Wide Electrocaloric Temperature Range Induced by Ferroelectric to Antiferroelectric Phase Transition
Abstract
1. Introduction
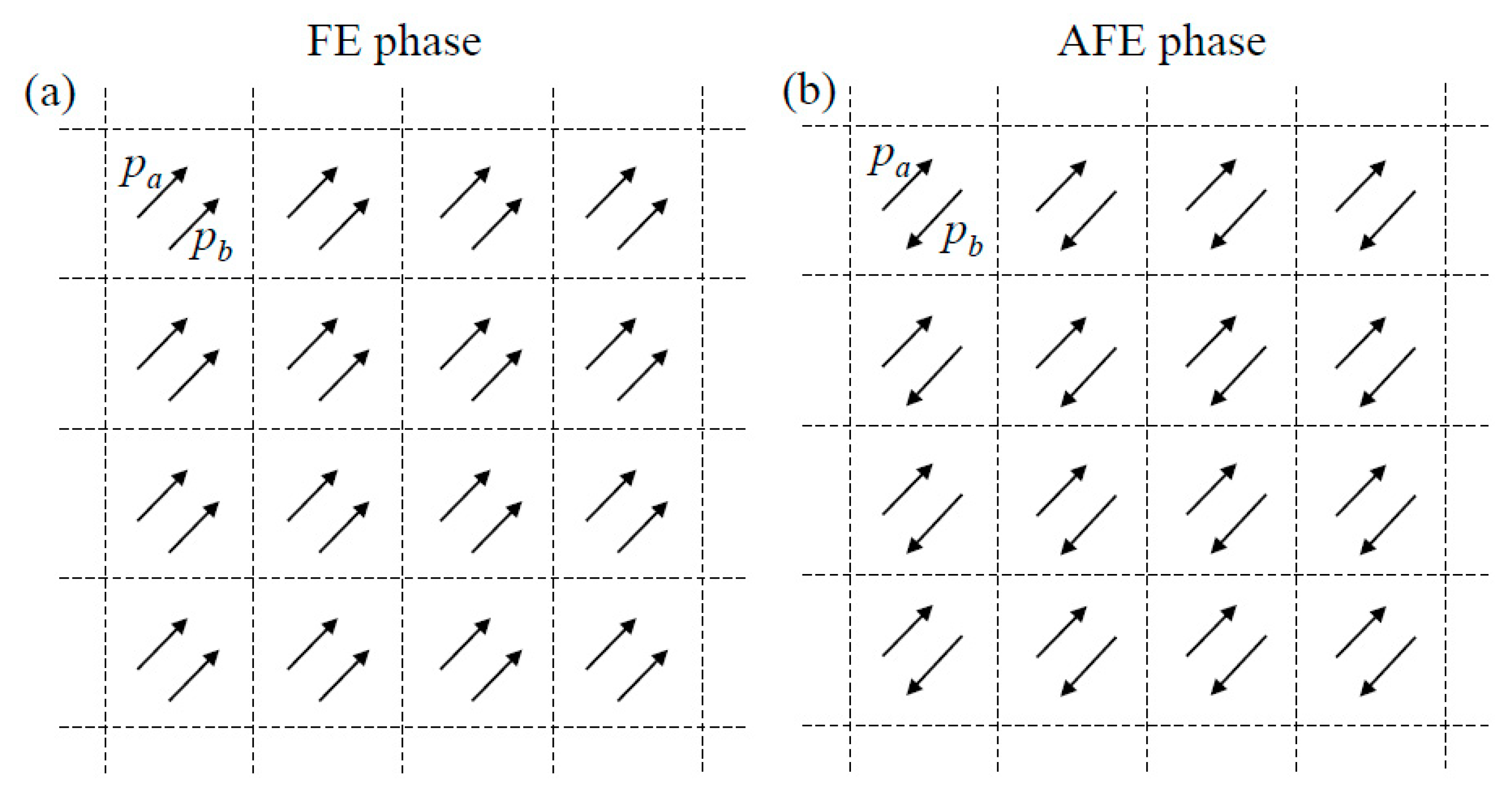
2. Theoretical Description
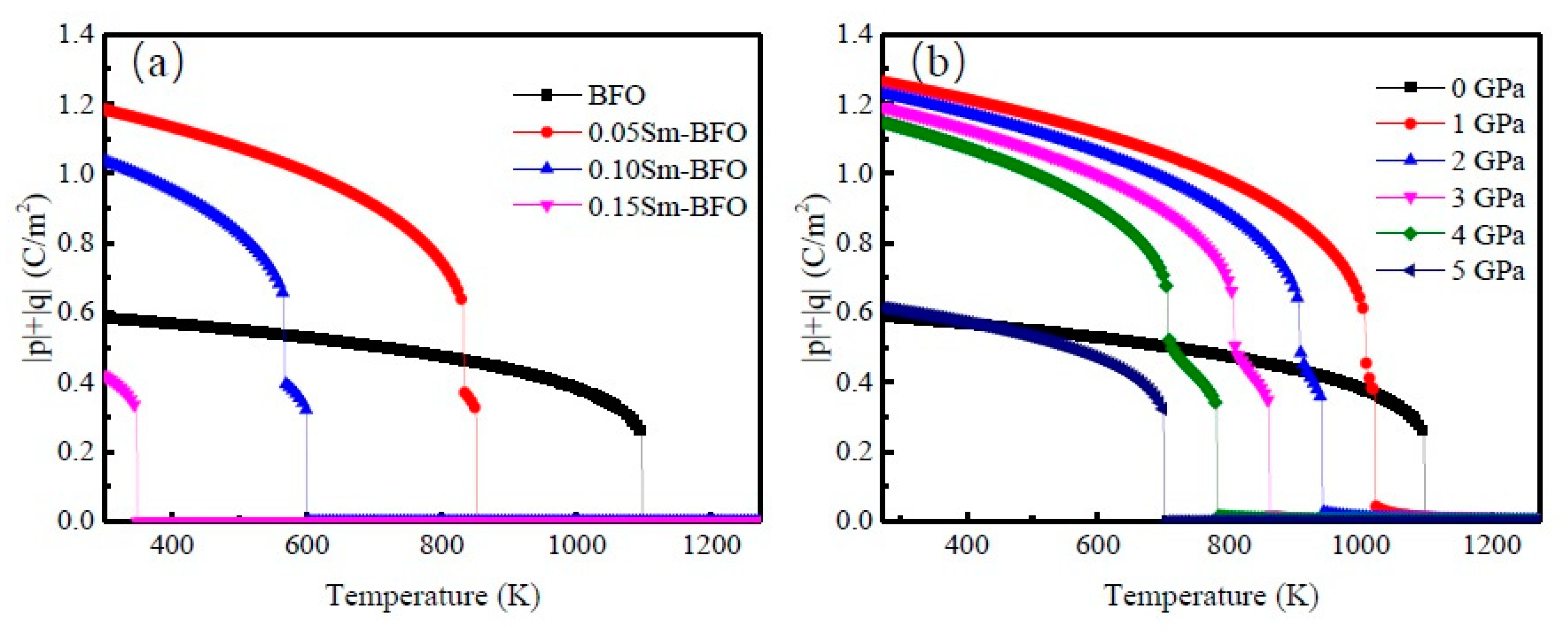
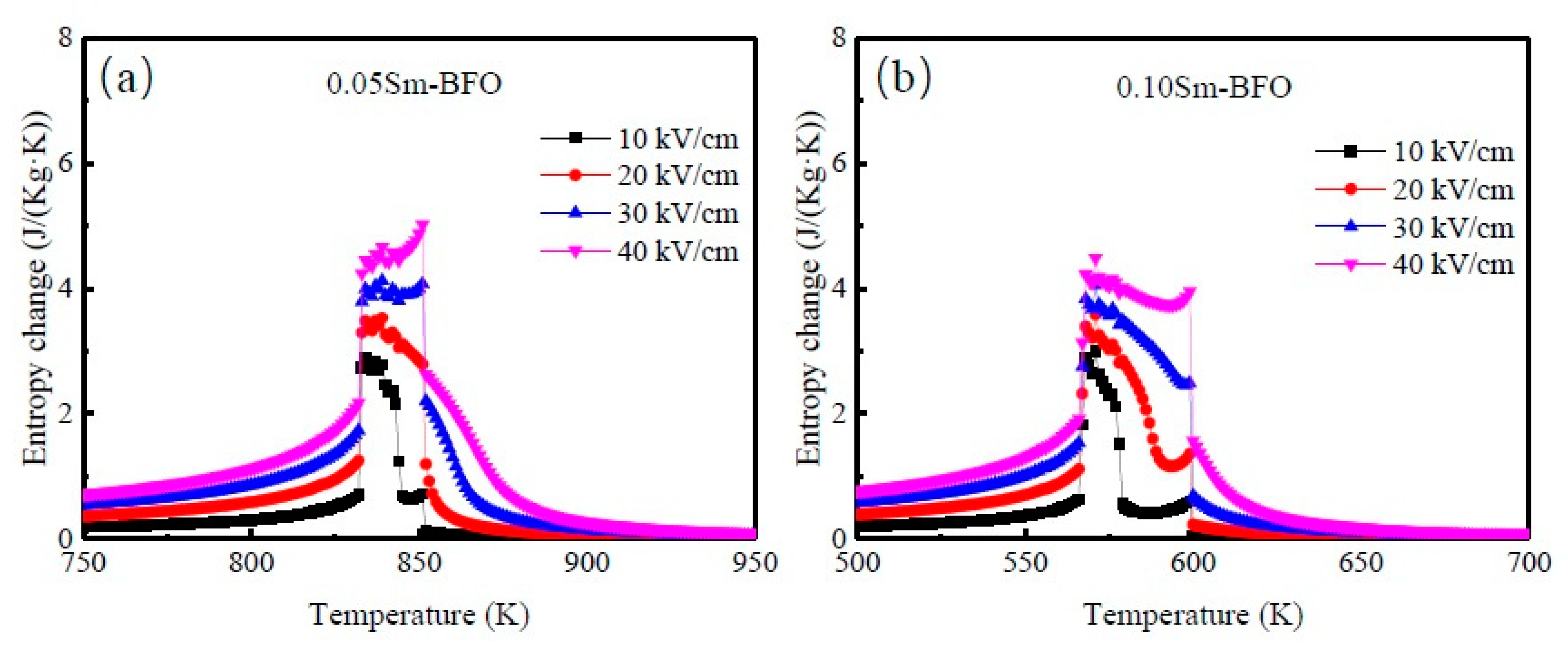
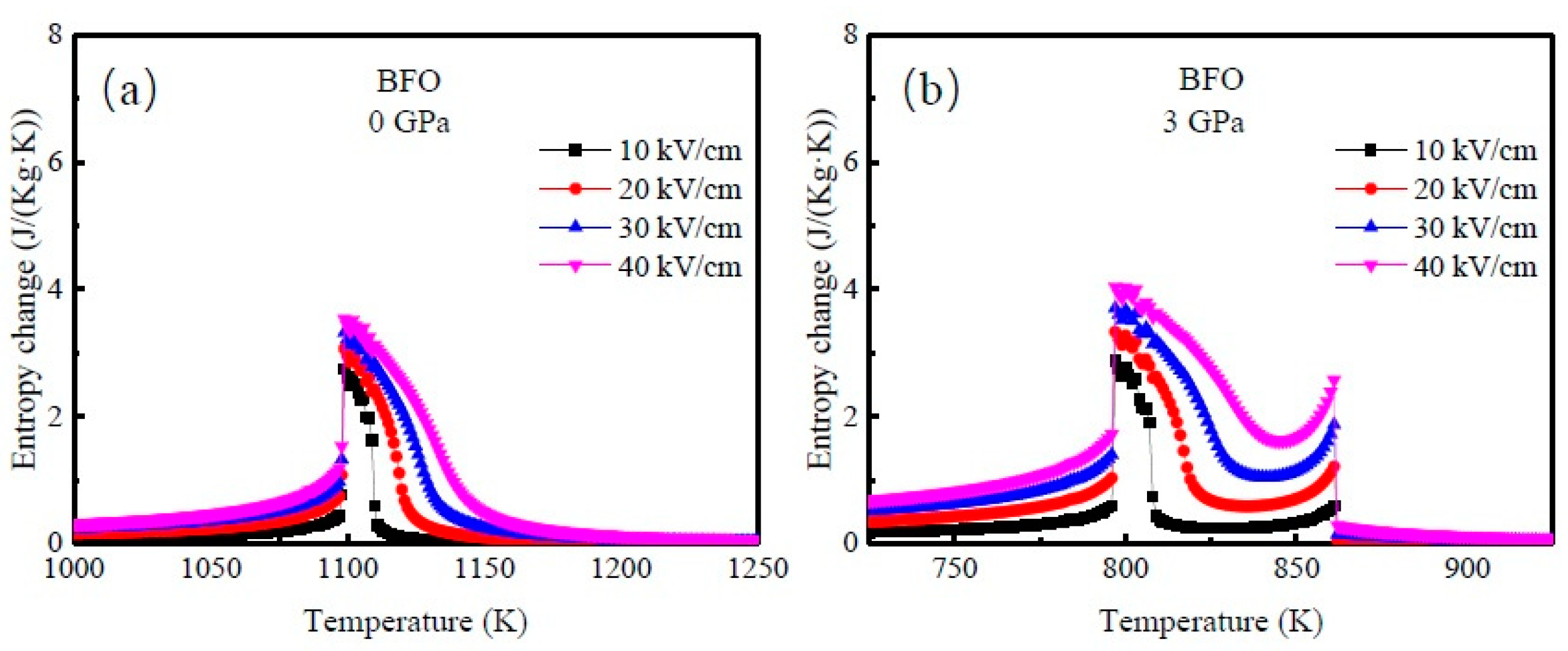
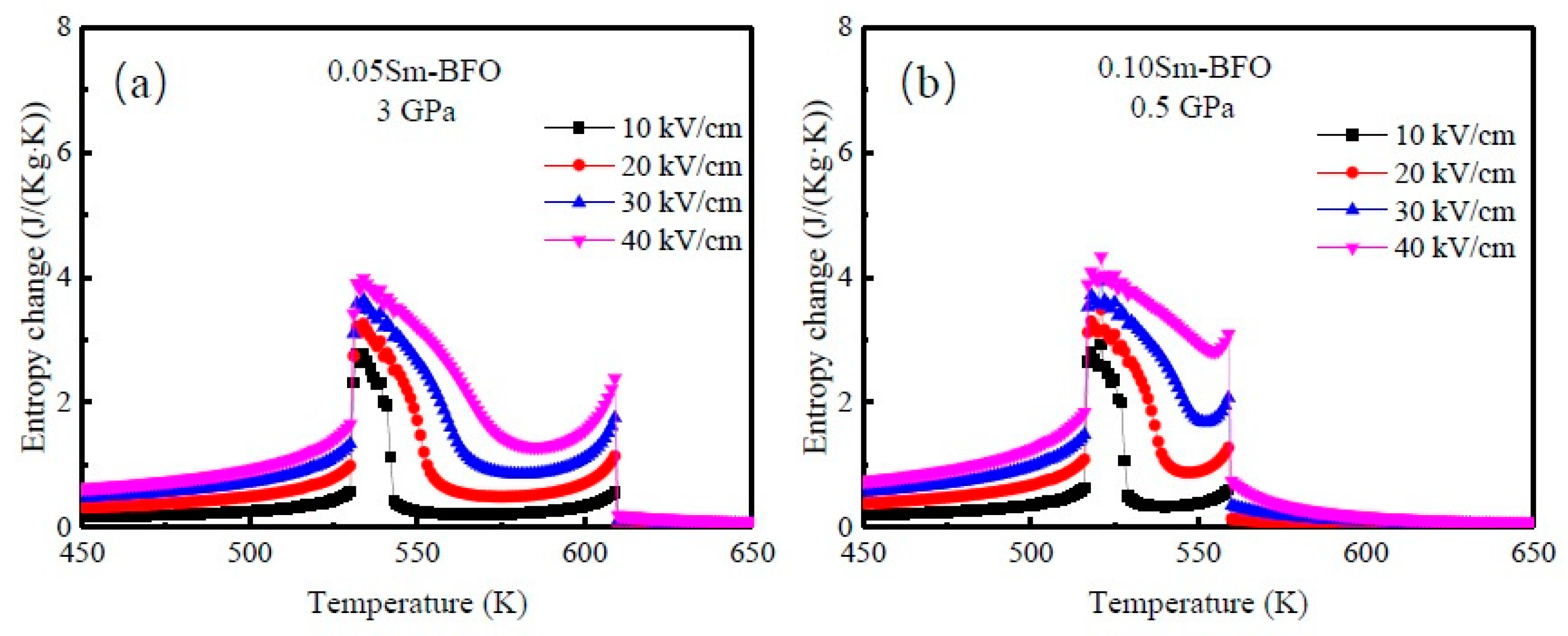
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Li, Q.; Gu, H.; Jiang, S.; Han, K.; Gadinski, M.R.; Haque, M.A.; Zhang, Q.; Wang, Q. Ferroelectric polymer nanocomposites for room-temperature electrocaloric refrigeration. Adv. Mater. 2015, 27, 1450–1454. [Google Scholar] [CrossRef]
- Guo, L.S.; Zhang, Q.M. Electrocaloric Materials for Solid-State Refrigeration. Adv. Mater. 2009, 21, 1983–1987. [Google Scholar]
- Quintero, M.; Ghivelder, L.; Gomez-Marlasca, F.; Parisi, F. Decoupling electrocaloric effect from Joule heating in a solid state cooling device. Appl. Phys. Lett. 2011, 99, 232908. [Google Scholar] [CrossRef]
- Chukka, R.; Cheah, J.; Chen, Z.; Yang, P.; Shannigrahi, S.; Wang, J.; Chen, L. Enhanced cooling capacities of ferroelectric materials at morphotropic phase boundaries. Appl. Phys. Lett. 2011, 98, 242902. [Google Scholar] [CrossRef]
- Karmanenko, S.F.; Pakhomov, O.V.; Prudan, A.M.; Starkov, A.S.; Eskov, A. Layered ceramic structure based on the electrocaloric elements working as a solid state cooling line. J. Eur. Ceram. Soc. 2007, 27, 3109–3112. [Google Scholar] [CrossRef]
