Effects of Some Hill Reaction-Inhibiting Herbicides on Nitrous Oxide Emission from Nitrogen-Input Farming Soil
Abstract
:1. Introduction
2. Materials and Methods
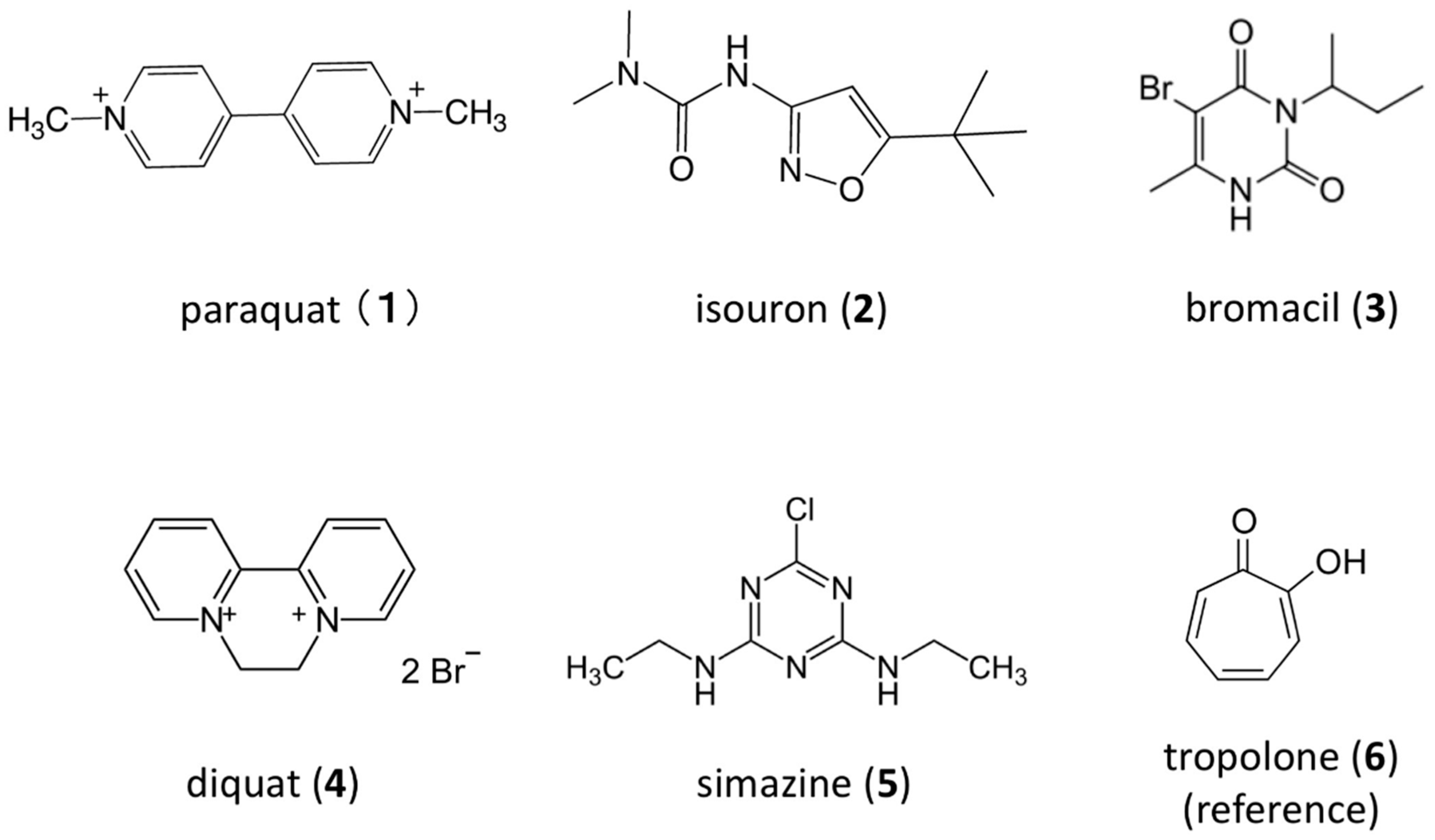
2.1. Chemicals
2.2. N2O Emitting Soil for N2O Emission Assay
2.3. Culture-Based N2O-Emission Inhibition Assay Using Hill Reaction Inhibitors towards an Incomplete Denitrifier Isolated from the Andisol
2.4. Soil Bed-Based N2O Emission with Sucrose Supplementation
2.5. Soil Bed-Based N2O Emission Inhibition Assay
2.6. Quantitative Measurement of N2O in Headspace
2.7. Effects of Hill Reaction-Inhibiting Herbicides on N2O Quenching Chitinophaga
2.8. Statistical Analysis
3. Results
3.1. Acceleration of N2O Emission in Soil Bed Culture Supplemented with Nitrogen Substrate and Sucrose
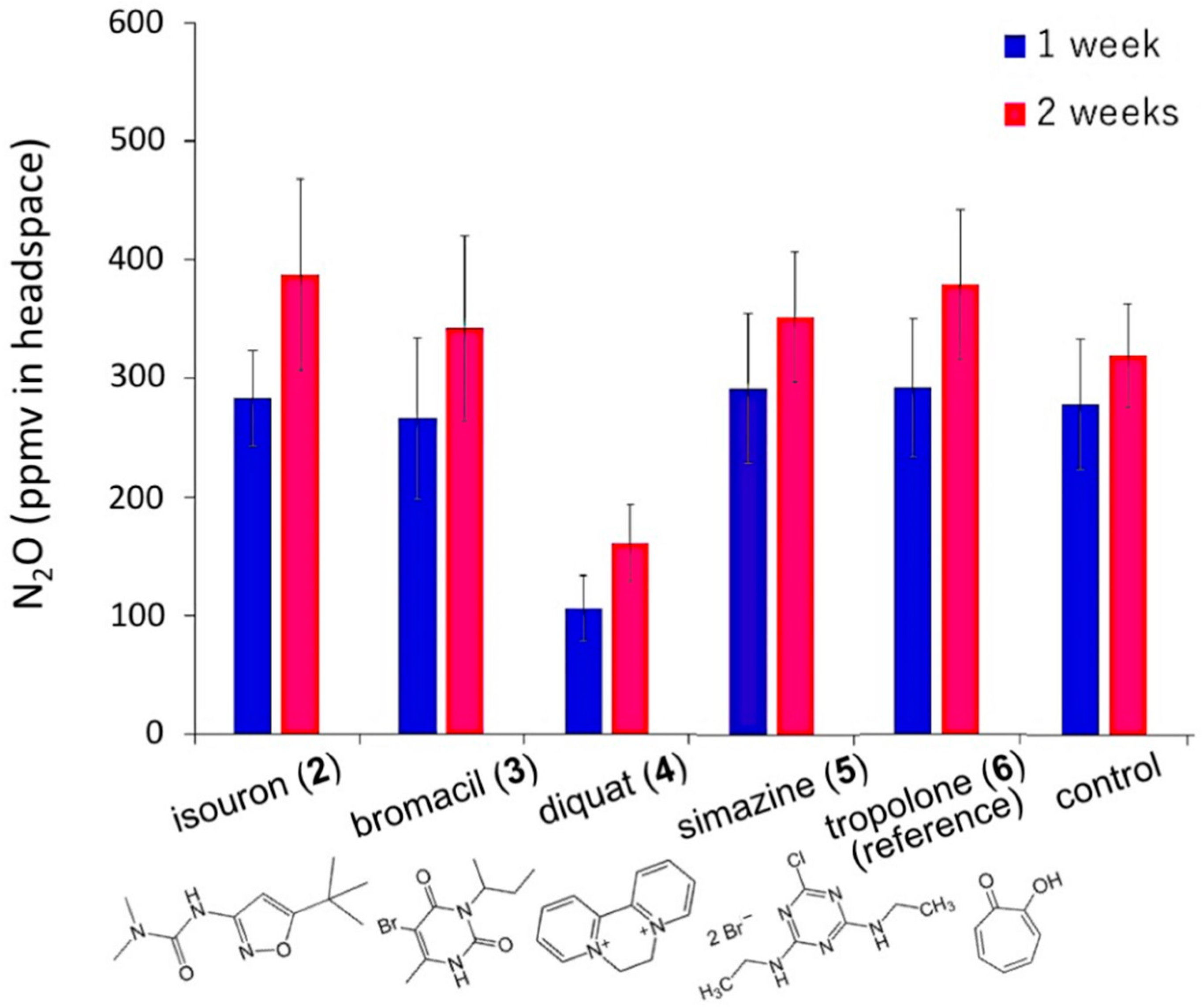
3.2. Suppression of N2O Emission from Soft Gel Medium Adding Hill Reaction-Inhibiting Herbicides
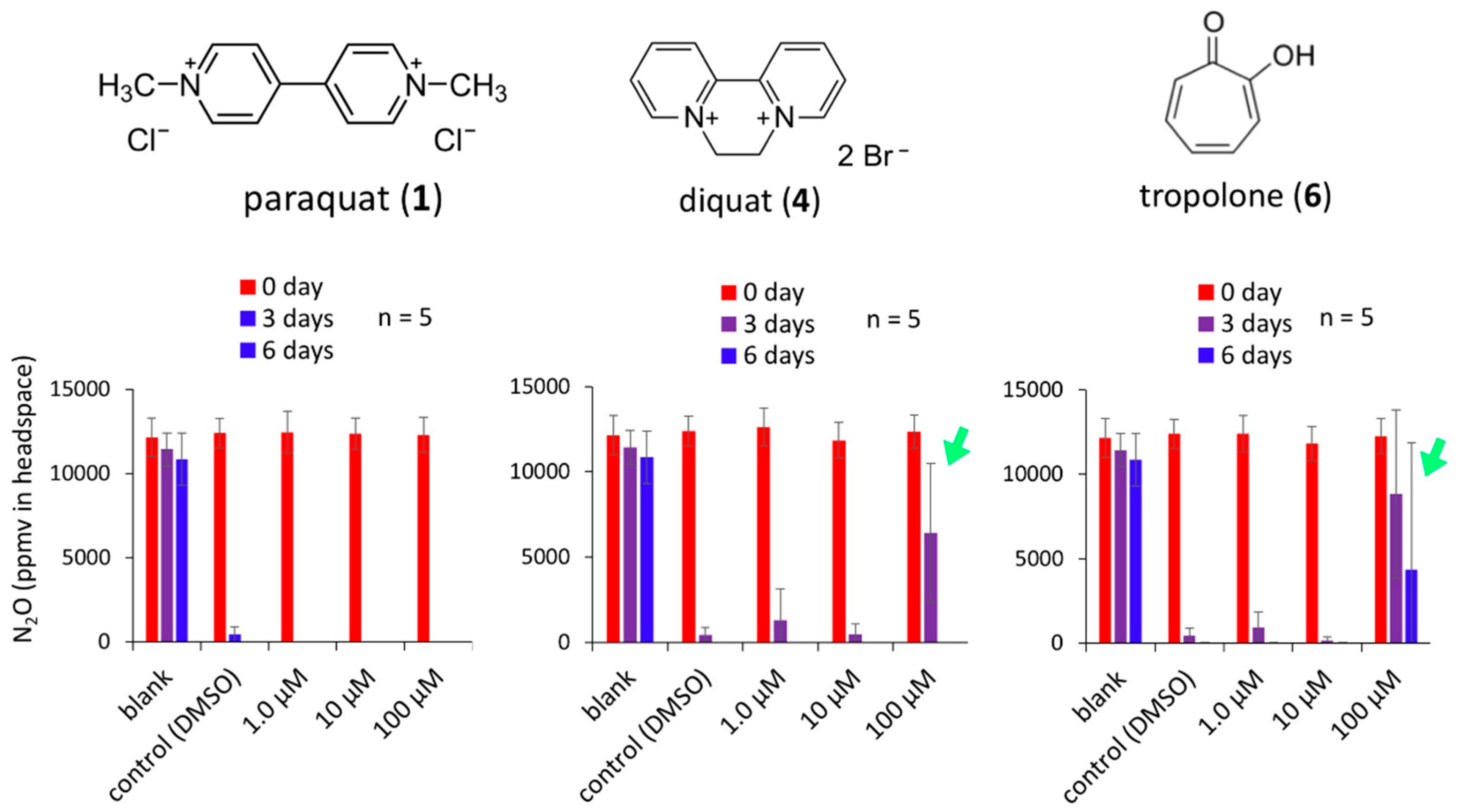
3.3. Suppression of N2O Emission from an Autoclaved Soil of N2O Emission Hotspot Andisol Followed by Inoculation with Pseudomonas sp. 05CFM15-6D
3.4. Suppressing Action of the Herbicides on Actively N2O Quenching Chitinophaga Isolated from an Andisol Corn Farm Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Groenigen, J.W.; Huygens, D.; Boeckx, P.; Kuyper, T.W.; Lubbers, I.M.; Rütting, T.; Groffman, P.M. The soil N cycle: New insights and key challenges. Soil 2015, 1, 235–256. [Google Scholar] [CrossRef]
