Multiscale Characterisation of Cortical Bone Tissue
Abstract
Featured Application
Abstract
1. Introduction
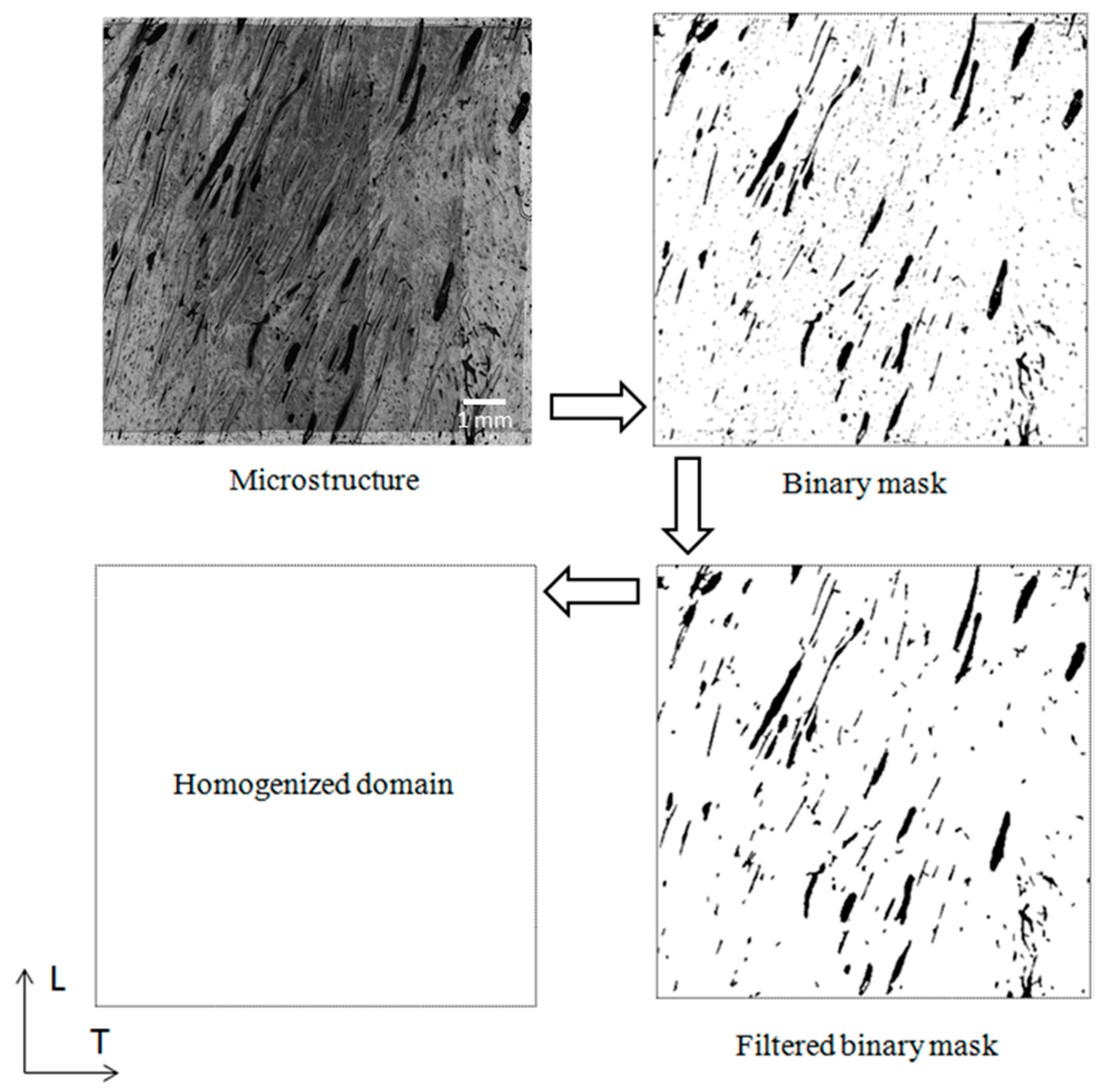
2. Materials and Methods
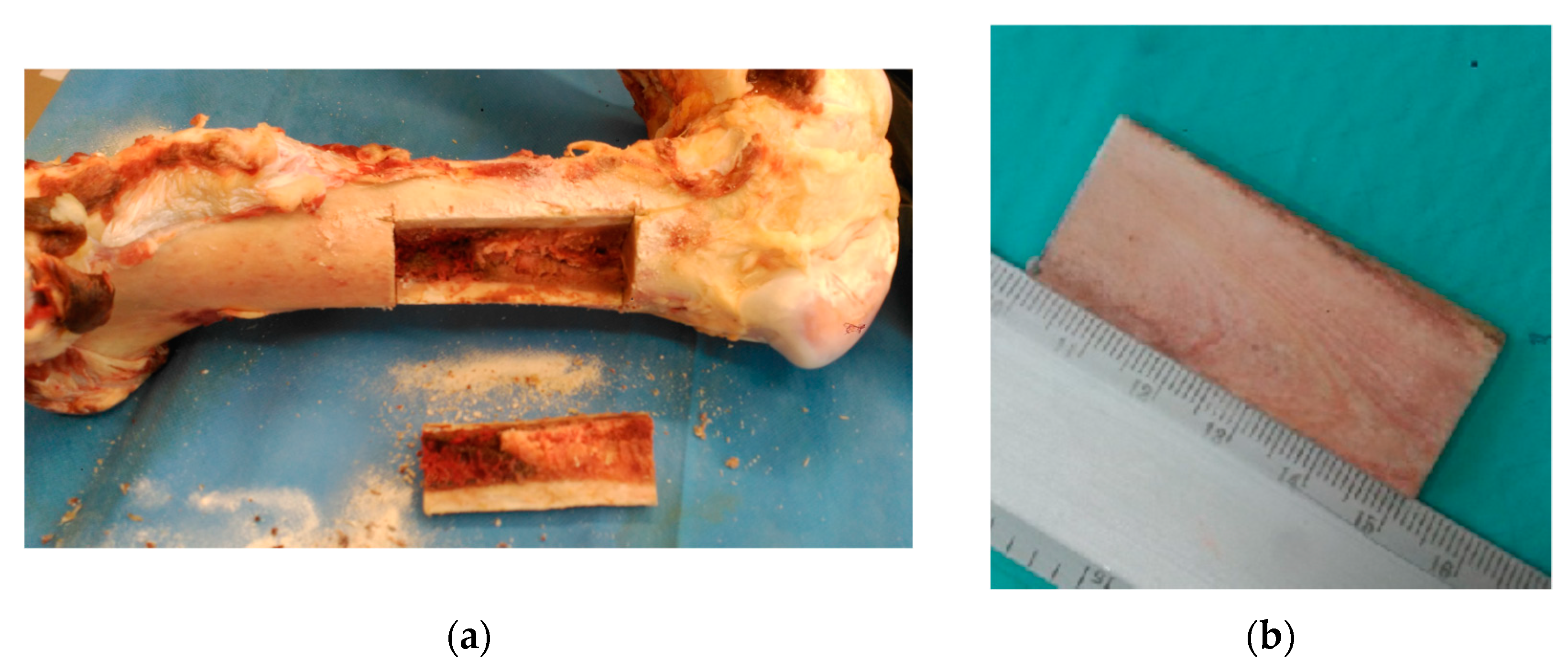
