Abstract
The penetration of optical clearing agents (OCAs) is restricted by the natural barrier function of the stratum corneum (SC) of the skin, which can be breached by physical and chemical methods to enhance the transcutaneous delivery of OCAs. To breach the barrier function of SC, we carried out the in vivo experimental study to enhance the optical clearing effect of PEG-400 by laser irradiation in conjunction with a chemical penetration enhancer thiazone. We compared the reflectance spectra of skin without laser irradiation or thiazone. Mono-treatment of thiazone could not significantly enhance the optical clearing efficacy of the skin. After 60 min, the reflectance spectrum decreased by only approximately 10%. With the combined treatment, the reflectance spectrum decreased by approximately 30% after 10 min. Subsequently, the effect of laser dose on the enhancement of optical clearing efficacy was studied. The optimal irradiation dose was determined. The reflectance of skins irradiated by a laser dose at 0.7 J/cm2 decreased by approximately 10% and were 20% lower than those at 0.5 and 0.9 J/cm2. The laser at 0.5 J/cm2 could not damage the SC completely, whereas the laser at 0.9 J/cm2 influenced the epidermis and dermis; thus, the reflectance of skin samples irradiated by 0.9 J/cm2 did not decrease.
1. Introduction
The skin is the largest and outermost organ. As a typical turbid medium, the skin exhibits high scattering characteristics that limit the penetration depth of light, which is detrimental to in vivo optical imaging. The optical clearing technique can significantly improve the quality of biomedical optical imaging. Introducing hyperosmotic and high refractive optical clearing agents (OCAs) into the dermis can decrease the scattering and increase the light penetration depth by matching the refractive indexes of visible components, which are mostly collagen fibers and elastic fibers, and invisible components, such as liquid matrix, in the skin []. Early studies on skin tissue photometry were mainly performed on in vitro skin, for example, immersion has been used to enhance the effect of optical clearing []. As a common method, OCAs are intradermally injected to improve the optical clearing of in vivo skin effectively []. However, this method is not widely available because OCAs with high concentration can induce visible skin lesions, such as edema, suppuration, or scarring. In view of this effect, many studies have focused on the enhancement of optical clearing effect by different physical and chemical methods, such as removal of stratum corneum (SC) using sandpaper [] and perforation of the skin []. However, these methods are difficult to quantify in operating strength, easily damage the skin, and show poor experimental repeatability. Thus, the efficacy of optical clearing is not satisfactory.
Achieving optical clearing by skin surface application is relatively safe and has been studied by many researchers. The penetration of OCAs is restricted by the natural barrier function of SC of the skin. Many physical and chemical methods have been proposed to enhance the transcutaneous delivery of OCAs to breach the barrier function of SC. Chemical enhancers include alcohol, propylene glycol (PG), azone, thiazone, dimethylsulfoxide, fatty acids, and oleic acid [,,,]. The polar molecules of these chemical enhancers can bind to a variety of organic compounds, such as lipid molecules in SC with a small molecular weight and high permeability, which is beneficial to enhance the transcutaneous delivery of drugs and cosmetics. Wang et al. [] compared thiazone with azone and PG and found that thiazone possesses the highest penetration-enhancing effect for in vivo skin. Zhong and Guo [] applied a mixture of PEG-400 and thiazone on in vivo rat skin and then monitored the changes of reflectance to measure the optical clearing efficacy by VIS-NIR spectrometer. They found that the reflectance spectrum of in vivo skin with the implementation of the mixture is significantly lower than that with pure PEG-400. Wang et al. [] found that no histopathologic or microstructural changes in rat skin are induced by administration of the mixture of PEG-400 and thiazone. Rui et al. [] proposed a method to obtain the best optical clearer based on the above method. In these research works, the SC of in vivo skin must be stripped with tape several times, which is a physical method to remove a part of SC. So far, the chemical methods are useful but cannot separately breach the barrier function of SC completely and enhance the penetration of OCAs. Thus, these techniques must be combined with physical methods.
Many physical methods are used to increase the permeability of SC by removing a part of SC or increasing the penetration rate of OCAs, including the use of abrasive sandpaper [], flashlamp [], ultrasound [], microneedles [], pressure [], iontophoresis [], electroporation [], and microdermabrasion []. Compression, stretching, and UV irradiation have also been used to reduce the scattering properties of the skin []. Nevertheless, these methods would inevitably cause some invasion and damage to the active epidermis and dermis of the skin. In comparison, the non-contact light-irradiation is safer. Liu et al. [,] compared different light sources and found that the 1064 nm Nd:YAG laser irradiation with appropriate dose is able to increase penetration of glycerol in the skin and improve optical clearing of skin. Besides, the light in the near infrared band will not produce obvious side effects on the skin. However, the effect of laser irradiation on the penetration of different OCAs has not been systematically studied in vivo.
