Partition of Cu and Pb in a Two-Stage Fluidized-Bed Waste Gasification System
Abstract
1. Introduction
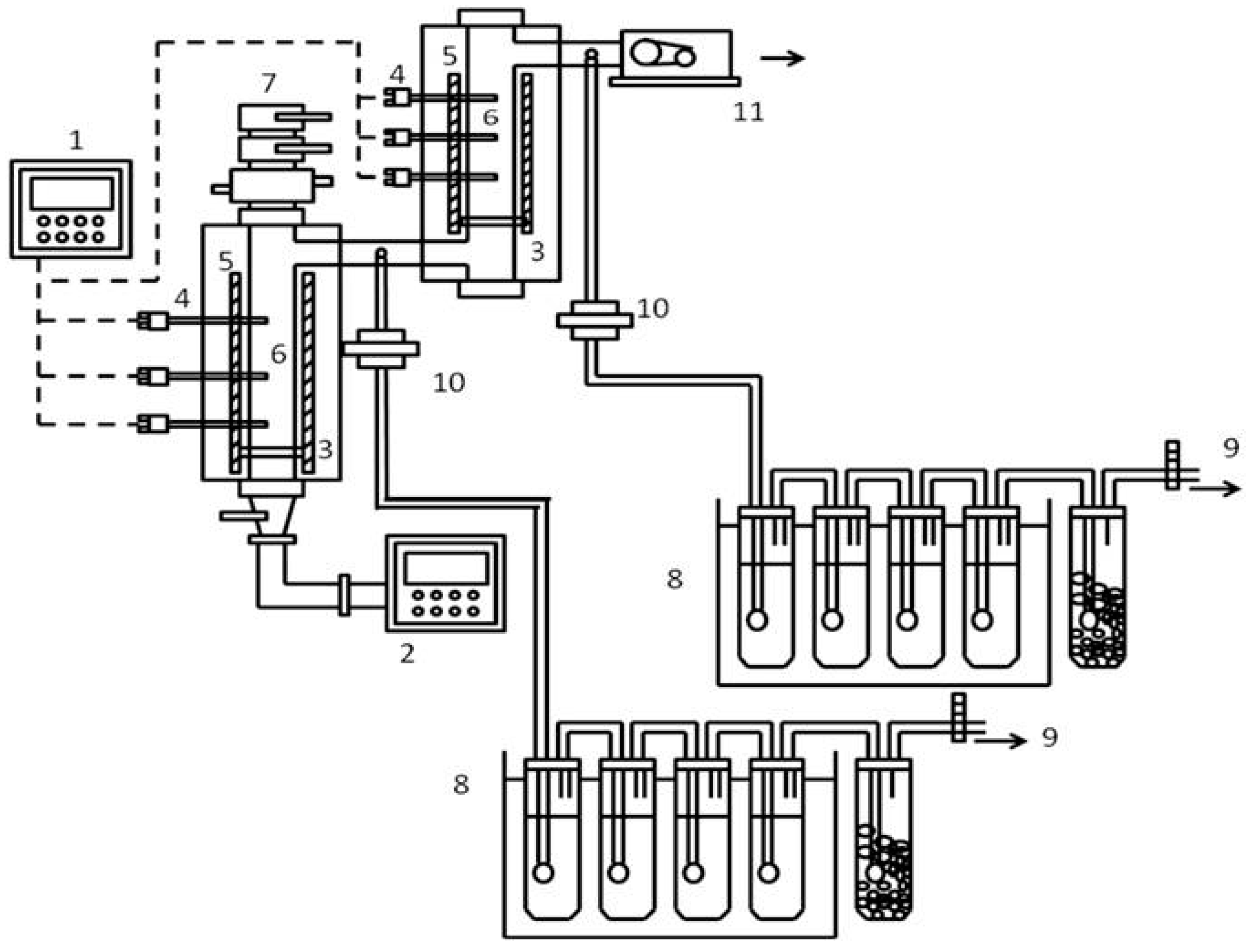
2. Materials and Methods
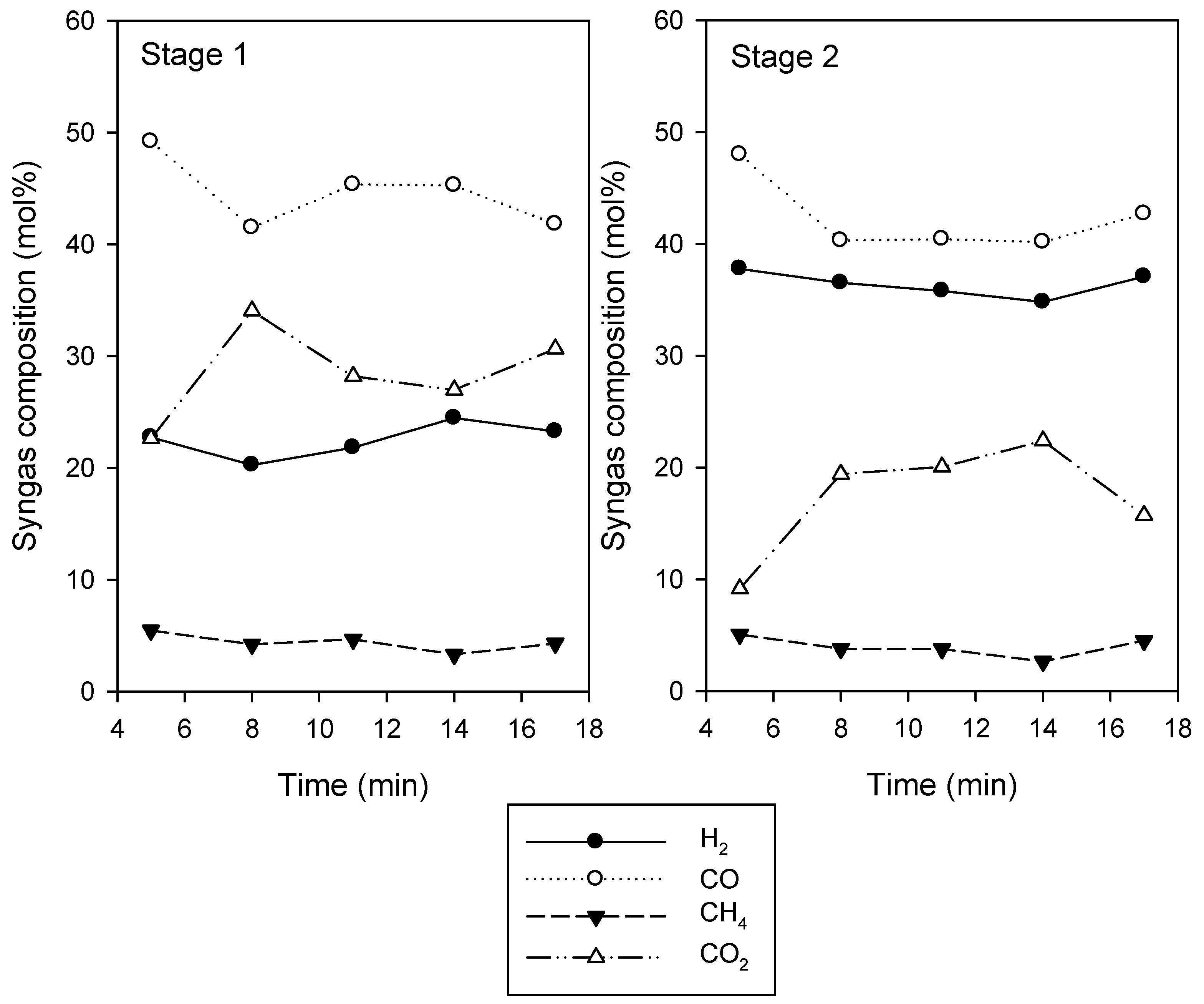
3. Results and Discussion
3.1. Influence of Different Operating Temperatures on Heavy Metal Concentrations in Bed Material and Fly Ash
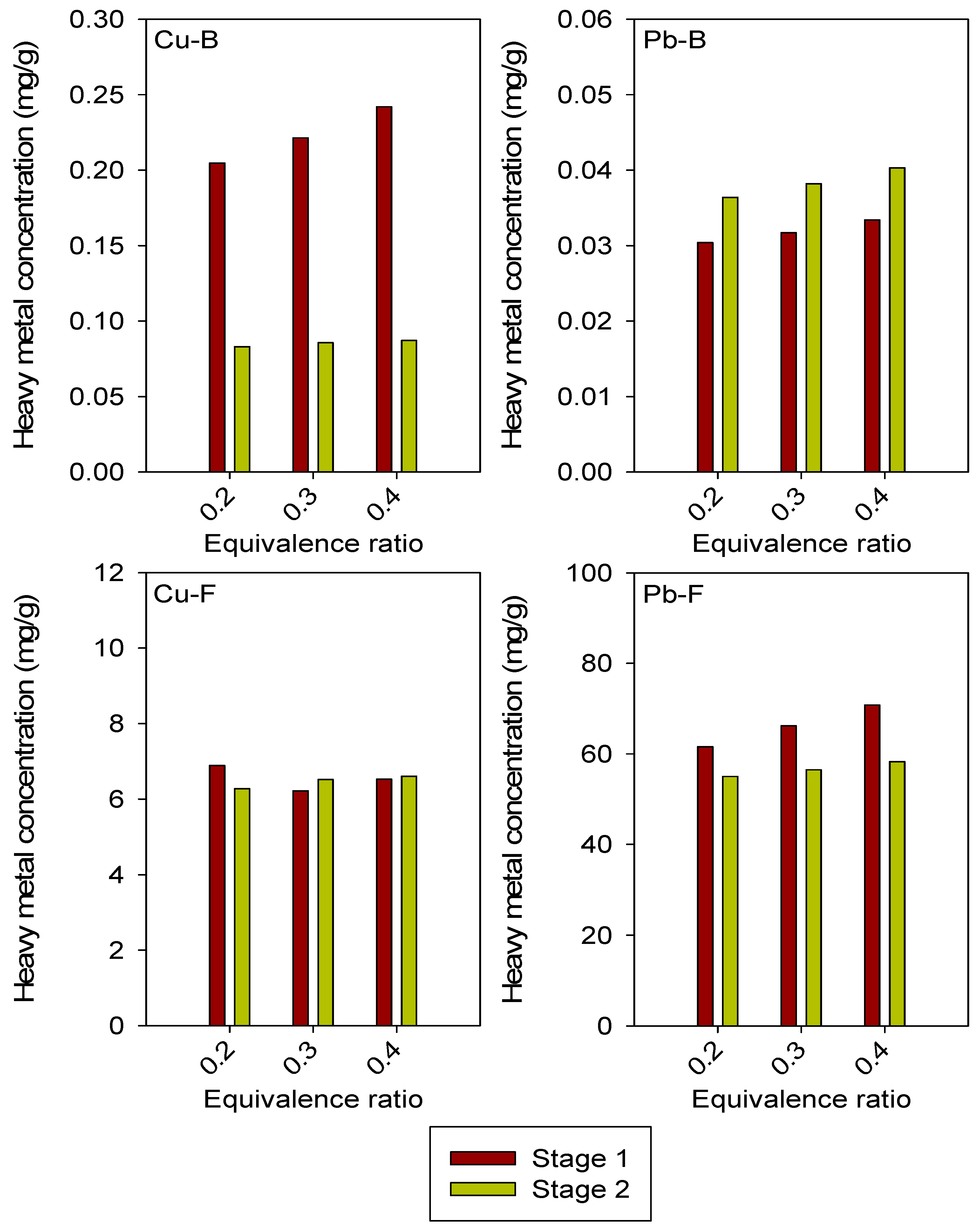
3.2. Influence of Different Equivalence Ratio Values on Heavy Metal Concentrations in Bed Material and Fly Ash
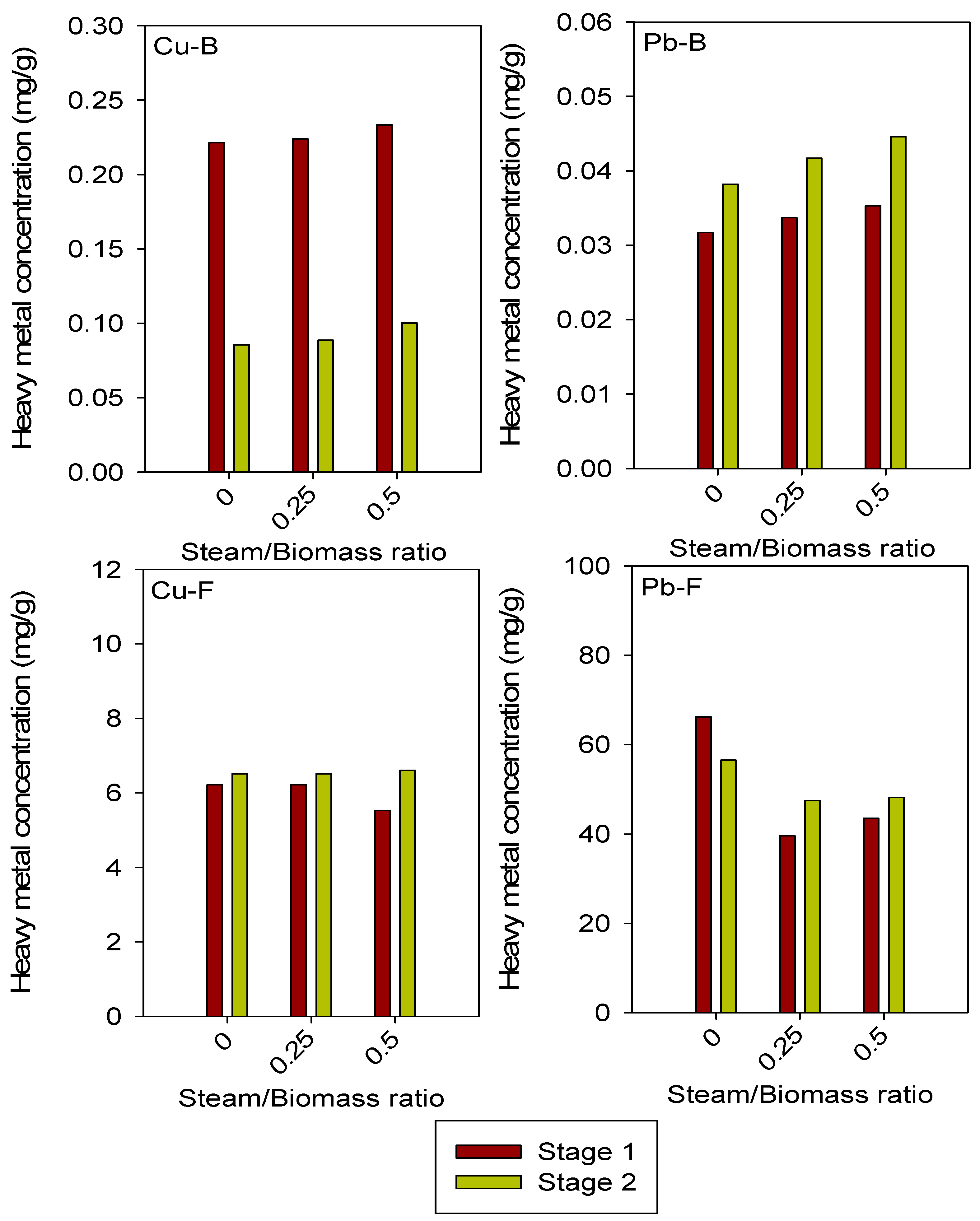
3.3. Influence of Different Steam/Biomass Ratio Values on Heavy Metal Concentrations in Bed Material and Fly Ash
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colpan, C.O.; Hamdullahpur, F.; Dincer, I.; Yoo, Y. Effect of gasification agent on the.performance of solid oxide fuel cell and biomass gasification systems. Int. J. Hydrog. Energy 2010, 356, 5001–5009. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Kumar, A.; Eskridge, K.; Jones, D.D.; Hanna, M.A. Steam-air fluidized bed gasification of distillers grains: Effects of steam to biomass ratio, equivalence ratio and gasification temperature. Bioresour. Technol. 2009, 100, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Alauddin, Z.A.B.Z.; Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Gasification of lignocellulosic biomass in fluidized beds for renewable energy development: A review. Renew. Sustain. Energy Rev. 2010, 14, 2852–2862. [Google Scholar] [CrossRef]
