Separation of N–C5H12–C9H20 Paraffins Using Boehmite by Inverse Gas Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Boehmite
2.2. Characterization Techniques
2.3. IGC Determinations
3. Results
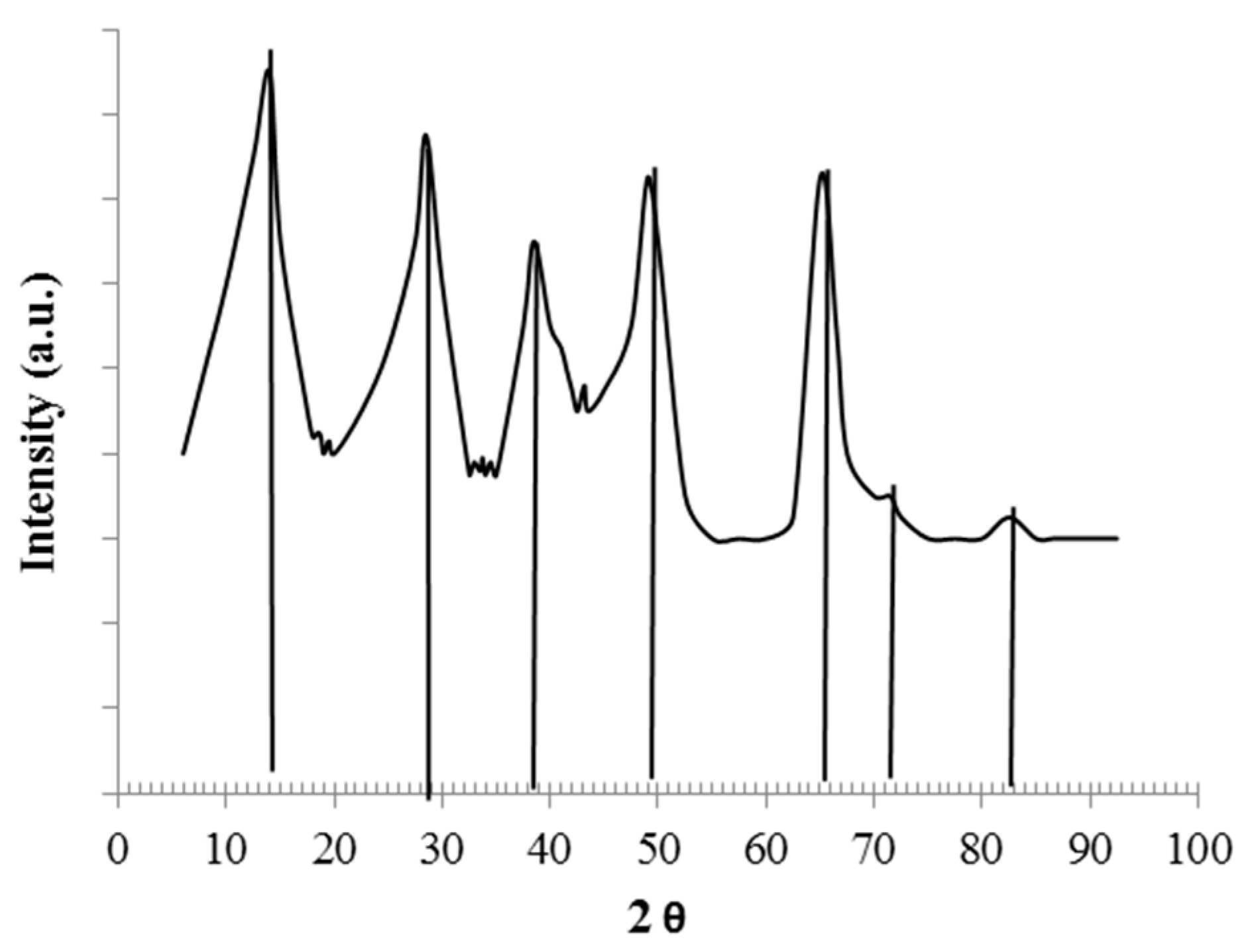
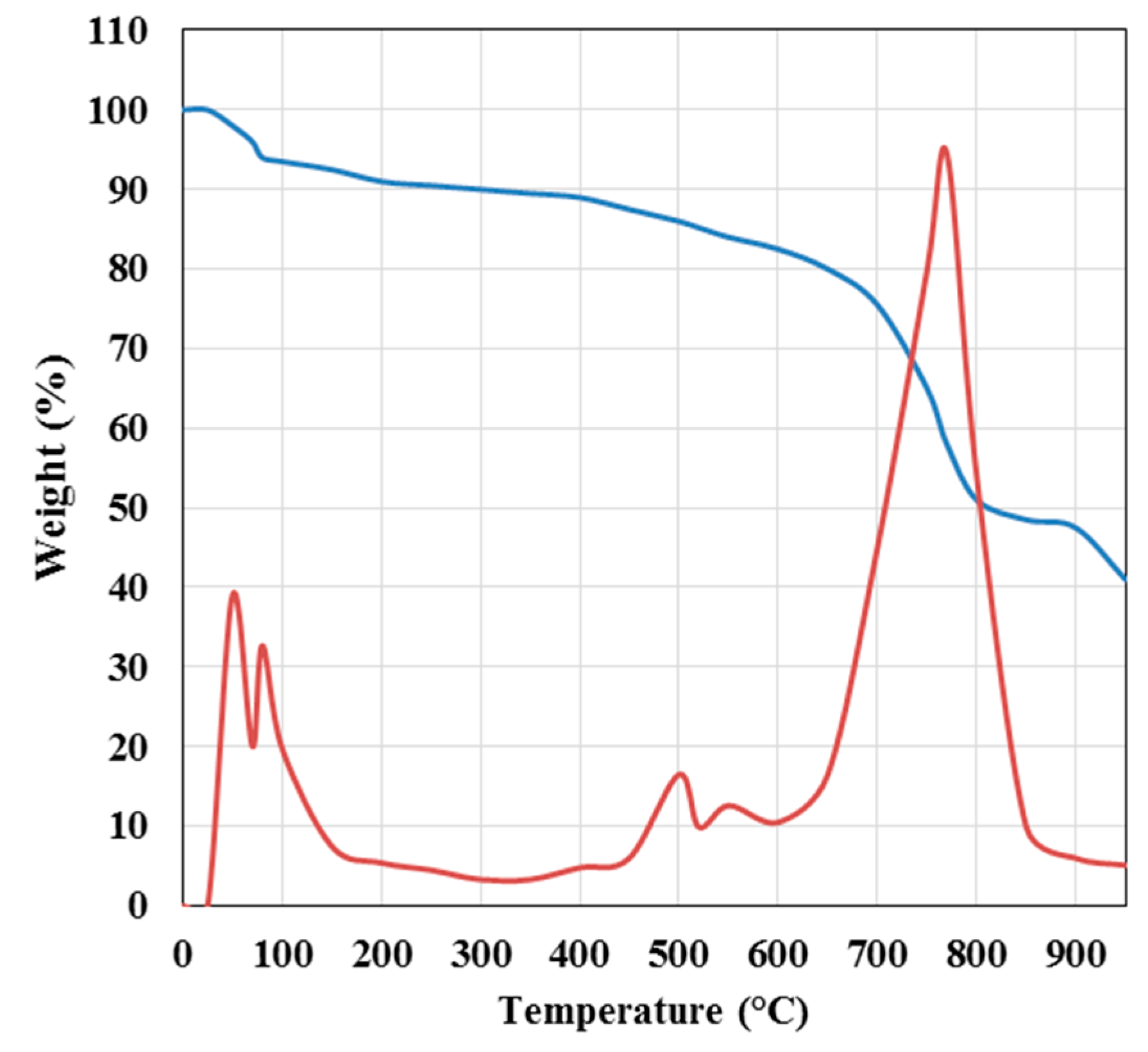
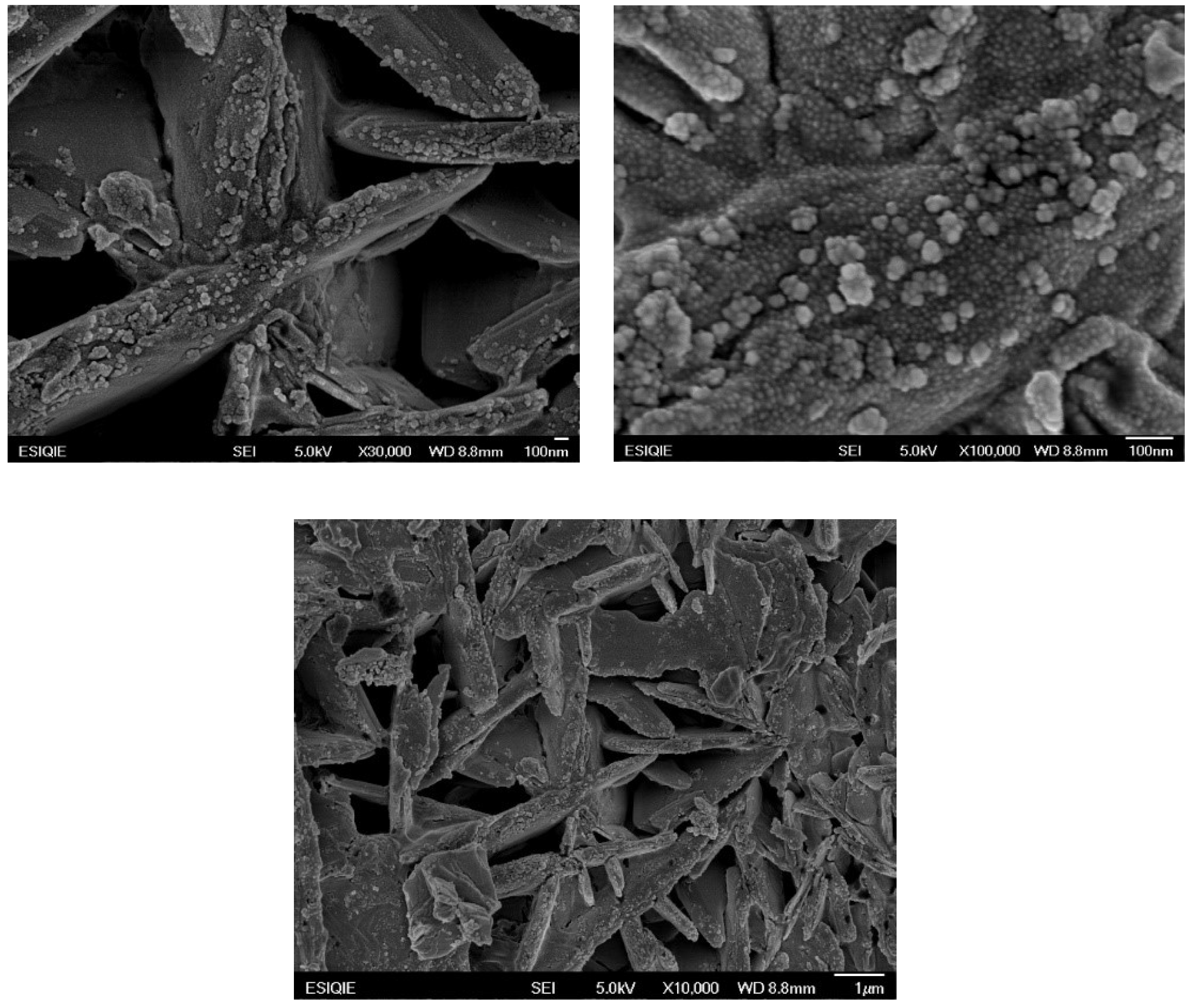
3.1. Samples Characterization
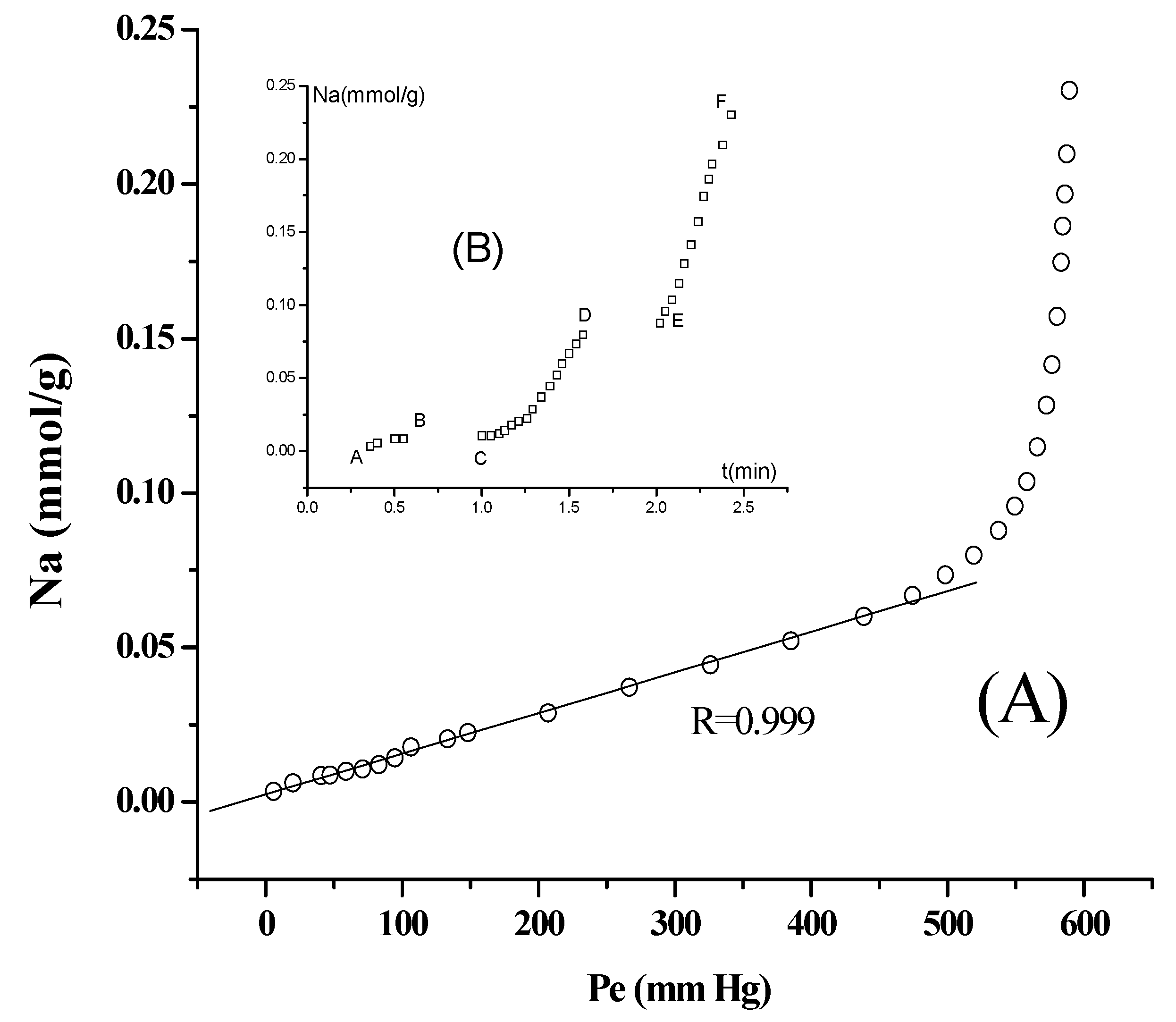
3.2. Separation of n-Paraffins C5–C9 by IGC
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, D.Y.; Lin, Y.S.; Li, Y.C.; Shieh, D.L.; Lin, J.L. Synthesis of mesoporous pseudoboehmite and alumina templated with 1-hexadecyl-2,3-dimethyl-imidazolium chloride. Microporous Mesoporous Mater. 2008, 108, 276–282. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Han, B.; Xu, B.; Liu, X.; Yan, Z. Effects of synthetic conditions on the textural structure of pseudo-boehmite. J. Colloid Interface Sci. 2016, 469, 1–7. [Google Scholar] [CrossRef]
- Al-Khattaf†, S. The Influence of Alumina on the Performance of FCC Catalysts during Hydrotreated VGO Catalytic Cracking. Energy Fuels 2002, 17, 62–68. [Google Scholar] [CrossRef]
- Kwak, J.H.; Mei, D.; Peden, C.H.F.; Rousseau, R.; Szanyi, J. (100) facets of γ-Al2O3: The active surfaces for alcohol dehydration reactions. Catal. Lett. 2011, 141, 649–655. [Google Scholar] [CrossRef]