Laser irradiation can promote the penetration of glycerol into the in vivo skin, but the optical clearing efficacy is not satisfied. The quantitative study on the effect of physicochemical combined penetration enhancement technology is still lacking. To find a safe way to enhance the optical clearing of skin, VIS-NIR spectrometer was employed in this study to monitor the reflectance spectra of in vivo rat skin to determine the effect of laser-irradiation in conjunction with a chemical penetration enhancer. Taking the original reflectance as control, the reflectance with mono-treatment of chemical penetration enhancer (thiazone) and mono-treatment of laser irradiation was compared with the combination of these two strategies. And then, we measured the reflectance and transmittance of in vitro skin to prove that the combined treatment can improve the penetration depth of light in skin.
2. Materials and Methods
2.1. Experiments In Vivo
All experimental protocols and animal handling procedures were approved based on the comments of the laboratory animal care committee of Xi’an Jiaotong University Health Science Center. The corresponding ethical approval code is NO.XJTULAC2018-540. As shown in Figure 1, VIS-NIR spectrometer was used to monitor the changes in reflectance spectra of the rat skin to study the enhancement of optical clearing effect by laser irradiation in conjunction with a chemical penetration enhancer. The system was composed of the light source (SL-1), reflection probe (R400-7), spectrometer (USB-2000, Ocean Optics, USA), and the computer software Spectra Suit. The probe consisted of a bundle of seven optical fibers with six illumination fibers around one read fiber, each of which was 400 mm in diameter. The core-to-core distance between the illumination fiber and the read fiber was 487 mm. Before the experiment, the system was preheated for approximately 30 min and then standardized by a reflectance standard made up of barium sulfate. During the experiment, the probe was vertical and soft touched to in vivo dorsal skin, and the reflectance was then saved. The average value of each measurement was determined three times.
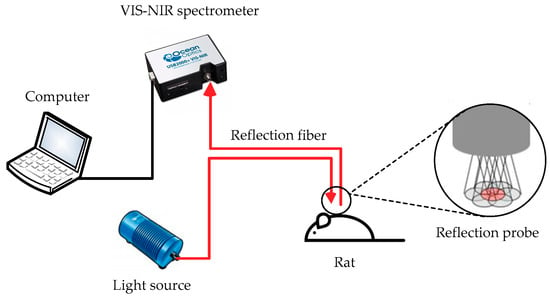
Figure 1.
Reflection spectrum measurement system diagram.
In this in vivo experiment, a total of 36 female Sprague–Dawley rats with 100 g to 120 g body weight were obtained from Xi’an Jiaotong University Health Science Center and fed under standard rodent feeding condition. Before the experiment, animals were intraperitoneally anesthetized using 10% chloral hydrate. The dorsal hair was shaved, and the residue hair was removed using 8% NaS applied for 1–2 min until the hair could be easily wiped off. The back of the rats was then cleaned with water. Measurements were delayed after 15 min of hair removal to dry the skin.
To study the effect of the chemical penetration enhancer, we prepared the OCA as follows. First, thiazone (99% purity, Wuhan Hengwo Technology Co., Ltd., China) was heated and melted in a 40 °C water bath. The chemical reagent PEG-400 (analytical reagent, Tianjin Kermel Chemical Reagent Co., Ltd., China) was then mixed with melting thiazone at a volume ratio of 9:1. PEG400 is a high refractive reagent with a refractive index of 1.47, which is similar to the refractive index of collagen fibers in the skin, and has amphipathic molecules. Thiazone is a new type of chemical penetration enhancer that can damage the SC to increase skin permeability. Thiazone is a white crystal at room temperature with a melting point of 40 °C and a refractive index of 1.576.
Q-switched 1064 nm Nd:YAG laser (WON-COSJET TR, WON Technology Co., Ltd., Korea) was adopted for the investigation of conjunction effect with thiazone, and the parameters were set, as shown in Table 1.

Table 1.
Laser parameters.