- Ožbolt, M.; Kitanovski, A.; Tušek, J.; Poredoš, A. Electrocaloric refrigeration: Thermodynamics, state of the art and future perspectives. Int. J. Refrig. 2014, 40, 174–188. [Google Scholar] [CrossRef]
- Correia, T.M.; Kar-Narayan, S.; Young, J.S.; Scott, J.F.; Mathur, N.D.; Whatmore, R.W.; Zhang, Q. PST thin films for electrocaloric coolers. J. Phys. D Appl. Phys. 2011, 44, 165407. [Google Scholar] [CrossRef]
- Lu, S.G.; Rožič, B.; Zhang, Q.M.; Kutnjak, Z.; Pirc, R.; Lin, M.; YLi, X.; Lee, G. Comparison of directly and indirectly measured electrocaloric effect in relaxor ferroelectric polymers. Appl. Phys. Lett. 2010, 97, 202901. [Google Scholar] [CrossRef]
- Khodayari, A.; Mohammadi, S. Solid-State Cooling Line Based on the Electrocaloric Effect. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 503–508. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, G.; Zhang, X.; Jiang, S.; Zeng, Y.; Wang, Q. Relaxor ferroelectric-based electrocaloric polymer nanocomposites with a broad operating temperature range and high cooling energy. Adv. Mater. 2015, 27, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, Z.; Zhao, S. Giant Negative and Positive Electrocaloric Effects Coexisting in Lead-Free Na0.5Bi4.5Ti4O15 Films Over a Broad Temperature Range. Phys. Status. Solidi RRL 2018, 12, 1700443. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Z.; Lu, Q.; Zhao, S. Giant negative electrocaloric effect over a broad temperature range in lead-free based Bi0.5(K0.15Na0.85)0.05TiO3 relaxor ferroelectric films. J. Alloy. Compd. 2018, 756, 62–67. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Shi, S.; Yin, Y.; Li, H.; Wang, Q.; Zhang, Y.; Bian, J.; Rajput, S.S.; Long, C.; et al. Large electrocaloric efficiency over a broad temperature span in lead-free BaTiO3-based ceramics near room temperature. Appl. Phys. Lett. 2017, 111, 202902. [Google Scholar] [CrossRef]
- Han, F.; Bai, Y.; Qiao, L.J.; Guo, D. A systematic modification of the large electrocaloric effect within a broad temperature range in rare-earth doped BaTiO3 ceramics. J. Mater. Chem. C 2016, 4, 1842–1849. [Google Scholar] [CrossRef]
- Geng, W.P.; Liu, Y.; Meng, X.J.; Bellaiche, L.; Scott, J.F.; Dkhil, B.; Jiang, A. Giant Negative Electrocaloric Effect in Antiferroelectric La-Doped Pb(ZrTi)O3 Thin Films Near Room Temperature. Adv. Mater. 2015, 27, 3165–3169. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Ye, H.; Zhang, Y.; Gu, H.; Li, X.; Randall, C.A.; Zhang, Q.M. Giant Electrocaloric Response Over A Broad Temperature Range in Modified BaTiO3 Ceramics. Adv. Funct. Mater. 2014, 24, 1300–1305. [Google Scholar] [CrossRef]