2.1. Sample Preparation
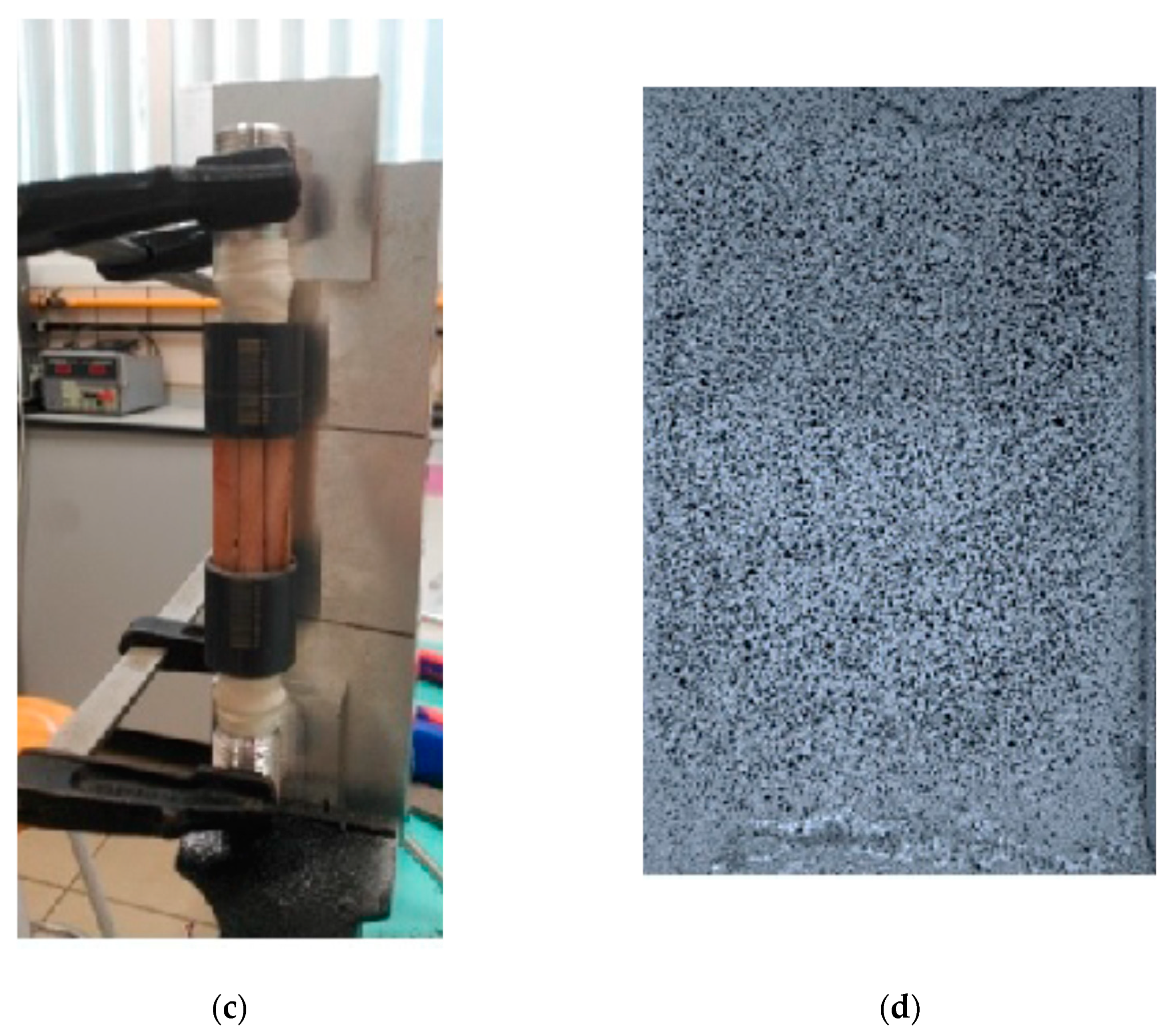
