Na2Ti7O15 Nanowires with an Oriented Tunnel Structure and High Mechanical Stability: A Potential Anode of Sodium-Ion Batteries and Gas Sensing Materials
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrothermal Synthesis
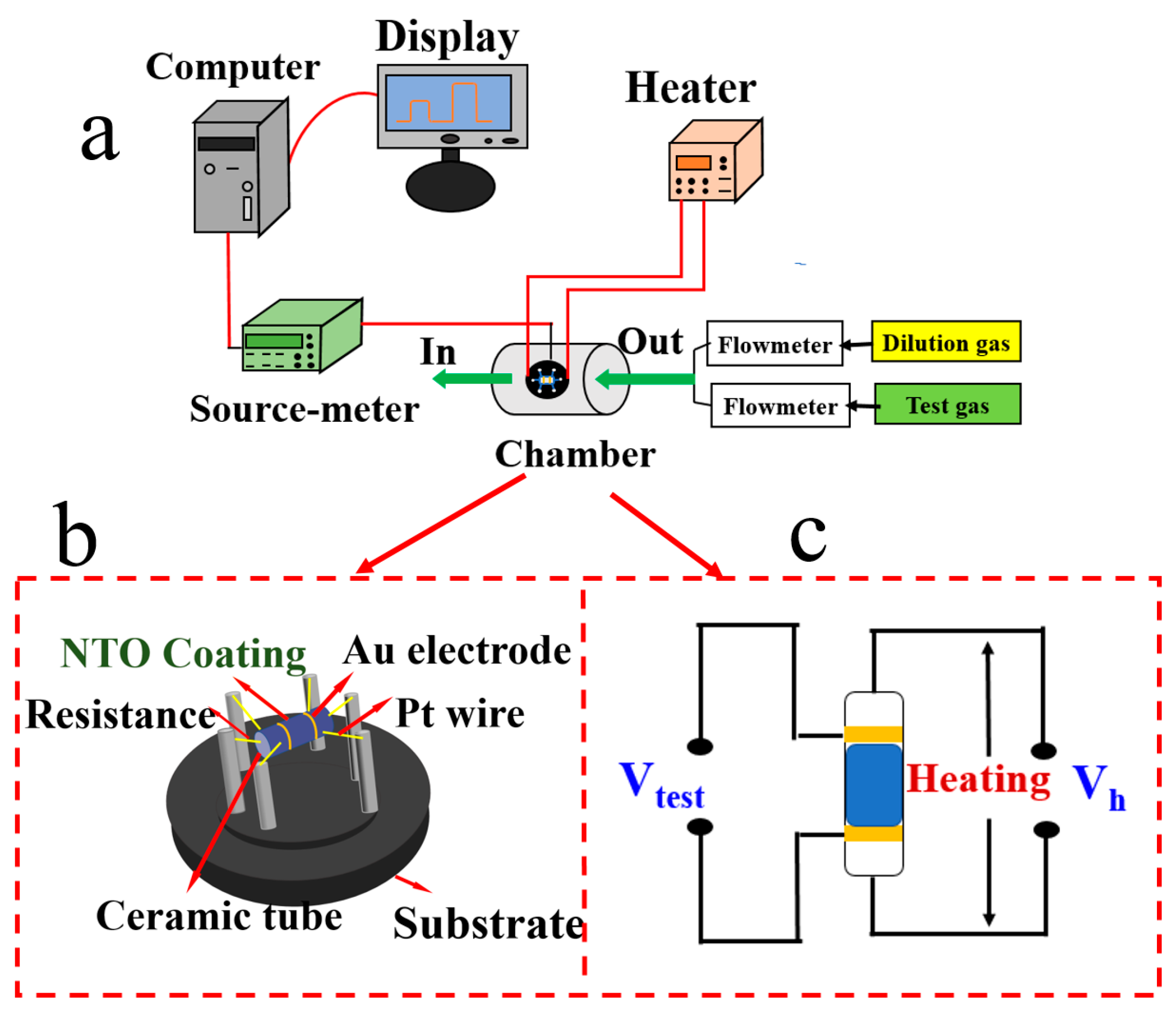
2.2. Structural and Mechanical Characterization
3. Results and Discussion
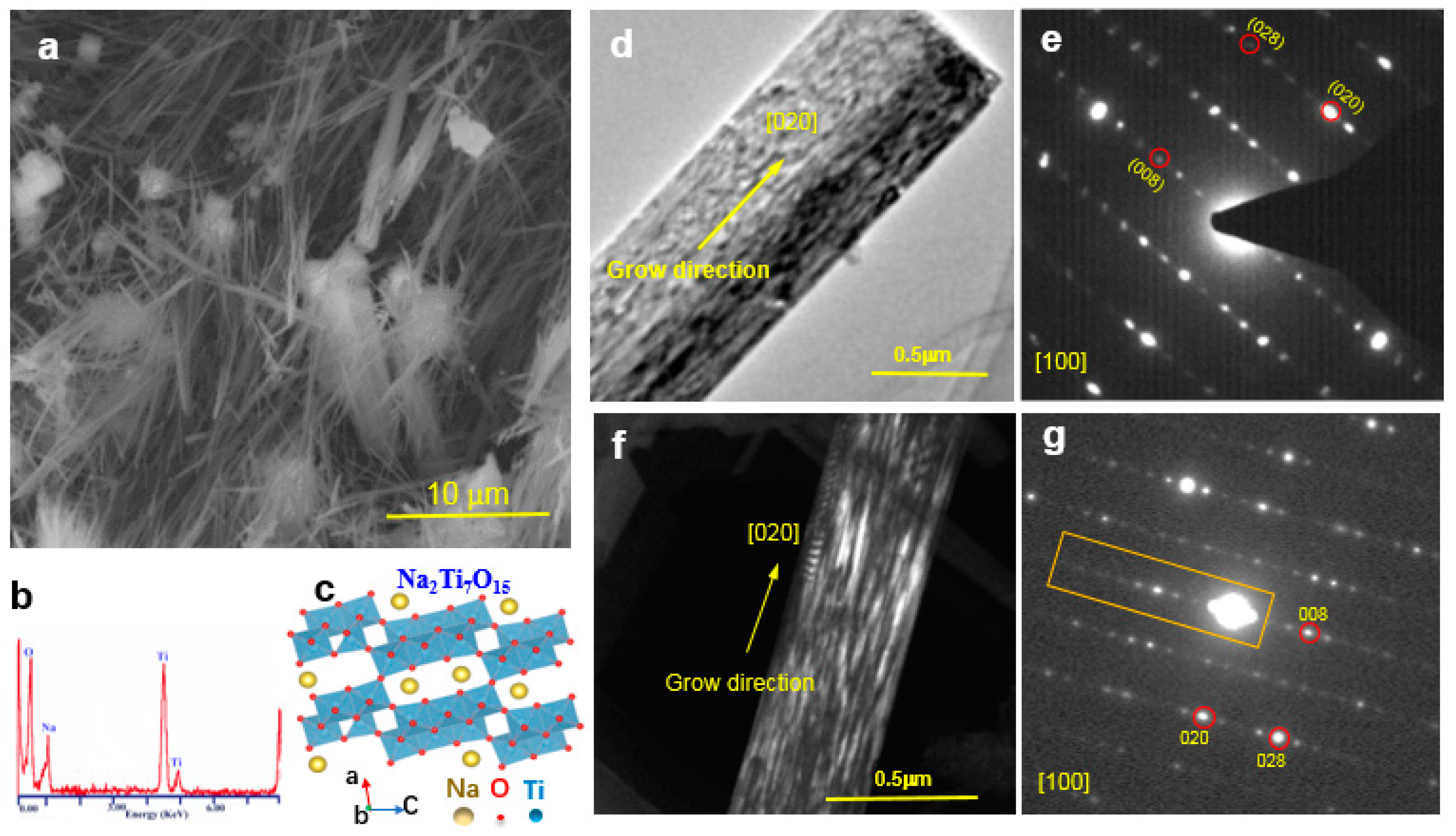
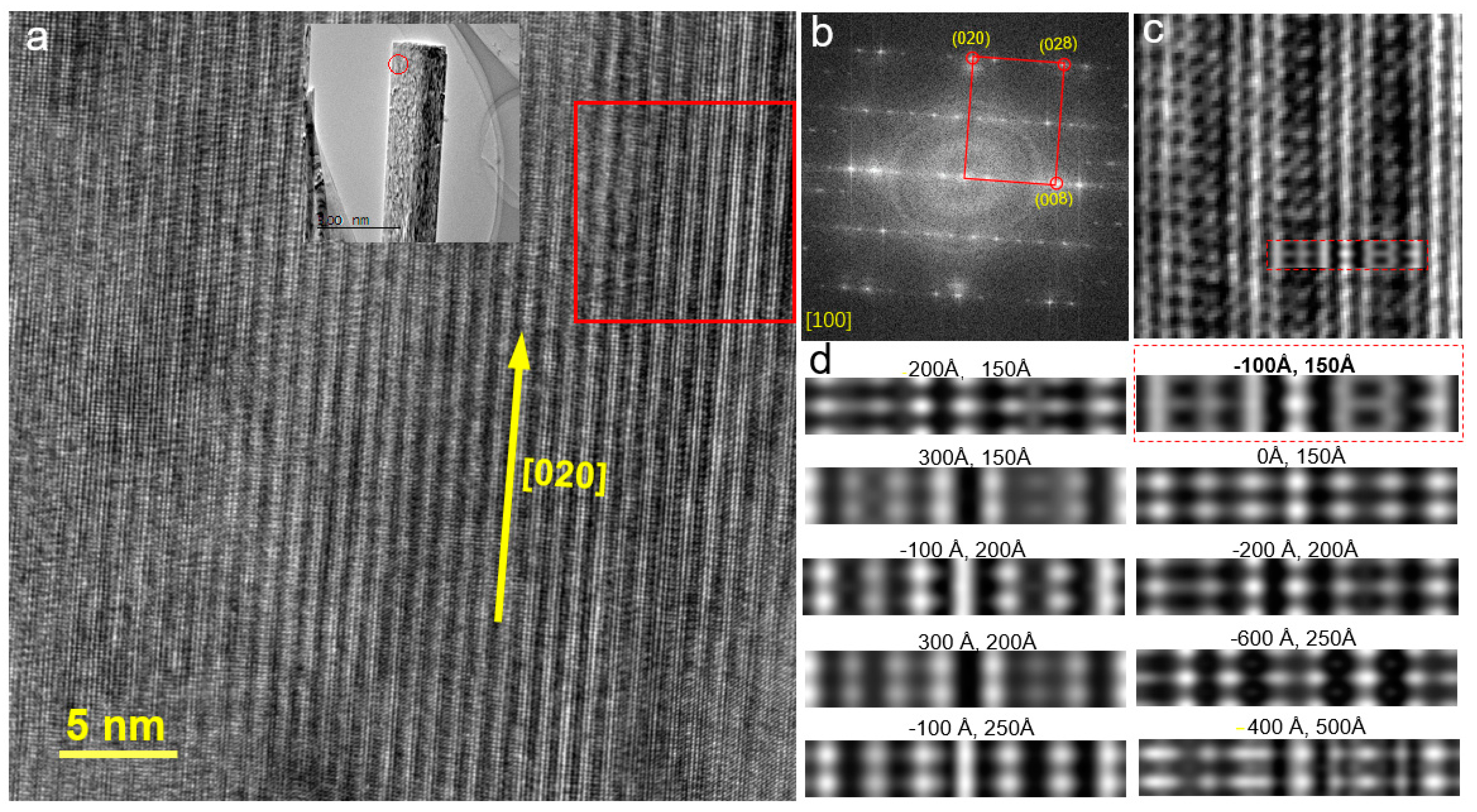
3.1. Morphology and Structural Analysis
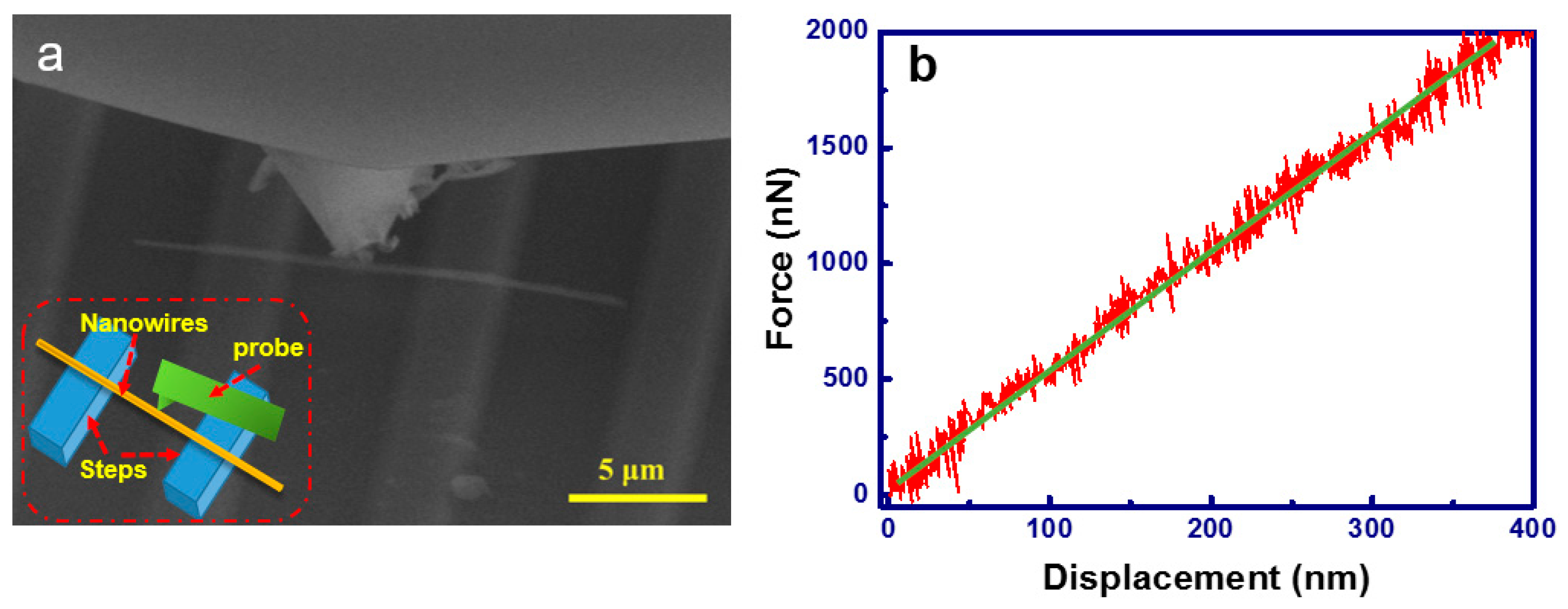
3.2. Mechanical Properties
3.3. GasSensitive Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, C.J.; Hua, W.B.; Zhang, Z.; Zhong, B.H.; Yang, Z.G.; Feng, G.L.; Xiang, W.; Wu, Z.G.; Guo, X.D. Design and synthesis of layered na2ti3o7 and tunnel na2ti6o13 hybrid structures with enhanced electrochemical behavior for sodium-ion batteries. Adv. Sci. 2018, 5, 1800519. [Google Scholar] [CrossRef]
- Zou, G.D.; Zhang, Q.R.; Fernandez, C.; Huang, G.; Huang, J.Y.; Peng, Q.M. Heterogeneous ti3sic2@c-containing na2ti7o15 architecture for high-performance sodium storage at elevated temperatures. ACS Nano 2017, 11, 12219–12229. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Jiang, H.F.; Liu, T.; Liu, X.Q.; Meng, G.Y. Preparation and photocatalytic activity of alkali titanate nano materials a(2)ti(n)o(2n+1) (a = li, na and k). Mater. Res. Bull. 2007, 42, 334–344. [Google Scholar] [CrossRef]