- Cao, W.P.; Li, W.L.; Dai, X.F.; Zhang, T.D.; Sheng, J.; Hou, Y.F.; Fei, W.D. Large electrocaloric response and high energy-storage properties over a broad temperature range in lead-free NBT-ST ceramics. J. Eur. Ceram. Soc. 2016, 36, 593–600. [Google Scholar] [CrossRef]
- Sun, X.; Huang, H.; Ma, X.; Wen, Y.; Dang, Z.M. Tunable Temperature Range of Stress-Enhanced Electrocaloric Effects in Composition Gradient Bilayers. J. Ceram. Sci. Technol. 2018, 9, 201–208. [Google Scholar]
- Zhao, Y.; Liu, X.Q.; Wu, J.W.; Wu, S.Y.; Chen, X.M. Electrocaloric effect in relaxor ferroelectric Ba(Ti1-xYx)O3-x/2 ceramics over a broad temperature range. J. Alloy. Compd. 2017, 729, 57–63. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.B.; Li, B.; Zhong, X.L.; Lou, X.J.; Zhou, Y.C. Sizable electrocaloric effect in a wide temperature range tuned by tensile misfit strain in BaTiO3 thin films. J. Appl. Phys. 2011, 109, 126102. [Google Scholar] [CrossRef]
- Zhang, J.X.; Li, Y.L.; Wang, Y.; Liu, Z.K.; Chen, L.Q.; Chu, Y.H.; Zavaliche, F.; Ramesh, R. Effect of substrate-induced strains on the spontaneous polarization of epitaxial BiFeO3 thin films. J. Appl. Phys. 2007, 101, 114105. [Google Scholar] [CrossRef]
