) | (34.8 ± 1.50) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Q.; Wang, D.; Yan, X. NO-CH4-SCR Over Core-Shell MnH-Zeolite Composites. Appl. Sci. 2019, 9, 1773. https://doi.org/10.3390/app9091773
Li Y, Wang Q, Wang D, Yan X. NO-CH4-SCR Over Core-Shell MnH-Zeolite Composites. Applied Sciences. 2019; 9(9):1773. https://doi.org/10.3390/app9091773
Chicago/Turabian StyleLi, Yixiao, Quanhua Wang, Ding Wang, and Xiaoliang Yan. 2019. "NO-CH4-SCR Over Core-Shell MnH-Zeolite Composites" Applied Sciences 9, no. 9: 1773. https://doi.org/10.3390/app9091773
APA StyleLi, Y., Wang, Q., Wang, D., & Yan, X. (2019). NO-CH4-SCR Over Core-Shell MnH-Zeolite Composites. Applied Sciences, 9(9), 1773. https://doi.org/10.3390/app9091773