The specific steps of the experiment were as follows. First, the left side of the back skin of the rats was the experimental group, and the right side as the control and both were divided into three areas. The original reflectance spectra of six areas of the rat skin were then measured for reference. Second, the reflectance spectra of the skin were measured in the left experimental area after laser treatment, and then the OCA was applied to the three parts for 10 min, after that the agent was wiped off to eliminate the specular reflection, measured for a total of 6 times for 60 min. The control area on the right side was treated with non-laser treatment or without the addition of thiazone in PEG-400. Each different experiment was repeated at least 6 times in 6 different rats. Each representative spectra is an average of all the 18 spectra from 6 rats because each rats’ back skin was divided into 6 areas in two conditions.
2.2. Experiments In Vitro
A UV/VIS/NIR spectrophotometer (Cary 5000, Varian Company, USA) with a single integrating sphere was employed to measure the diffuse reflection spectrum and transmission spectrum of the tissue-like phantoms at 400–1000 nm.
The early steps of skin preparation are the same as in vivo. After hair removal, rats with prepared skin were sacrificed, and full-thickness skin was excised from the dorsal region. After the adipose layer had been removed, skin samples were flattened and sandwiched between the two glass slides with a dimension of 30 mm × 30 mm × 1 mm. All samples were divided into experimental and control group, with three samples in each group. Afterward, the native transmittance and reflectance spectra were also measured. The central region with a diameter of 30 mm of experimental samples was exposed to a Q-switched Nd:YAG laser, whose parameters and dose were the same as in vivo study. All skin samples were then treated with OCA on the epidermal side in the central region, with moistened gauze which was immersed in saline solution attaching the dermal side, and were covered with culture dishes. After that, the reflectance and transmittance spectra were measured from OCA treating time of 60 min. The control samples were treated with non-laser treatment or without the addition of thiazone in PEG-400.
3. Results and Discussion
3.1. Laser Irradiation and Thiazone Treatment-Induced Changes in Reflectance Spectra of Skin In Vivo
Figure 2a–c show the typical reflectance spectra of a rat skin at native state after 0.5 J/cm2 laser irradiation and 10 and 60 min after the followed topical application of PEG-400 with or without thiazone; Figure 2d shows the relative change of the reflectance in 60 min at 0.5 J/cm2 with different treatment. No change in reflectance without laser irradiation is observed. Figure 2a indicates that the mono-treatment of thiazone slightly changes reflectance statistically. The mono-treatment of thiazone did not enable PEG-400 to exert its optical clearing effect without laser irradiation. In Figure 2b,c compared with the reflectance spectrum of native skin, the decrease in reflectance after laser irradiation were 2.5%, 0.8%, and 1.4% at 475, 575, and 615 nm, respectively. After the laser irradiation, OCA is applied to the skin. The significant decrease is observed after OCA treatment. Figure 2c indicates that Nd:YAG laser irradiation reduces the reflectance of short wavelengths (400–600 nm), and the following treatment of PEG-400 with thiazone can further decrease the values by approximately 10% in 10 min and 25.2%, 23.7%, and 17.6% at 475, 575, and 615 nm wavelength in 60 min, respectively. Figure 2b indicates that the reflectance only decreases by 17.9%, 20.8%, and 13.8% without thiazone. Compared with mono-treatment of laser irradiation, combined penetration enhancement makes the penetration rate of the OCA fast, and the final optical clearing efficacy is improved.
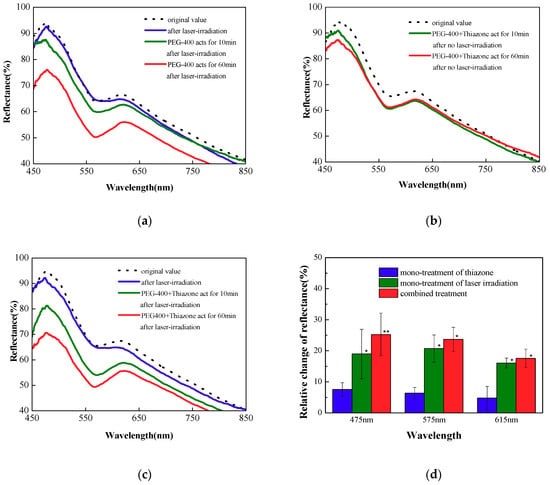
Figure 2.
Reflectance spectra of in vivo rat skin with (a) mono-treatment of thiazone without laser irradiation, (b) mono-treatment of laser irradiation at 0.5 J/cm2 without thiazone, (c) combined treatment at 0.5 J/cm2. (d) The relative change of the reflectance in 60 min at 0.5 J/cm2 with different treatment, * p < 0.05; ** p < 0.01.