- Keyvanloo, K.; Mardkhe, M.K.; Alam, T.M.; Bartholomew, C.H.; Woodfield, B.F.; Hecker, W.C. Supported Iron Fischer–Tropsch Catalyst: Superior Activity and Stability Using a Thermally Stable Silica-Doped Alumina Support. ACS Catal. 2014, 4, 1071–1077. [Google Scholar] [CrossRef]
- Benítez Guerrero, M.; Pérez-Maqueda, L.A.; Castro, P.P.; Pascual Cosp, J. Alúminas porosas: El método de bio-réplica para la síntesis de alúminas estables de alta superficie específica. Bol. Soc. Esp. Ceram. Vidrio 2013, 52, 251–267. [Google Scholar] [CrossRef]
- Sanchez-Valente, J.; Bokhimi, X.; Toledo, J. Synthesis and catalytic properties of nanostructured aluminas obtained by sol–gel method. Appl. Catal. A Gen. 2004, 264, 175–181. [Google Scholar] [CrossRef]
- García, G.; Falco, M.; Crespo, P.; Cabrera, S.; Sedran, U. Characterization and catalytic evaluation of aluminum oxides obtained by the atrane route. Catal. Today 2011, 166, 60–66. [Google Scholar] [CrossRef]
- Chen, J.-F.; Shao, L.; Guo, F.; Wang, X.-M. Synthesis of nano-fibers of aluminum hydroxide in novel rotating packed bed reactor. Chem. Eng. Sci. 2003, 58, 569–575. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, A.-X.; Luo, C.; Sun, L.-D.; Zhang, Y.-W.; Duan, W.-T.; Liu, H.-C.; Yan, C.-H. Facile Synthesis for Ordered Mesoporous γ-Aluminas with High Thermal Stability. J. Am. Chem. Soc. 2008, 130, 3465–3472. [Google Scholar] [CrossRef]