- Ollivier, J.; Töwe, S.; Bannert, A.; Hai, B.; Kastl, E.-M.; Meyer, A.; Su, M.X.; Kleineidam, K.; Schloter, M. Nitrogen turnover in soil and global change. FEMS Microbiol. Ecol. 2011, 78, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbarao, G.V.; Ban, T.; Kishii, M.; Ito, O.; Samejima, H.; Wang, H.Y.; Pearse, S.J.; Gopalakrishnan, S.; Nakahara, K. Can biological nitrification inhibition (BNI) genes from perennial Leymus racemosus (Triticeae) combat nitrification in wheat farming? Plant Soil 2007, 299, 55–64. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Ishikawa, T.; Ono, H.; Yoshida, M.; Yoshihashi, T.; Zhu, Y.; Zakir, H.A.K.M.; Deshpande, S.P.; Hash, C.T.; et al. Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 2013, 366, 243–259. [Google Scholar] [CrossRef]
- Tesfamariam, T.; Yoshinaga, H.; Deshpande, S.P.; Pinnamaneni, R.P.; Sahrawat, K.L.; Ando, Y.; Nakahara, K.; Hash, C.T.; Subbarao, G.V. Biological nitrification inhibition in sorghum: The role of sorgoleone production. Plant Soil 2014, 379, 325–335. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.P.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, A.T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Ward, B.B.; Devol, A.H.; Rich, J.J.; Chang, B.X.; Bulow, S.E.; Naik, H.; Pratihary, A.; Jayakumar, A. Denitrification as the dominant nitrogen loss process in the Arabian Sea. Nature 2009, 461, 78–82. [Google Scholar] [CrossRef]
- Kara, E.E.; Arli, M.; Uygur, V. Effects of the herbicide Topogard on soil respiration, nitrification, and denitrification in potato-cultivated soils differing in pH. Biol. Fertil. Soils 2004, 39, 474–478. [Google Scholar]
- Weller, S.; Fischer, A.; Willibald, G.; Navé, B.; Kiese, R. N2O emissions from maize production in South-West Germany and evaluation of N2O mitigation potential under single and combined inhibitor application. Agric. Ecosyst. Environ. 