- Kaneko, K.; Li, L.; Matsushima, A.; Sato, H.; Shimizu, T.; Kim, H.; Takarada, T. Biomass Volatile Decomposition with a Novel Ni Loaded Brown Coal Char at Extremely Low Temperature. J. Chem. Eng. Jpn. 2016, 49, 294–299. [Google Scholar] [CrossRef]
- Kobayashi, N.; Suami, A.; Itaya, Y. Co-gasification Behavior of Woody Biomass and Coal in an Entrained Down-Flow Gasifier. J. Chem. Eng. Jpn. 2017, 50, 862–870. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S.; Guo, X.; He, M. Hydrogen-rich gas from catalytic steam gasification of biomass in a fixed bed reactor: Influence of temperature and steam on gasification performance. Int. J. Hydrog. Energy 2009, 34, 2191–2194. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Ollero, P.; Leckner, B. Optimization of char and tar conversion in fluidized bed biomass gasifiers. Fuel 2013, 103, 42–52. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C. A novel reforming method for hydrogen production from biomass steam gasification. Bioresour. Technol. 2009, 100, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, S.P.; Xie, D.Y.; Yan, Y.J. A novel integrated process for hydrogen production from biomass. Int. J. Hydrog. Energy. 2014, 39, 1274–1279. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air–steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef]
- Chiang, K.Y.; Chien, K.L.; Lu, C.H. Hydrogen energy production from disposable chopsticks by a low temperature catalytic gasification. Int. J. Hydrog. Energy 2012, 37, 15672–15680. [Google Scholar] [CrossRef]