- Voelkel, A.; Strzemiecka, B.; Adamska, K.; Milczewska, K. Inverse gas chromatography as a source of physiochemical data. J. Chromatogr. A 2009, 1216, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Batko, K.; Voelkel, A. Inverse gas chromatography as a tool for investigation of nanomaterials. J. Colloid Interface Sci. 2007, 315, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Papirer, E.; Perrin, J.-M.; Siffert, B.; Philipponneau, G. Surface characteristics of aluminas in relation with polymer adsorption. J. Colloid Interface Sci. 1991, 144, 263–270. [Google Scholar] [CrossRef]
- Onjia, A.E.; Milonjić, S.K.; Rajaković, L.V. Inverse gas chromatography of chromia. Part I. Zero surface coverage. J. Serbian Chem. Soc. 2001, 66, 259–271. [Google Scholar] [CrossRef]
- Onjia, A.E.; Milonjić, S.K.; Rajaković, L.V. Inverse gas chromatography of chromia. Part II. Finite surface coverage. J. Serbian Chem. Soc. 2002, 67, 165–178. [Google Scholar] [CrossRef]
- Rials, T.G.; Simonsen, J. Investigating interphase development in woodpolymer composites by inverse gas chromatography. Compos. Interfaces 2000, 7, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Sain, M. The effect of chemically coated nanofiber reinforcement on biopolymer based nanocomposites. BioResources 2007, 2, 371–388. [Google Scholar]