- Stengl, V.; Bakardjieva, S.; Subrt, J.; Vecernikova, E.; Szatmary, L.; Klementova, M.; Balek, V. Sodium titanate nanorods: Preparation, microstructure characterization and photocatalytic activity. Appl. Catal. B-Environ. 2006, 63, 20–30. [Google Scholar] [CrossRef]
- Yada, M.; Inoue, Y.; Uota, M.; Torikai, T.; Watari, T.; Noda, I.; Hotokebuchi, T. Plate, wire, mesh, microsphere, and microtube composed of sodium titanate nanotubes on a titanium metal template. Langmuir 2007, 23, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Teng, H. Nanotube formation from a sodium titanate powder via low-temperature acid treatment. Langmuir 2008, 24, 3434–3438. [Google Scholar] [CrossRef]
- Li, H.; Fei, H.L.; Liu, X.; Yang, J.; Wei, M.D. In situ synthesis of Na2Ti7O15 nanotubes on a ti net substrate as a high performance anode for na-ion batteries. Chem. Commun. 2015, 51, 9298–9300. [Google Scholar] [CrossRef]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Adsorptive removal of heavy metals from water using sodium titanate nanofibres loaded onto gac in fixed-bed columns. J. Hazard. Mater. 2015, 287, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Cabello, M.; Ortiz, G.F.; Lopez, M.C.; Alcantara, R.; Gonzalez, J.R.; Tirado, J.L.; Stoyanova, R.; Zhecheva, E. Self-organized sodium titanate/titania nanoforest for the negative electrode of sodium-ion microbatteries. J. Alloy. Compd. 2015, 646, 816–826. [Google Scholar] [CrossRef]