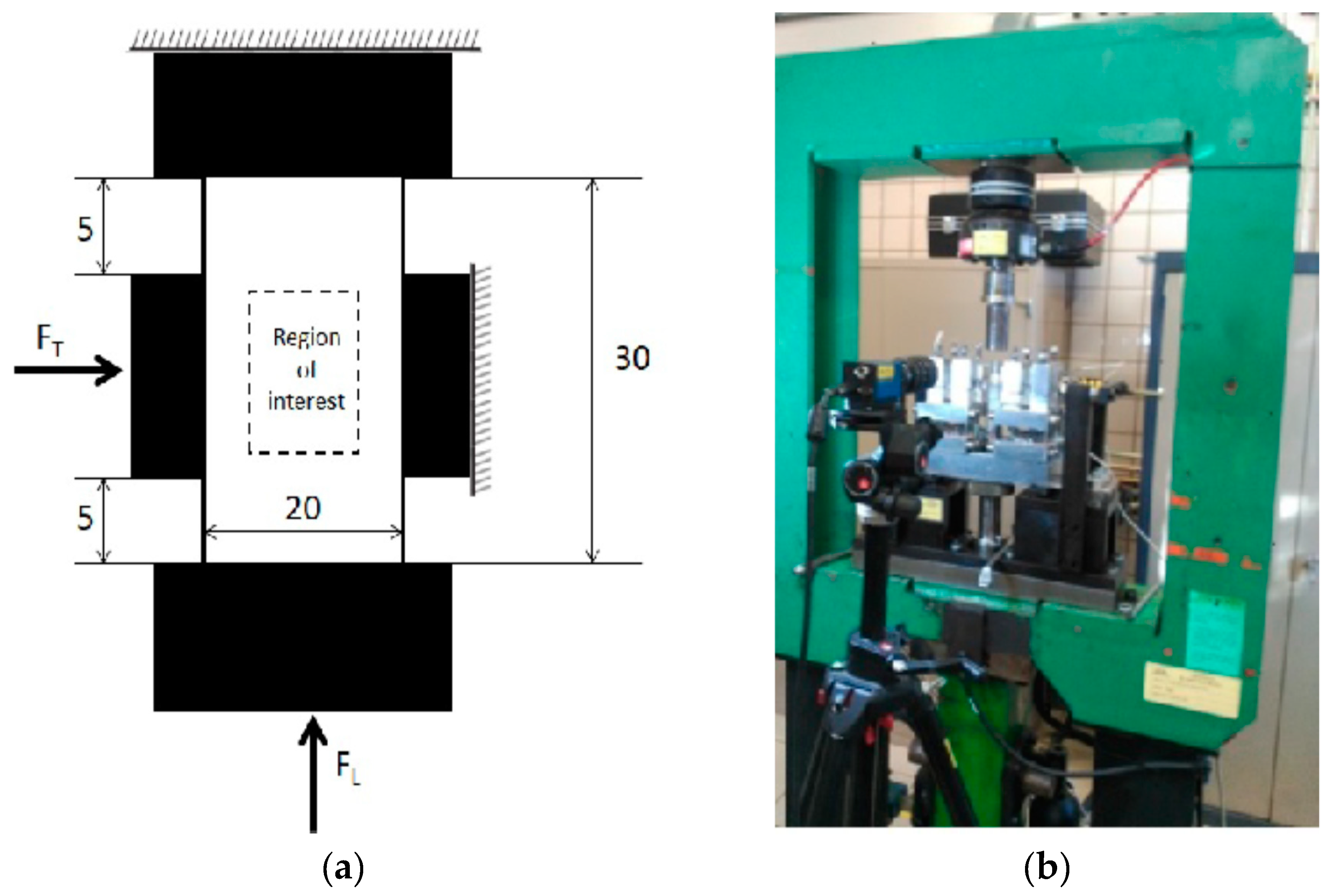
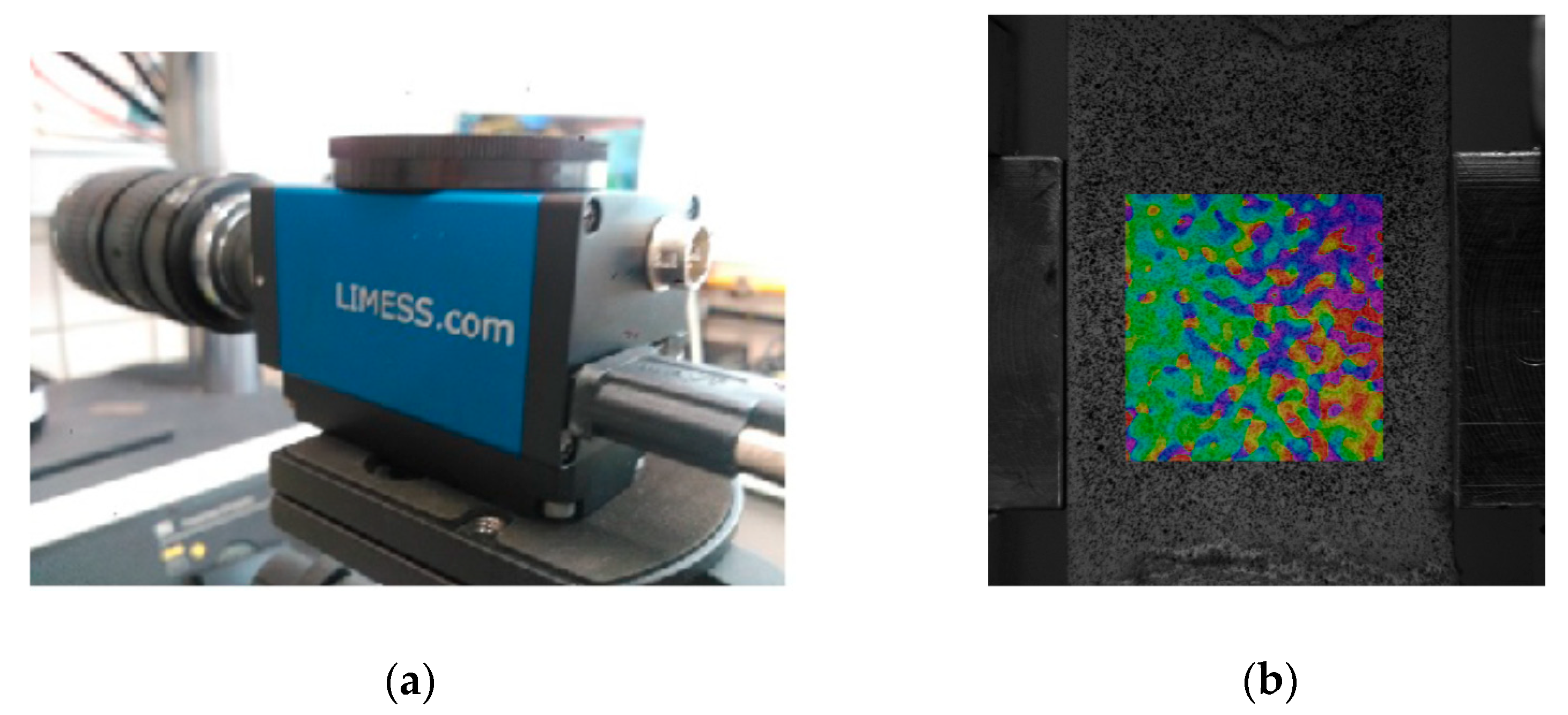
2.2. Experimental Tests