- Autie-Castro, G.; Autie, M.; Reguera, E.; Santamaría-González, J.; Moreno-Tost, R.; Rodríguez-Castellón, E.; Jiménez-López, A. Adsorption and separation of light alkane hydrocarbons by porous hexacyanocobaltates (III). Surf. Interface Anal. 2009, 41, 730–734. [Google Scholar] [CrossRef]
- Rivera, A.; Farías, T.; de Ménorval, L.C.; Autié-Castro, G.; Yee-Madeira, H.; Contreras, J.L.; Autié-Pérez, M. Acid natural clinoptilolite: Structural properties against adsorption/separation of n-paraffins. J. Colloid Interface Sci. 2011, 360, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hechevarría, H.M.; Labadie Suarez, J.M.; Santamaría-González, J.; Infantes-Molina, A.; Autié-Castro, G.; Cavalcante, C.L.; Rodríguez-Castellón, E.; Autie-Pérez, M. Adsorption and separation of propane and propylene by Cuban natural volcanic glass. Mater. Chem. Phys. 2015, 168, 132–137. [Google Scholar] [CrossRef]
- Autie-Pérez, M.; Infantes-Molina, A.; Cecilia, J.A.; Labadie-Suárez, J.M.; Rodríguez-Castellón, E. Separation of light liquid paraffin C5-C9 with Cuban volcanic glass previously used in copper elimination from water solutions. Appl. Sci. 2018, 8, 295. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Menendez, I. Separation of hydrocarbon gas mixtures using phenolic resin-based carbon membranes. Sep. Purif. Technol. 2002, 28, 29–41. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K. In Situ Synthesis of Thin Zeolitic–Imidazolate Framework ZIF-8 Membranes Exhibiting Exceptionally High Propylene/Propane Separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.G.; Lin, Y.S. Granulation of Sol-Gel-Derived Nanostructured Alumina. AIChE J. 1997, 43, 505–514. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, Y.S. Sol-Gel Synthesis of Silicalite/γ-Alumina Granules. Ind. Eng. Chem. Res. 2000, 39, 4944–4948. [Google Scholar] [CrossRef]
- Dubinin, M.; Stoeckli, H. Homogeneous and heterogeneous micropore structures in carbonaceous adsorbents. J. Colloid Interface Sci. 1980, 75, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Dubinin, M.M. Generalization of the theory of volume filling of micropores to nonhomogeneous microporous structures. Carbon N. Y. 1985, 23, 373–380. [Google Scholar] [CrossRef]
- Rivera, A.; Farías, T.; Charles de Meorval, L.; AutiePeez, M.; Lam, A. Natural and Sodium Clinoptilolites Submitted to Acid Treatments: Experimental and Theoretical Studies. J. Phys. Chem. C 2013, 117, 4079–4088. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, M.K.; Rhee, C.K.; Kim, W.W. Control of hydrolytic reaction of aluminum particles for aluminum oxide nanofibers. Mater. Sci. Eng. A 2004, 375–377, 1263–1268. [Google Scholar] [CrossRef]
- Aguado, J.; Escola, J.M.; Castro, M.C.; Paredes, B. Sol–gel synthesis of mesostructured γ-alumina templated by cationic surfactants. Microporous Mesoporous Mater. 2005, 83, 181–192. [Google Scholar] [CrossRef]