2019, 269, 215–223. [Google Scholar] [CrossRef]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2018, 2, 410–416. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Bergaust, L.; Mao, Y.J.; Bakken, L.R.; Frostegard, A. Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrous oxide reductase in Paracoccus denitrificans. Appl. Environ. Microbiol. 2010, 76, 6387–6396. [Google Scholar] [CrossRef]
- Liu, B.B.; Morkved, P.T.; Frostegard, A.; Bakken, L.R. Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol. Ecol. 2010, 72, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Frostegård, Å.; Bakken, L.R. Impaired reduction of N2O to N2 in acid soils is due to a posttranscriptional interference with the expression of nosZ. mBio 2014, 5, e01383-14. [Google Scholar] [CrossRef]
- Stępniewski, W.; Stępniewska, Z. Selected oxygen-dependent process-response to soil management and tillage. Soil Tillage Res. 2009, 102, 193–200. [Google Scholar] [CrossRef]
- Leppelt, T.; Dechow, R.; Gebbert, S.; Freibauer, A.; Lohila, A.; Augustin, J.; Drösler, M.; Fiedler, S.; Glatzel, S.; Höper, H.; et al. Nitrous oxide emission budgets and land-use-driven hotspots for organic soils in Europe. Biogeosciences 2014, 11, 6595–6612. [Google Scholar] [CrossRef] [Green Version]
- Melling, L.; Hatano, R.; Goh, K.J. Nitrous oxide emissions from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Sci. Plant Nutr. 2007, 53, 792–805. [Google Scholar] [CrossRef] [Green Version]
- Akhira, N.I.M.; Kusin, F.M.; Mohamat-Yusuff, F.; Awang, M.; Ashaaria, Z.H. Impact of nitrogen fertilizer application on nitrous oxide emission in oil palm plantation. Procedia Environ. Sci. 2015, 30, 315–319. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Hatano, R.; Hashidoko, Y. Effects of methyl viologen dichloride and other chemicals on nitrous oxide (N2O) emission and repression by pseudomonad denitrifiers isolated from corn farmland soil in Hokkaido, Japan. J. Pestic. Sci. 2014, 39, 115–120. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Isoda, R.; Wang, M.; Hatano, R.; Hashidoko, Y. Physiological and genotypic characteristics of nitrous oxide (N2O)-emitting Pseudomonas species isolated from dent corn Andisol farmland in Hokkaido, Japan. Microbes Environ. 2016, 31, 93–103. [Google Scholar] [CrossRef]
- Tollefson, J. Intensive farming may ease climate change. Nature 2010, 465, 853. [Google Scholar] [CrossRef]
- Tyler, H.L.; Locke, M.A.; Duke, S.O. Effect of weed management. In Weed Control: Sustainability, Hazards, and Risks in Cropping Systems Worldwide; Korres, N.E., Burgos, N.R., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 32–61. [Google Scholar]
- Funderburk, H.H., Jr.; Lawrence, J.M. Mode of action and metabolism of diquat and paraquat. Weeds 1964, 12, 259–264. [Google Scholar] [CrossRef]
- Dodge, A.D. The mode of action of the bipyridylium herbicides, paraquat and diquat. Endeavour 1971, 30, 130–135. [Google Scholar] [CrossRef]
- Katayanagi, N.; Sawamoto, T.; Hayakawa, A.; Hatano, R. Nitrous oxide and nitric oxide fluxes from cornfield, grassland, pasture and forest in a watershed in Southern Hokkaido, Japan. Soil Sci. Plant Nutr. 2008, 54, 662–680. [Google Scholar] [CrossRef] [Green Version]
- Takeda, H.; Takahashi, N.; Hatano, R.; Hashidoko, Y. Active N2O emission from bacterial microbiota of Andisol farmland and characterization of some N2O emitters. J. Basic Microbiol. 2012, 52, 477–486. [Google Scholar] [CrossRef]