3.2. Combined Treatment of Different Doses of Laser Irradiation and Thiazone-Induced Changes in Reflectance Spectra of Skin In Vivo
Figure 3a,b show the effect of laser irradiation dose on the combined penetration enhancement; Figure 3c,d show the relative change of the reflectance in 60 min at 0.7 and 0.9 J/cm2 with different treatment. As shown in Figure 3a, when the laser irradiation dose increases to 0.7 J/cm2, the decrease in reflectance after laser irradiation were 5.2%, 5.1%, and 7.0% at 475, 575, and 615 nm, respectively. The combined penetration enhancement method achieves the best optical clearing effect at 10 min (the green solid line), and the reflectance of the skin decreases significantly, between 450–600 nm, by 30.9%, 28.7% and 28.2% at 475, 575, and 615 nm, respectively. By comparing the reflectance spectrum with the combined penetration enhancement method with that of the mono-treatment of laser irradiation at 10 min (the red solid line with square symbols), the penetration rate of the combined penetration enhancement method becomes increasingly fast. By comparing the combined penetration enhancement method with thiazone mono-treatment (the green solid line with triangle symbols), the thiazone mono-treatment cannot achieve any optical clearing effect. This effect is similar to the situation at 0.5 J/cm2 laser irradiation dose. The situation is different when the laser irradiation dose is further increased to 0.9 J/cm2. Figure 3b indicates that elevated laser dose causes more significant changes in reflectance, and further treatment of PEG-400 with thiazone cannot reduce the reflectance. Optical clearing effect is almost ineffective statistically, but the reflectance of the skin decreases more significantly after laser irradiation than other doses, especially at 575 nm with an 8.9% decrease. Though increased irradiation dose decreases the reflectance in short wavelengths, the reflectance is not further reduced after the treatment of the OCA. This result may be due to the scattering and absorption of the skin to light, leading to the thermal damage of the epidermis and the dermis, which forms the thermal solidification area that is not conducive to the penetration of the OCA.
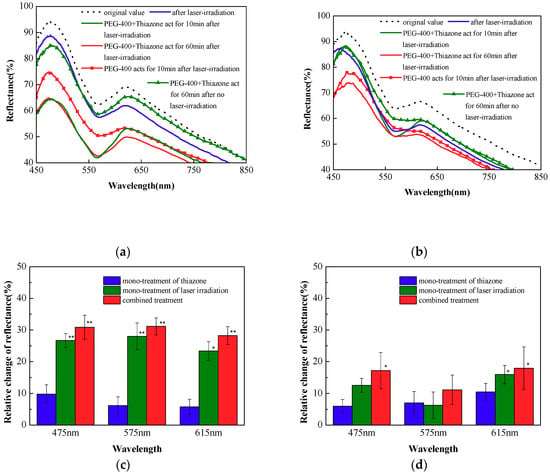
Figure 3.
Reflectance spectra of in vivo rat skin with three different treatments at (a) 0.7 and (b) 0.9 J/cm2. The relative change of the reflectance in 60 min at (c) 0.7 and (d) 0.9 J/cm2 with different treatment, * p < 0.05; ** p < 0.01.
As shown in Figure 4, we further compared the skin reflectance spectrum at 60 min for the three combined penetration enhancement methods with different laser irradiation dose to evaluate the final effect of combined penetration enhancement. The blue, green, and red solid lines represent the reflectance spectra of the skin 60 min after the application of PEG-400 and thiazone with doses of 0.5, 0.7, and 0.9 J/cm2. The difference between reflectance spectra between 450 and 600 nm is large. When the laser-irradiation dose is 0.7 J/cm2, the final optical clearing effect is most improved, and the reflectance compared with the original value is reduced by approximately 30% at 475, 575, and 615 nm. The maximum decrease of reflectance means the penetration of OCA into dermis is significantly increased after the laser irradiation. The reflectance at 0.9 J/cm2 dose compared with the original value decreases by 19.7% at 475 nm. Considering that laser irradiation reduced the reflectance by 7.2%, the optical clearing efficacy is worst. The possible reason can be explained as: too high laser dose may cause a thermal coagulation area and affect the normal physiological state of the skin, which may be an obstruction of transdermal delivery. Therefore, the optimum laser energy density in combined treatment exists.