- Gregorio, F.D.; Zaccariello, L. Fluidized bed gasification of a packaging derived fuel: Energetic, environmental and economic performances comparison for waste-to-energy plants. Energy 2012, 42, 331–341. [Google Scholar] [CrossRef]
- Zainal, Z.A.; Rifau, A.; Quadir, G.A.; Seetharamu, K.N. Experimental investigation of a downdraft biomass gasifier. Biomass Bioenerg. 2002, 23, 283–289. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, G.; You, Y.; Xiao, B.; Liu, S.; He, P.; Guo, D.; Guo, X.; Zhang, G. Hydrogen-rich gas production by steam gasification of municipal solid waste (MSW) using NiO supported on modified dolomite. Int. J. Hydrog. Energy 2012, 37, 6503–6510. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Juárez, M.C.; Morales, M.P.; Muñoz, P.; Mendívil, M.A. Biomass gasification for electricity generation: Review of current technology barriers. Renew. Sustain. Energy Rev. 2013, 18, 174–183. [Google Scholar] [CrossRef]
- Dascomb, J.; Krothapalli, A.; Fakhrai, R. Thermal conversion efficiency of producing hydrogen enriched syngas from biomass steam Gasification. Int. J. Hydrog. Energy 2013, 38, 11790–11798. [Google Scholar] [CrossRef]
- Ge, H.; Guo, W.; Shen, L.; Song, T.; Xiao, J. Biomass gasification using chemical looping in a 25 kWth reactor with natural hematite as oxygen carrier. Chem. Eng. J. 2016, 286, 174–183. [Google Scholar] [CrossRef]
- Baba, A.; Gurdal, G.; Sengunalp, F. Leaching characteristics of fly ash from fluidized bed combustion thermal power plant: Case study: Çan (Çanakkale-Turkey). Fuel Process. Technol. 2010, 91, 1073–1080. [Google Scholar] [CrossRef]