- Hashidoko, Y.; Takakai, F.; Toma, Y.; Darung, U.; Melling, L.; Tahara, S.; Hatano, R. Emergence and behaviors of acid-tolerant Janthinobacterium sp. that evolves N2O from deforested tropical peatland. Soil Biol. Biochem. 2008, 40, 116–125. [Google Scholar] [CrossRef]
- Hara, S.; Hashidoko, Y.; Desyatkin, R.; Hatano, R.; Tahara, S. High rate of N2-fixation by East Siberian cryophilic soil bacteria measured by acetylene reduction in nitrogen-poor medium solidified with gellan gum. Appl. Environ. Microbiol. 2009, 75, 2811–2819. [Google Scholar] [CrossRef]
- Takatsu, Y.; Achiwa, N.; Vainio, L.; Tahvanainen, T.; Hashidoko, Y. Relationship of nitrous oxide production and consumption by collapsed palsa. In Proceedings of the International Conference for Soil Science, New Orleans, LA, USA, 25–26 January 2018. [Google Scholar]
- Azegami, K.; Nishiyama, K.; Kato, H. Effect of iron limitation on “Pseudomonas plantarii” growth and tropolone and protein production. Appl. Environ. Microbiol. 1988, 54, 844–847. [Google Scholar]
- Nishizawa, M.; Sakai, S.; Konno, U.; Nakahara, N.; Takaki, Y.; Saito, Y.; Imachi, H.; Tasumi, E.; Makabe, A.; Koba, K.; et al. Nitrogen and oxygen isotope effects of ammonia oxidation by thermophilic Thaumarchaeota from a geothermal water stream. Appl. Environ. Microbiol. 2016, 82, 4492–4504. [Google Scholar] [CrossRef]
- Lau, S.Y.L.; Takatsu, Y.; Melling, L.; Hashidoko, Y. Chitinophaga spp., potent N2O quenchers from deep soils of oil palm plantation located in reclaimed tropical peatland in Sarawak, Malaysia, and Andisol corn farmland in Hokkaido, Japan. manuscript in preparation.