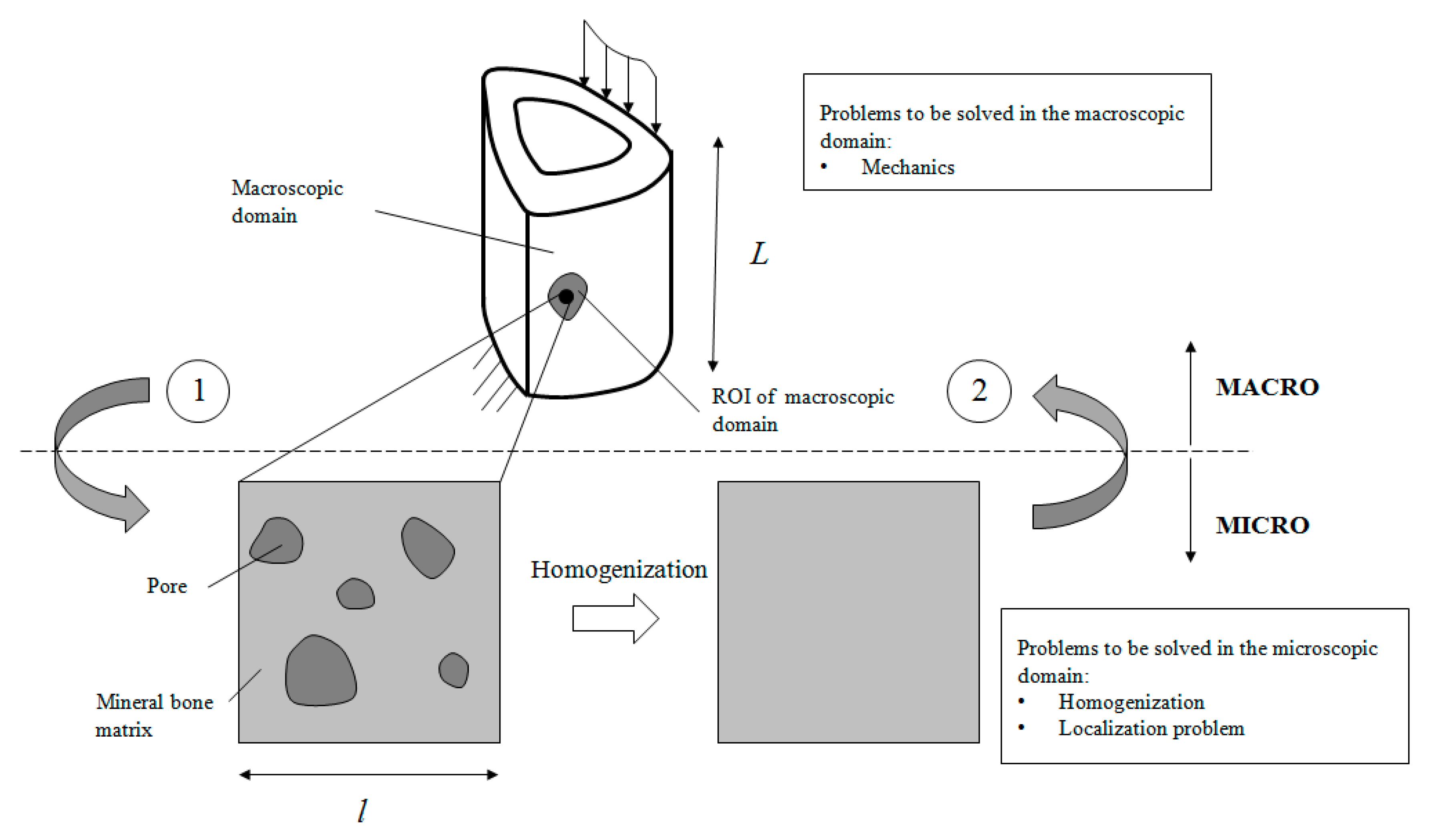
3. Mathematical Approach
3.1. Localisation
3.2. Homogenisation