- Yao, J.; Li, W.B.; Kong, Q.N.; Wu, Y.Y.; He, R.; Shen, D.S. Content, mobility and transfer behavior of heavy metals in MSWI bottom ash in Zhejiang province, China. Fuel 2010, 89, 616–622. [Google Scholar] [CrossRef]
- Zhang, H.; He, P.J.; Shao, L.M. Implication of heavy metals distribution for a municipal solid waste management system—A case study in Shanghai. Sci. Total Environ. 2008, 402, 257–267. [Google Scholar] [CrossRef]
- Barton, R.G.; Clark, W.D.; Seeker, W.R. Fate of metals in waste combustion system. Combust. Sci. Technol. 1990, 74, 327–342. [Google Scholar] [CrossRef]
- Lin, C.L. Effects of fluidized parameters on capture of heavy metal in various bed-material size distributions. Fuel Process. Technol. 2013, 106, 149–159. [Google Scholar] [CrossRef]
- Lin, C.L.; Wu, M.H.; Weng, W.C. Effect of the type of bed material in two-stage fluidized bed gasification reactors on hydrogen gas synthesis and heavy metals distribution. Int. J. Hydrog. Energy 2019, 44, 5633–5639. [Google Scholar] [CrossRef]
- Hiraoka, M.; Takeda, N. Behavior of hazardous substances in stabilization and solidification processes of industrial wastes. In Toxic and Hazardous Waste Disposal; Pojasek, R.B., Ed.; Ann Arbor Science: Ann Arbor, MI, USA, 1980; Volume 3, pp. 107–124. [Google Scholar]
- Gerstle, R.W.; Albrinck, D.N. Atmospheric emissions of metals from sewage sludge incineration. J. Air Pollut. Control assoc. 1982, 32, 1113–1123. [Google Scholar] [CrossRef]