- Sun, X.; Huang, H.; Wang, J.; Wen, Y.; Dang, Z.-M. Strain-induced broadening temperature range of electrocaloric effects in ferroelectric superlattices. J. Alloy. Compd. 2019, 777, 821–827. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, G.; Ma, X.; Liang, D.; Wang, J.; Liu, Y.; Wang, Q.; Chen, L.-Q. Size effects of electrocaloric cooling in ferroelectric nanowires. J. Am. Ceram. Soc. 2018, 101, 1566–1575. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Huang, H.; Wang, J.; Li, Q.; Chen, L.Q.; Wang, Q. Toward Wearable Cooling Devices: Highly Flexible Electrocaloric Ba0.67Sr0.33TiO3 Nanowire Arrays. Adv. Mater. 2016, 28, 4811–4816. [Google Scholar] [CrossRef] [PubMed]
- Mischenko, A.S.; Zhang, Q.; Scott, J.F.; Whatmore, R.W.; Mathur, N.D. Giant electrocaloric effect in thin-film PbZr0.95Ti0.05O3. Science 2006, 311, 1270–1271. [Google Scholar]
- Neese, B.; Chu, B.J.; Lu, S.G.; Wang, Y.; Furman, E.; Zhang, Q.M. Large Electrocaloric Effect in Ferroelectric Polymers Near Room Temperature. Science 2008, 321, 821–823. [Google Scholar] [CrossRef]
- Saranya, D.; Chaudhuri, A.R.; Parui, J.; Krupanidhi, S.B. Electrocaloric effect of PMN–PT thin films near morphotropic phase boundary. Bull. Mater. Sci. 2009, 32, 259–262. [Google Scholar] [CrossRef]
- Zheng, G.P.; Uddin, S.; Zheng, X.C.; Yang, J.H. Structural and electrocaloric properties of multiferroic-BiFeO3 doped 0.94Bi0.5Na0.5TiO3-0.06BaTiO3 solid solutions. J. Alloy. Compd. 2016, 663, 249–255. [Google Scholar] [CrossRef]