- Frost, R.L.; Gobac, Ž.Ž.; López, A.; Xi, Y.; Scholz, R.; Lana, C.; Lima, R.M.F. Characterization of the sulphate mineral coquimbite, a secondary iron sulphate from Javier Ortega mine, Lucanas Province, Peru - Using infrared, Raman spectroscopy and thermogravimetry. J. Mol. Struct. 2014, 1063, 251–258. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, A.; Wang, X.; Zhang, T. Morphologically controlled synthesis of mesoporous alumina. Microporous Mesoporous Mater. 2007, 100, 35–44. [Google Scholar] [CrossRef]
- Park, Y.K.; Tadd, E.H.; Zubris, M.; Tannenbaum, R. Size-controlled synthesis of alumina nanoparticles from aluminum alkoxides. Mater. Res. Bull. 2005, 40, 1506–1512. [Google Scholar] [CrossRef]
- Busca, G. The surface of transitional aluminas: A critical review. Catal. Today 2014, 226, 2–13. [Google Scholar] [CrossRef]
- Ram, S. Infrared spectral study of molecular vibrations in amorphous, nanocrystalline and AlO(OH) · αH2O bulk crystals. Infrared Phys. Technol. 2001, 42, 547–560. [Google Scholar] [CrossRef]
- Kanti Naskar, M.; Chatterjee, M. Boehmite Nanoparticles by the Two-Reverse Emulsion Technique. J. Am. Ceramic Soc. 2005, 88, 3322–3326. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Lin, J.; Song, H.; Luo, J.; Elssfah, E.M.; Ammar, E.; Huang, Y.; Ding, X.; Gao, J.; Qi, S.; Tang, C. Self-Assembly of Flowerlike AlOOH (Boehmite) 3D Nanoarchitectures. J. Phys. Chem. 2006, 110, 