- Ramirez-Salgado, J.; Djurado, E.; Fabry, P. Synthesis of sodium titanate composites by sol-gel method for use in gas potentiometric sensors. J. Eur. Ceram. Soc. 2004, 24, 2477–2483. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.P.; Li, G.R.; Yan, T.Y.; Zhu, H.Y. Electrochemical lithium storage of sodium titanate nanotubes and nanorods. Electrochim. Acta 2008, 53, 7061–7068. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Oh, S.H.; Finones, R.R.; Daraio, C.; Chen, L.H.; Jin, S.H. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials 2005, 26, 4938–4943. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhu, M.K.; Hou, Y.D.; Wang, R.Z.; Yan, H.; Liu, L.Y. Structural modulation of na0.5bi0.5tio3 in hydrothermal synthesis. Int. J. Appl. Ceram. Technol. 2016, 13, 569–578. [Google Scholar] [CrossRef]
- Setinc, T.; Spreitzer, M.; Logar, M.; Suvorov, D. Hydrothermal synthesis of nanosized na0.5bi0.5tio3. J. Am. Ceram. Soc. 2011, 94, 3793–3799. [Google Scholar] [CrossRef]
- Tao, D.D.; Fang, Z.X.; Qiu, M.; Li, Y.; Huang, X.; Ding, K.N.; Chen, W.K.; Su, W.Y.; Zhang, Y.F. First-principles study of na2+xti7o15 as anode materials for sodium-ion batteries. J. Alloy. Compd. 2016, 689, 805–811. [Google Scholar] [CrossRef]