- Peng, B.L.; Fan, H.Q.; Zhang, Q. A Giant Electrocaloric Effect in Nanoscale Antiferroelectric and Ferroelectric Phases Coexisting in a Relaxor Pb0.8Ba0.2ZrO3 Thin Film at Room Temperature. Adv. Funct. Mater. 2013, 23, 2987–2992. [Google Scholar] [CrossRef]
- Wu, M.; Song, D.; Vats, G.; Ning, S.; Guo, M.; Zhang, D.; Xue, D.; Pennycook, S.J.; Lou, X. Defect-controlled electrocaloric effect in PbZrO3 thin films. J. Mater. Chem. C 2018, 6, 10332–10340. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, Q.; Lyu, Y.; Liu, L.; Lou, X.; Shaw, C.; Huang, H.; Wang, Z. Thermal strain induced large electrocaloric effect of relaxor thin film on LaNiO3/Pt composite electrode with the coexistence of nanoscale antiferroelectric and ferroelectric phases in a broad temperature range. Nano Energy 2018, 47, 285–293. [Google Scholar] [CrossRef]
- Xue, F.; Liang, L.; Gu, Y.; Takeuchi, I.; Kalinin, S.V.; Chen, L.Q. Composition- and pressure-induced ferroelectric to antiferroelectric phase transitions in Sm-doped BiFeO3 system. Appl. Phys. Lett. 2015, 106, 012903. [Google Scholar] [CrossRef]
- Liao, Z.; Xue, F.; Sun, W.; Song, D.; Zhang, Q.; Li, J.-F.; Chen, L.-Q.; Zhu, J. Reversible phase transition induced large piezoelectric response in Sm-doped BiFeO3 with a composition near the morphotropic phase boundary. Phys. Rev. B 2017, 95, 214101. [Google Scholar] [CrossRef]