- Soni, C.G.; Wang, Z.; Dalai, A.K.; Pugsley, T.; Fonstad, T. Hydrogen production via gasification of meat and bone meal in two-stage fixed bed reactor system. Fuel 2009, 88, 920–925. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, F.; Li, H.; Wang, Y.; Dong, L.; Yu, J.; Xu, G. Pilot verification of a low-tar two-stage coal gasification process with a fluidized bed pyrolyzer and fixed bed gasifier. Appl. Energy. 2014, 115, 9–16. [Google Scholar] [CrossRef]
- Lin, C.L.; Weng, W.C. Effects of different operating parameters on the syngas composition in a two-stage gasification process. Renew. Energy 2017, 109, 135–143. [Google Scholar] [CrossRef]
- Lin, C.L.; Wey, M.Y.; You, S.D. The effect of particle size distribution on minimum fluidization velocity at high temperature. Powder Technol. 2002, 126, 297–301. [Google Scholar] [CrossRef]
- Jakob, A.; Stucki, S.; Kuhn, P. Evaporation of heavy metals during the heat treatment of municipal solid waste incinerator fly ash. Environ. Sci. Technol. 1995, 29, 2429–2436. [Google Scholar] [CrossRef]
- Sun, C.J.; Li, M.G.; Gau, S.H.; Wang, Y.H.; Jan, Y.L. Improving the mechanical characteristics and restraining heavy metal evaporation from sintered municipal solid waste incinerator fly ash by wet milling. J. Hazard. Mater. 2011, 195, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wey, M.Y.; Ou, W.Y.; Liu, Z.S.; Tseng, H.H.; Yang, W.Y.; Chiang, B.C. Pollutants in incineration flue gas. J. Hazard. Mater. 2001, 82, 247–262. [Google Scholar] [CrossRef]