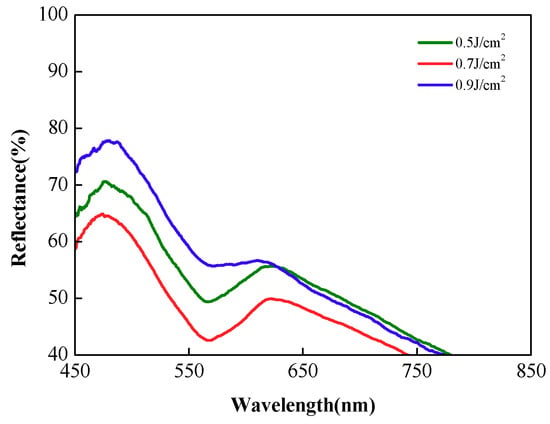
Figure 4.
Reflectance spectra of in vivo rat skin with combined treatment after different laser irradiation doses at 60 min.
3.3. Changes in Transmittance of Skin In Vitro with Different Treatments
In this study, the reflectance and transmittance of in vitro skin were measured by a spectrophotometer with an integrating sphere setup. Figure 5a,b show typical spectra of the reflectance and transmission of in vitro skin with different treatment in 60 min, respectively. Oscillations at 800 nm in spectra are created by light source change of the spectrophotometer. The reflectance and transmittance are used to measure the optical clearing efficacy: the lower the reflectance, the higher the transmittance and the better the optical clearing efficacy. The combined penetration enhancement method at 0.7 J/cm2 achieves the best optical clearing effect. In Figure 5a, the reflectance of the skin decreases significantly and by 33.9%, 35.9%, and 33.4% relatively at 475, 575, and 615 nm, respectively, and this can match the in vivo results approximately. In Figure 5b, the transmittance increases 70.0%, 167%, and 101% at 475, 575, and 615 nm respectively. These conclusions are also matched: (1) the mono-treatment of thiazone causes insignificant optical clearing effect; (2) the laser dose of 0.9 J/cm2 causes the worst optical clearing effect; (3) the efficacy of combined treatment is a little better than the mono-treatment of laser-irradiation in 60 min.
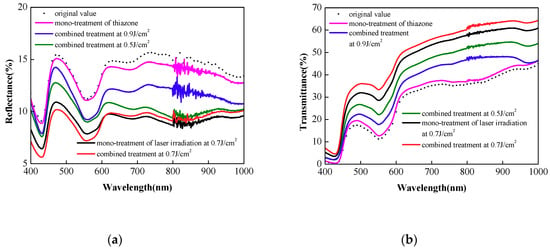
Figure 5.
Typical reflectance (a) and transmittance (b) spectra of in vitro rat skin with different treatment in 60 min.
4. Conclusions
This work investigated the enhancement of the optical clearing effect by laser irradiation in conjunction with a chemical penetration enhancer by using VIS-NIR spectrometer in vivo experiments. The conclusions are as follows:
- Mono-treatment of laser irradiation is less effective than combined treatment, and insignificant optical clearing effect is observed with the mono-treatment of thiazone.
- Combined treatment with a lower dose of 1064 nm Nd:YAG laser irradiation achieves improved optical clearing effect. Under the effect of 0.5 J/cm2 laser irradiation dose, the reflectance decreases by 25.2%, 23.7%, and 17.6% at 475, 575, and 615 nm, respectively.
- The optimum laser irradiation dose exists in the combined treatment. The combined penetration enhancement at 0.7 J/cm2 dose achieves the best optical clearing effect in only 10 min. The final optical clearing effect is also the best, decreasing by approximately 30% of the original value.
Author Contributions
Conceptualization, X.L. and B.C.; Methodology, B.C., and X.L.; Formal Analysis, X.L.; Investigation, X.L.; Data Curation, X.L.; Writing-Original Draft Preparation, X.L.; Writing-Review & Editing, B.C.; Supervision, B.C.; Project Administration, B.C.; Funding Acquisition, B.C.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 51727811.