- Kyaw, K.M.; Toyota, K. Suppression of nitrous oxide production in soils applied with organic matter by the herbicides glyphosate and propanil. Soil Sci. Plant Nutr. 2007, 53, 441–447. [Google Scholar] [CrossRef]
- Bardon, C.; Poly, F.; Piola, F.; Pancton, M.; Comte, G.; Meiffren, G.; Haichar, F.Z. Mechanism of biological denitrification inhibition: Procyanidins induce an allosteric transition of the membrane-bound nitrate reductase through membrane alteration. FEMS Microbiol. Ecol. 2016, 92, fiw034. [Google Scholar] [CrossRef]
- Torralbo, F.; Menéndez, S.; Barrena, I.; Estavillo, J.M.; Marino, D.; González-Murua, C. Dimethyl pyrazol-based nitrification inhibitors effect on nitrifying and denitrifying bacteria to mitigate N2O emission. Sci. Rep. 2017, 7, 13810. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takatsu, Y.; Lau, S.Y.L.; Li, L.; Hashidoko, Y. Effects of Some Hill Reaction-Inhibiting Herbicides on Nitrous Oxide Emission from Nitrogen-Input Farming Soil. Appl. Sci. 2019, 9, 1903. https://doi.org/10.3390/app9091903
Takatsu Y, Lau SYL, Li L, Hashidoko Y. Effects of Some Hill Reaction-Inhibiting Herbicides on Nitrous Oxide Emission from Nitrogen-Input Farming Soil. Applied Sciences. 2019; 9(9):1903. https://doi.org/10.3390/app9091903
Chicago/Turabian StyleTakatsu, Yuta, Sharon Y. L. Lau, Li Li, and Yasuyuki Hashidoko. 2019. "Effects of Some Hill Reaction-Inhibiting Herbicides on Nitrous Oxide Emission from Nitrogen-Input Farming Soil" Applied Sciences 9, no. 9: 1903. https://doi.org/10.3390/app9091903
APA StyleTakatsu, Y., Lau, S. Y. L., Li, L., & Hashidoko, Y. (2019). Effects of Some Hill Reaction-Inhibiting Herbicides on Nitrous Oxide Emission from Nitrogen-Input Farming Soil. Applied Sciences, 9(9), 1903. https://doi.org/10.3390/app9091903