14249–14252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, F.; Lin, J.; Wei, S.Y.; Chen, D.; Gao, J.M.; Huang, Z.; Ding, X.X.; Tang, C. Nanoparticles assembly of boehmite nanofibers without a surfactant. Mater. Res. Bull. 2008, 43, 1709–1715. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, D.; Han, X.; Bao, X.; Frandsen, W.; Wang, D.; Su, D. Hydrothermal synthesis of microscale boehmite and gamma nanoleaves alumina. Mater. Lett. 2008, 62, 1297–1301. [Google Scholar] [CrossRef] [Green Version]
- Elizalde-González, M.P.; Pérez-Cruz, M.A. Interaction between organic vapors and clinoptilolite–mordenite rich tuffs in parent, decationized, and lead exchanged forms. J. Colloid Interface Sci. 2007, 312, 317–325. [Google Scholar] [CrossRef]
- Christoph, G.G.; Corbato, C.E.; Hofmann, D.A.; Tettenhorst, R.T. Crystal Structure of Boehmite. Clays Clay Miner. 1979, 27, 81–86. [Google Scholar] [CrossRef]
- Hill, R.J. Hydrogen Atoms in Boehmite. a Single Crystal X-Ray Diffraction and Molecular Orbital Study. Clays Clay Miner. 1981, 29, 435. [Google Scholar] [CrossRef]
- Tettenhorst, R.; Hofmann, D.A. Crystal Chemistry of Boehmite. Clays Clay Miner. 1980, 28, 373–380. [Google Scholar] [CrossRef]

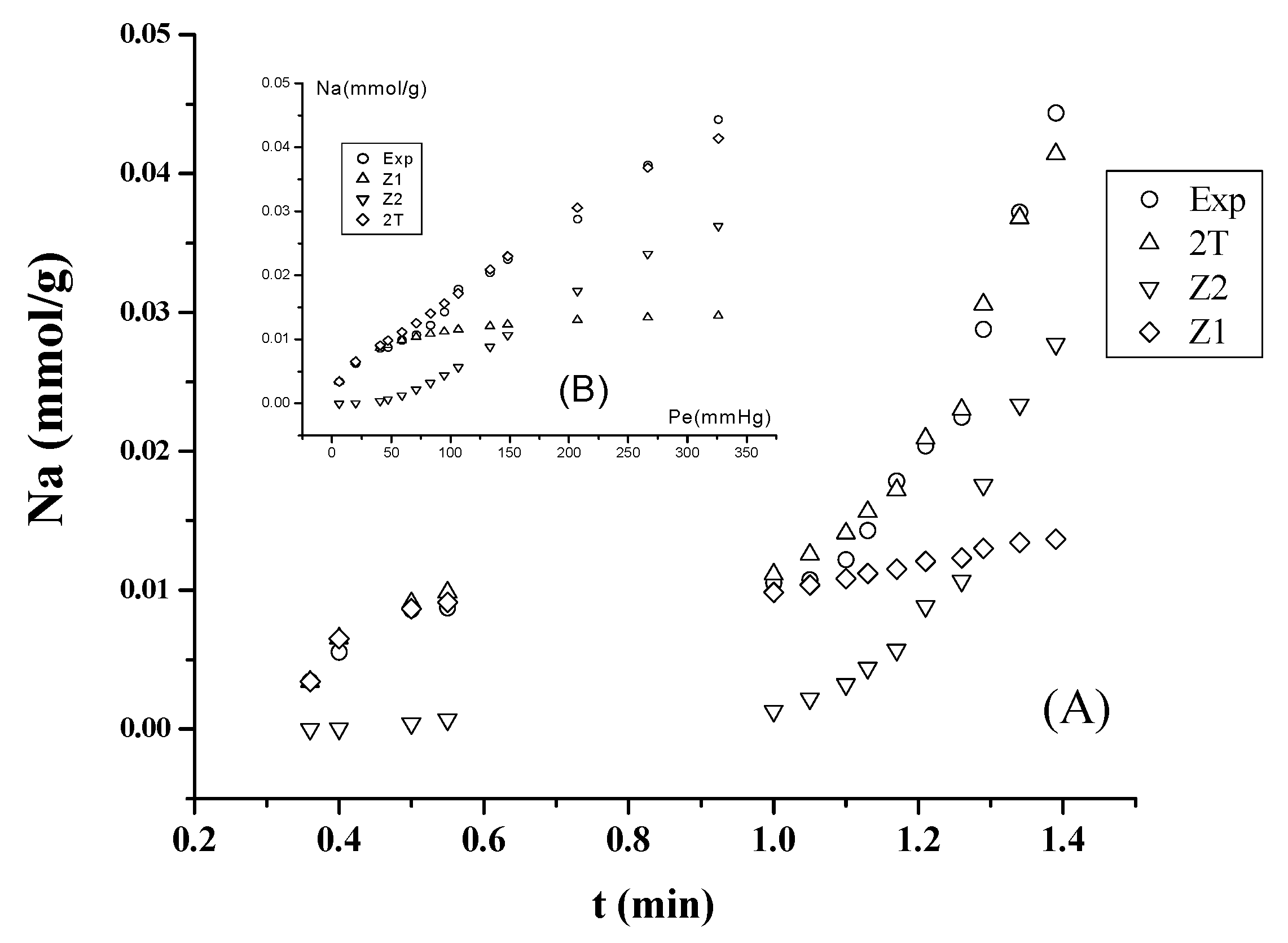
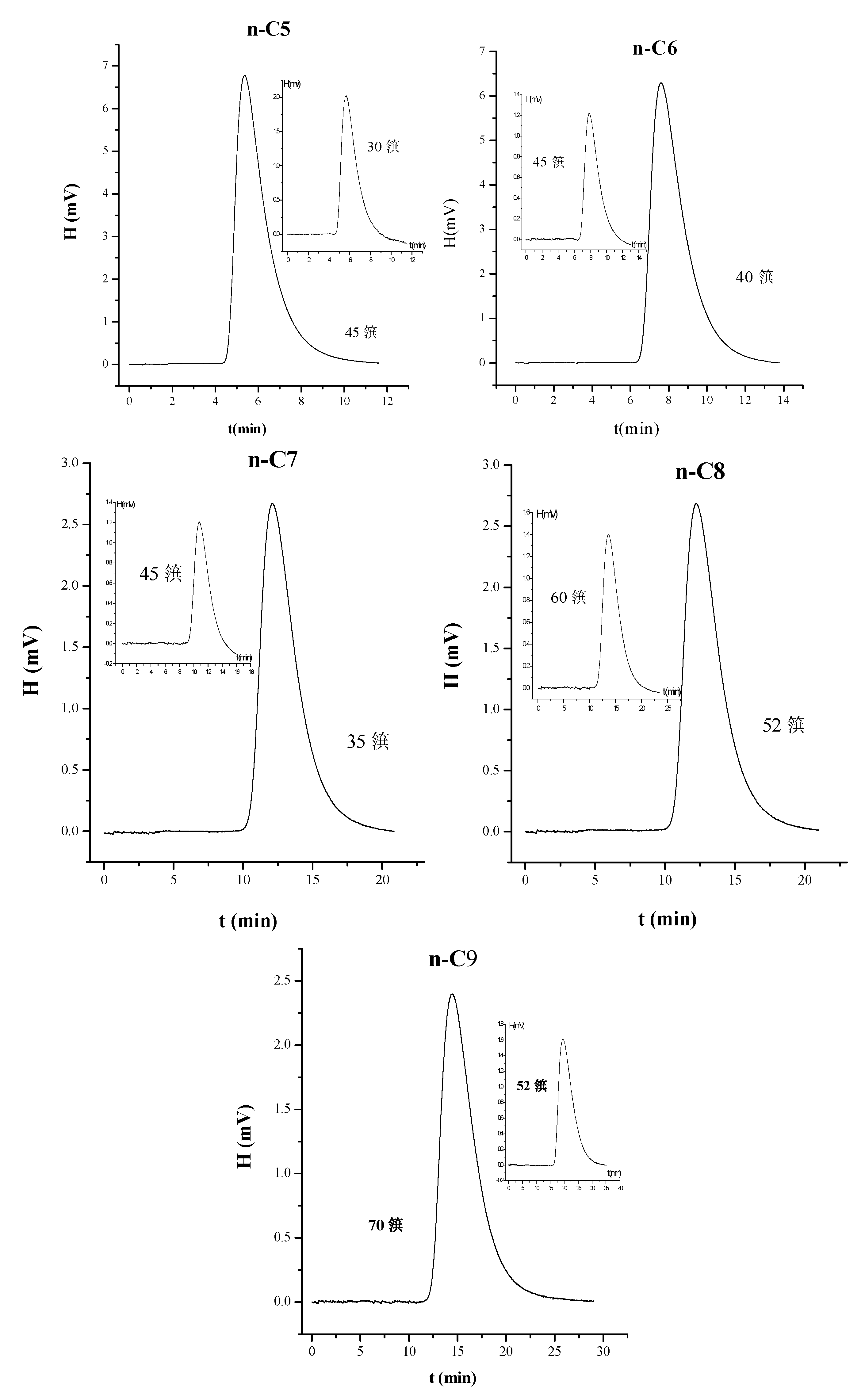
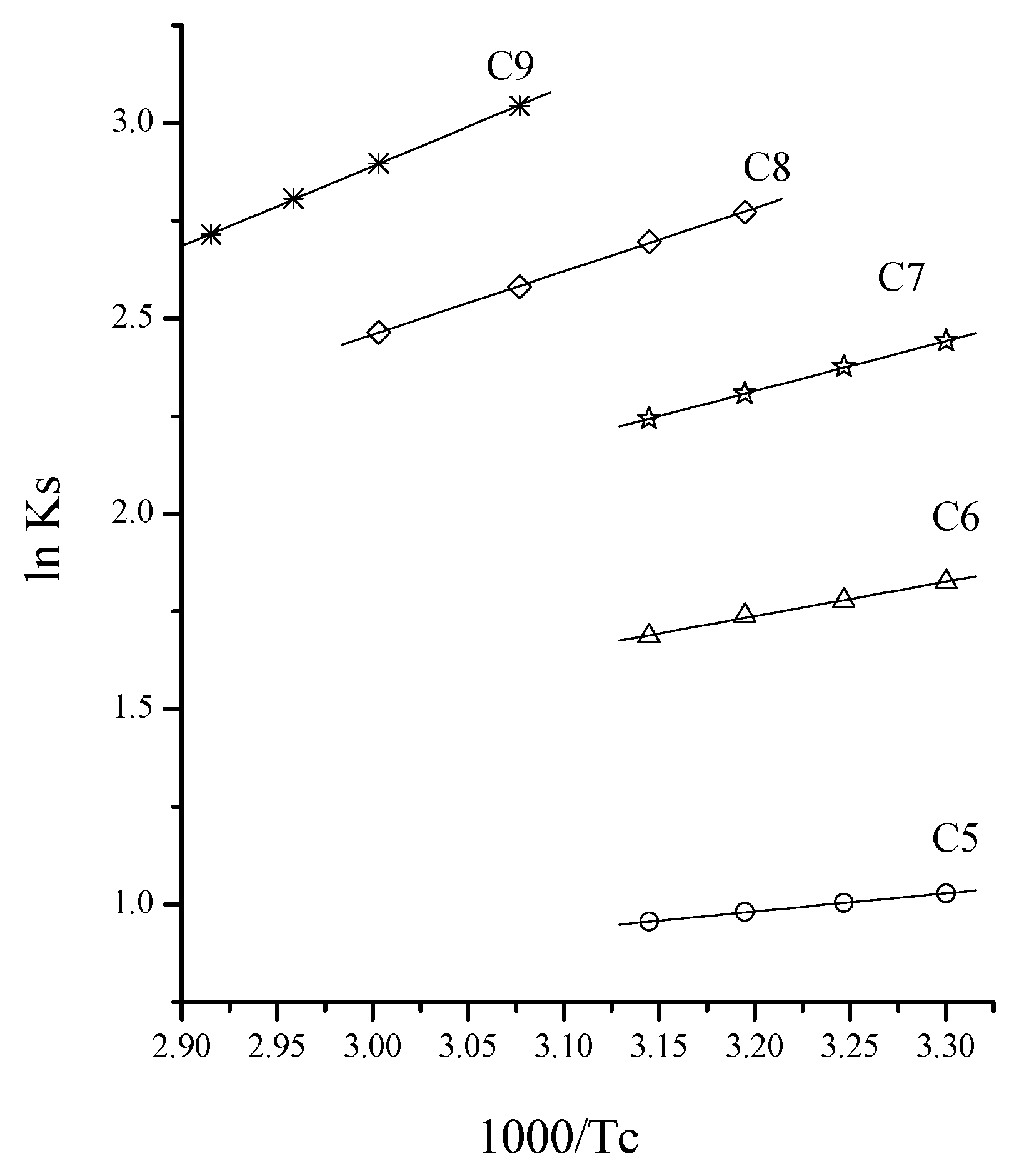
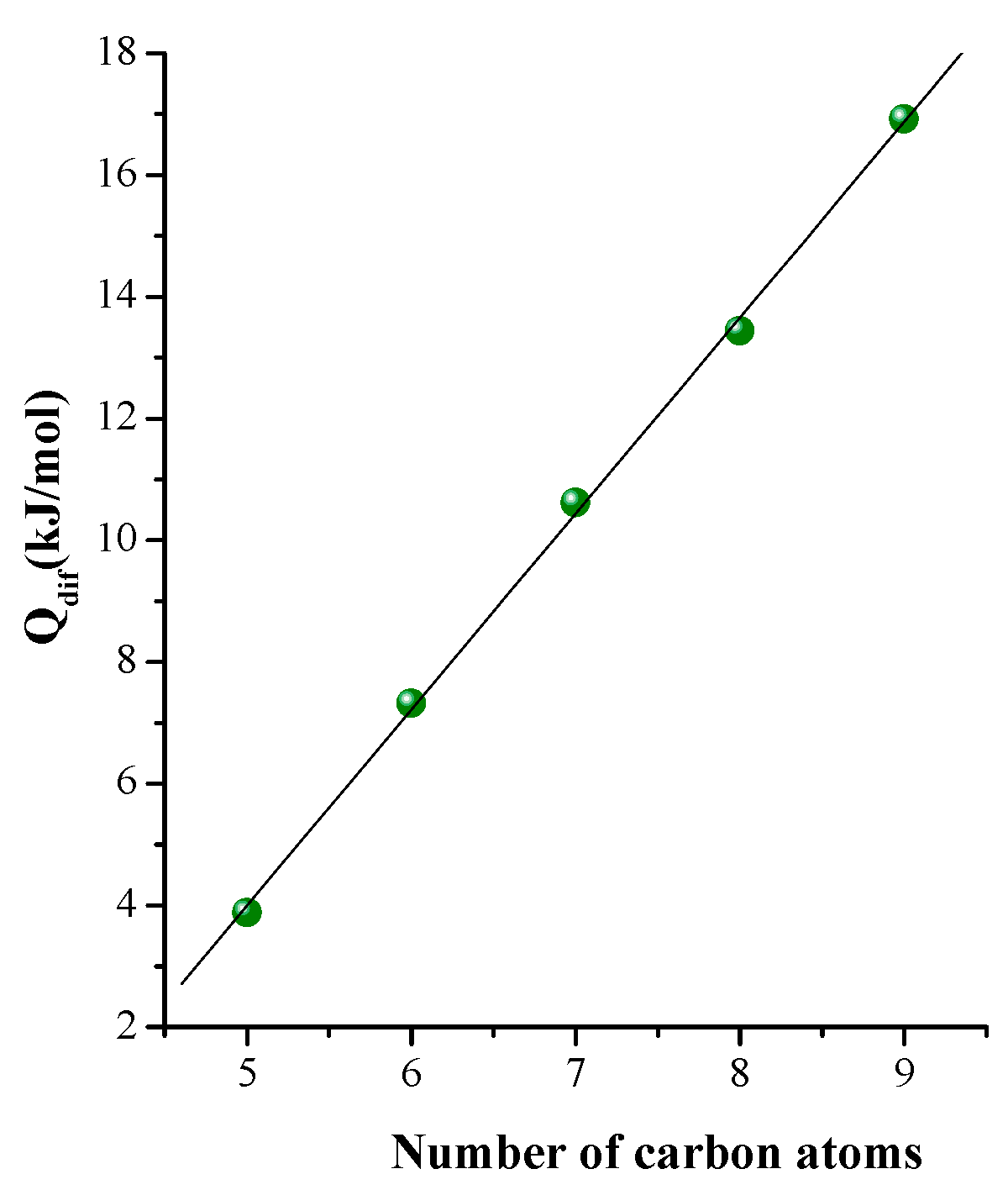
| Zone | Nm [mmol/g] | Ec [J/mol] | Dp [nm]. |