- Cheng, C.J.; Kan, D.; Lim, S.H.; McKenzie, W.R.; Munroe, P.R.; Salamanca-Riba, L.G.; Withers, R.L.; Takeuchi, I.; Nagarajan, V. Structural transitions and complex domain structures across a ferroelectric-to-antiferroelectric phase boundary in epitaxial Sm-doped BiFeO3 thin films. Phys. Rev. B 2009, 80, 014109. [Google Scholar] [CrossRef]
- Marzouki, A.; Harzali, H.; Loyau, V.; Gemeiner, P.; Zehani, K.; Dkhil, B.; Bessais, L.; Megriche, A. Large magnetoelectric response and its origin in bulk Co-doped BiFeO3 synthesized by a stirred hydrothermal process. Acta Mater. 2018, 145, 316–321. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Z.; Li, T.; Li, Y. Microstructure and properties of Sm-substituted BiFeO3 ceramics. J. Rare Earths 2012, 30, 1123–1128. [Google Scholar] [CrossRef]
- Kittel, C. Theory of Antiferroelectric Crystals. Phys. Rev. 1951, 82, 729–732. [Google Scholar] [CrossRef]
- Okada, K. Phenomenological Theory of Antiferroelectric Transition I. Second-Order Transition. J. Phys. Soc. Jpn. 1969, 27, 420–428. [Google Scholar] [CrossRef]
- Cross, L.E. Antiferroelectric-Ferroelectric Switching in a Simple "Kittel" Antiferroelectric. J. Phys. Soc. Jpn. 1967, 23, 77–82. [Google Scholar] [CrossRef]
- Rabe, K.M. Functional Metal Oxides: New Science and Novel Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; p. 221. [Google Scholar]