Acknowledgments
Thanks for the technical support of the Second Affiliated Hospital of Xi’an Jiaotong University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tuchin, V.V.; Maksimova, I.L.; Zimnyakov, D.A.; Kon, I.L.; Mavlyutov, A.H.; Mishin, A.A. Light propagation in tissues with controlled optical properties. J. Biomed. Opt. 1997, 2, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Tsu, L.; Chen, E.; Ishak, T.S.; Iskandar, S.M.; Chess, S.; Nelson, J.S. Determination of chemical agent optical clearing potential using in vitro human skin. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2005, 36, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.; Tuchin, V.; Solovieva, A.; Stepanova, T.; Luo, Q.; Cheng, H. Skin backreflectance and microvascular system functioning at the action of osmotic agents. J. Phys. D Appl. Phys. 2003, 36, 1739. [Google Scholar] [CrossRef]
- Stumpp, O.; Chen, B.; Welch, A.J. Using sandpaper for noninvasive transepidermal optical skin clearing agent delivery. J. Biomed. Opt. 2006, 11, 041118. [Google Scholar] [CrossRef] [PubMed]
- Tuchin, V.; Altshuler, G.; Gavrilova, A.; Pravdin, A.; Tabatadze, D.; Childs, J.; Yaroslavsky, I. Optical clearing of skin using flashlamp-induced enhancement of epidermal permeability. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2006, 38, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, Q. Evaluation of skin optical clearing enhancement with azone as a penetration enhancer. Opt. Commun. 2007, 279, 223–228. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, J.; Zhi, Z.; Wen, X.; Luo, Q. Imaging dermal blood flow through the intact rat skin with an optical clearing method. J. Biomed. Opt. 2010, 15, 026008. [Google Scholar] [CrossRef]
- Bui, A.K.; McClure, R.A.; Chang, J.; Stoianovici, C.; Hirshburg, J.; Yeh, A.T.; Choi, B. Revisiting optical clearing with dimethyl sulfoxide (dmso). Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2009, 41, 142–148. [Google Scholar] [CrossRef]
- Peck, K.D.; Ghanem, A.-H.; Higuchi, W.I. Hindered diffusion of polar molecules through and effective pore radii estimates of intact and ethanol treated human epidermal membrane. Pharm. Res. 1994, 11, 1306–1314. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Duan, S.; Chen, Z.; Zhu, D. Improvement of In Vivo Rat Skin Optical Clearing with Chemical Penetration Enhancers. In Photonic Therapeutics and Diagnostics VII; International Society for Optics and Photonics: San Francisco, CA, USA, 2011. [Google Scholar]
- Zhong, H.; Guo, Z.; Wei, H.; Guo, L.; Wang, C.; He, Y.; Xiong, H.; Liu, S. Synergistic effect of ultrasound and thiazone–peg 400 on human skin optical clearing in vivo. Photochem. Photobiol. 2010, 86, 732–737. [Google Scholar] [CrossRef]
- Wang, J.; Shi, R.; Zhu, D. Switchable skin window induced by optical clearing method for dermal blood flow imaging. J. Biomed. Opt. 2012, 18, 061209. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Guo, L.; Zhang, C.; Feng, W.; Li, P.; Ding, Z.; Zhu, D. A useful way to develop effective in vivo skin optical clearing agents. J. Biophotonics 2017, 10, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, Q. Sonophoretic delivery for contrast and depth improvement in skin optical coherence tomography. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 56–61. [Google Scholar] [CrossRef]
- Yoon, J.; Son, T.; Choi, E.-h.; Choi, B.; Nelson, J.S.; Jung, B. Enhancement of optical skin clearing efficacy using a microneedle roller. J. Biomed. Opt. 2008, 13, 021103. [Google Scholar] [CrossRef]
- Rylander, C.G.; Milner, T.E.; Baranov, S.A.; Nelson, J.S. Mechanical tissue optical clearing devices: Enhancement of light penetration in ex vivo porcine skin and adipose tissue. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2008, 40, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Bose, V.G.; Langer, R.; Weaver, J.C. Electroporation of mammalian skin: A mechanism to enhance transdermal drug delivery. Proc. Natl. Acad. Sci. 1993, 90, 10504–10508. [Google Scholar] [CrossRef]
- Song, J.Y.; Kang, H.A.; Kim, M.Y.; Park, Y.M.; Kim, H.O. Damage and recovery of skin barrier function after glycolic acid chemical peeling and crystal microdermabrasion. Dermatol. Surg. 2004, 30, 390–394. [Google Scholar]
- Tuchin, V.V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis. SPIE J. Am. Opt. Eng. 2007. [Google Scholar] [CrossRef]
- Liu, C.; Zhi, Z.; Tuchin, V.V.; Luo, Q.; Zhu, D. Enhancement of skin optical clearing efficacy using photo-irradiation. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2010, 42, 132–140. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Yue, Y.; Luo, Q.; Zhu, D. 1064 nm-nd: Yag lasers with different output modes enhancing transdermal delivery: Physical and physiological mechanisms. J. Biomed. Opt. 2013, 18, 061228. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).