- Weadock, N.; Varongchayakul, N.; Wan, J.Y.; Lee, S.; Seog, J.; Hu, L.B. Determination of mechanical properties of the sei in sodium ion batteries via colloidal probe microscopy. Nano Energy 2013, 2, 713–719. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, T.-W.; Coskun, A.; Choi, J.W. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 2017, 357, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.F.; Bando, Y.; Kurashima, K.; Ogawa, H.; Inada, K. Pseudo-one-dimensional periodic domain boundary structures in alkali titanium oxides. J. Solid State Chem. 2001, 162, 128–137. [Google Scholar] [CrossRef]
- Huang, J.D.; Wei, Z.X.; Liao, J.Q.; Ni, W.; Wang, C.Y.; Ma, J.M. Molybdenum and tungsten chalcogenides for lithium/sodium-ion batteries: Beyond mos2. J. Energy Chem. 2019, 33, 100–124. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ren, J.; Gao, X.; Zhang, W.J.; Duan, H.P.; Wang, M.; Shui, J.L.; Xu, M. Self-adaptive electrode with swcnt bundles as elastic substrate for high-rate and long-cycle-life lithium/sodium ion batteries. Small 2018, 14, 1802913. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.Y.; Li, F.; Wang, J.R.; Qu, Y.Y.; Yang, Y.M.; Li, W.F.; Zhao, M.W. Prediction of a flexible anode material for li/na ion batteries: Phosphorous carbide monolayer (alpha-pc). Carbon 2019, 141, 444–450. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Hao, P.; Wang, J.; Zhang, Y. Effect of different oxide thickness on the bending young’s modulus of sio2@sic nanowires. Sci. Rep. 2016, 6, 18994. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.-Y.; Ding, Y.; Zhou, B.; Jia, N.-N.; Wang, K.; Zhang, Z.-H.; Sui, M.-L. Na2Ti7O15 Nanowires with an Oriented Tunnel Structure and High Mechanical Stability: A Potential Anode of Sodium-Ion Batteries and Gas Sensing Materials. Appl. Sci. 2019, 9, 1673. https://doi.org/10.3390/app9081673
Liu L-Y, Ding Y, Zhou B, Jia N-N, Wang K, Zhang Z-H, Sui M-L. Na2Ti7O15 Nanowires with an Oriented Tunnel Structure and High Mechanical Stability: A Potential Anode of Sodium-Ion Batteries and Gas Sensing Materials. Applied Sciences. 2019; 9(8):1673. https://doi.org/10.3390/app9081673
Chicago/Turabian StyleLiu, Li-Ying, Yang Ding, Bo Zhou, Ning-Ning Jia, Kuan Wang, Zhen-Hua Zhang, and Man-Ling Sui. 2019. "Na2Ti7O15 Nanowires with an Oriented Tunnel Structure and High Mechanical Stability: A Potential Anode of Sodium-Ion Batteries and Gas Sensing Materials" Applied Sciences 9, no. 8: 1673. https://doi.org/10.3390/app9081673
APA StyleLiu, L.-Y., Ding, Y., Zhou, B., Jia, N.-N., Wang, K., Zhang, Z.-H., & Sui, M.-L. (2019). Na2Ti7O15 Nanowires with an Oriented Tunnel Structure and High Mechanical Stability: A Potential Anode of Sodium-Ion Batteries and Gas Sensing Materials. Applied Sciences, 9(8), 1673. https://doi.org/10.3390/app9081673