- Lines, M.E.; Glass, A.M. Principles and Applications of Ferroelectrics and Related Materials; Clarendon Press: Oxford, UK, 1977. [Google Scholar]
- Bai, Y.; Zheng, G.P.; Ding, K.; Qiao, L.J.; Shi, S.Q.; Guo, D. The giant electrocaloric effect and high effective cooling power near room temperature for BaTiO3 thick film. J. Appl. Phys. 2011, 110, 094103. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Huang, H.; Jafri, H.M.; Wang, J.; Wen, Y.; Dang, Z.-M. Wide Electrocaloric Temperature Range Induced by Ferroelectric to Antiferroelectric Phase Transition. Appl. Sci. 2019, 9, 1672. https://doi.org/10.3390/app9081672
Sun X, Huang H, Jafri HM, Wang J, Wen Y, Dang Z-M. Wide Electrocaloric Temperature Range Induced by Ferroelectric to Antiferroelectric Phase Transition. Applied Sciences. 2019; 9(8):1672. https://doi.org/10.3390/app9081672
Chicago/Turabian StyleSun, Xiaohui, Houbing Huang, Hasnain Mehdi Jafri, Junsheng Wang, Yongqiang Wen, and Zhi-Min Dang. 2019. "Wide Electrocaloric Temperature Range Induced by Ferroelectric to Antiferroelectric Phase Transition" Applied Sciences 9, no. 8: 1672. https://doi.org/10.3390/app9081672
APA StyleSun, X., Huang, H., Jafri, H. M., Wang, J., Wen, Y., & Dang, Z.-M. (2019). Wide Electrocaloric Temperature Range Induced by Ferroelectric to Antiferroelectric Phase Transition. Applied Sciences, 9(8), 1672. https://doi.org/10.3390/app9081672