|---|---|---|---|
| 1 | 0.041 | 2481 | 5.2 |
| 2 | 0.056 | 669 | 19.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Larios, J.L.; Infantes-Molina, A.; Negrete-Melo, L.A.; Labadie-Suárez, J.M.; Yee-Madeira, H.T.; Autie-Pérez, M.A.; Rodríguez-Castellón, E. Separation of N–C5H12–C9H20 Paraffins Using Boehmite by Inverse Gas Chromatography. Appl. Sci. 2019, 9, 1810. https://doi.org/10.3390/app9091810
Contreras-Larios JL, Infantes-Molina A, Negrete-Melo LA, Labadie-Suárez JM, Yee-Madeira HT, Autie-Pérez MA, Rodríguez-Castellón E. Separation of N–C5H12–C9H20 Paraffins Using Boehmite by Inverse Gas Chromatography. Applied Sciences. 2019; 9(9):1810. https://doi.org/10.3390/app9091810
Chicago/Turabian StyleContreras-Larios, José L., Antonia Infantes-Molina, Luís A. Negrete-Melo, Juan M. Labadie-Suárez, Hernani T. Yee-Madeira, Miguel A. Autie-Pérez, and Enrique Rodríguez-Castellón. 2019. "Separation of N–C5H12–C9H20 Paraffins Using Boehmite by Inverse Gas Chromatography" Applied Sciences 9, no. 9: 1810. https://doi.org/10.3390/app9091810
APA StyleContreras-Larios, J. L., Infantes-Molina, A., Negrete-Melo, L. A., Labadie-Suárez, J. M., Yee-Madeira, H. T., Autie-Pérez, M. A., & Rodríguez-Castellón, E. (2019). Separation of N–C5H12–C9H20 Paraffins Using Boehmite by Inverse Gas Chromatography. Applied Sciences, 9(9), 1810. https://doi.org/10.3390/app9091810