- Chiang, B.C.; Wey, M.Y.; Yeh, C.L. Control of acid gases using a fluidized bed adsorber. J. Hazard. Mater. 2003, 101, 259–272. [Google Scholar] [CrossRef]
- Wey, M.Y.; Chen, K.H.; Liu, K.Y. The effect of ash and filter media characteristics on particle filtration efficiency in fluidized bed. J. Hazard. Mater. 2005, 121, 175–181. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass-a brief review. J. Hazard. Mater. 2013, 256–257, 56–66. [Google Scholar] [CrossRef] [PubMed]

| Run | Temperature (°C) | S/B | ER | |
|---|---|---|---|---|
| Stage 1 | Stage 2 | |||
| 1–3 | 700 | 900 | 0 | 0.2, 0.3, 0.4 |
| 4–6 | 800 | 900 | 0 | 0.2, 0.3, 0.4 |
| 7–9 | 900 | 900 | 0 | 0.2, 0.3, 0.4 |
| 10–12 | 700, 800, 900 | 900 | 0.25 | 0.3 |
| 13–15 | 700, 800, 900 | 900 | 0.5 | 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-L.; Chou, J.-D.; Weng, W.-C. Partition of Cu and Pb in a Two-Stage Fluidized-Bed Waste Gasification System. Appl. Sci. 2019, 9, 1576. https://doi.org/10.3390/app9081576
Lin C-L, Chou J-D, Weng W-C. Partition of Cu and Pb in a Two-Stage Fluidized-Bed Waste Gasification System. Applied Sciences. 2019; 9(8):1576. https://doi.org/10.3390/app9081576
Chicago/Turabian StyleLin, Chiou-Liang, Jing-Dong Chou, and Wang-Chang Weng. 2019. "Partition of Cu and Pb in a Two-Stage Fluidized-Bed Waste Gasification System" Applied Sciences 9, no. 8: 1576. https://doi.org/10.3390/app9081576
APA StyleLin, C.-L., Chou, J.-D., & Weng, W.-C. (2019). Partition of Cu and Pb in a Two-Stage Fluidized-Bed Waste Gasification System. Applied Sciences, 9(8), 1576. https://doi.org/10.3390/app9081576