3.3. Variational Formulation
3.4. Multiscale Approach
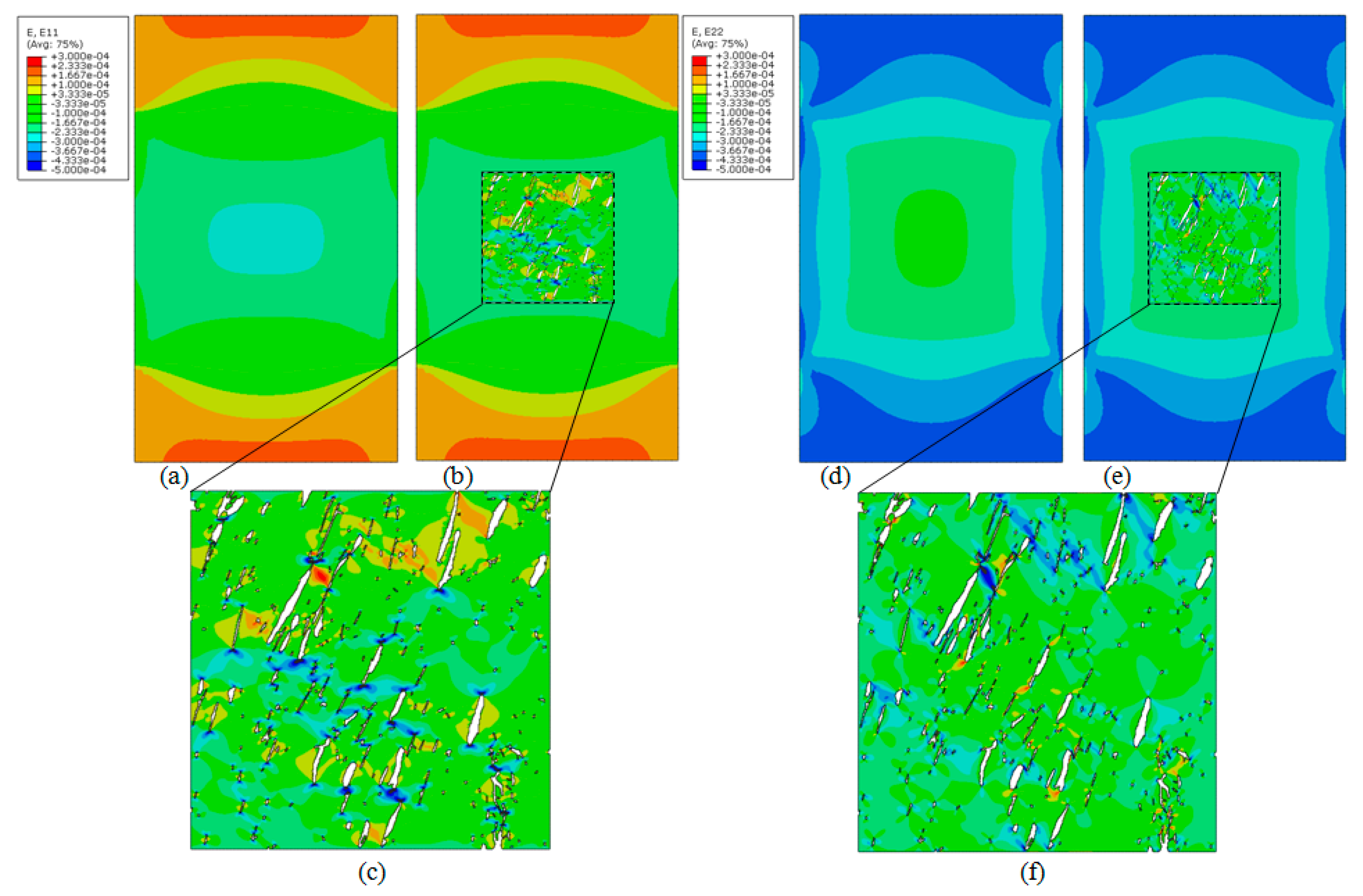
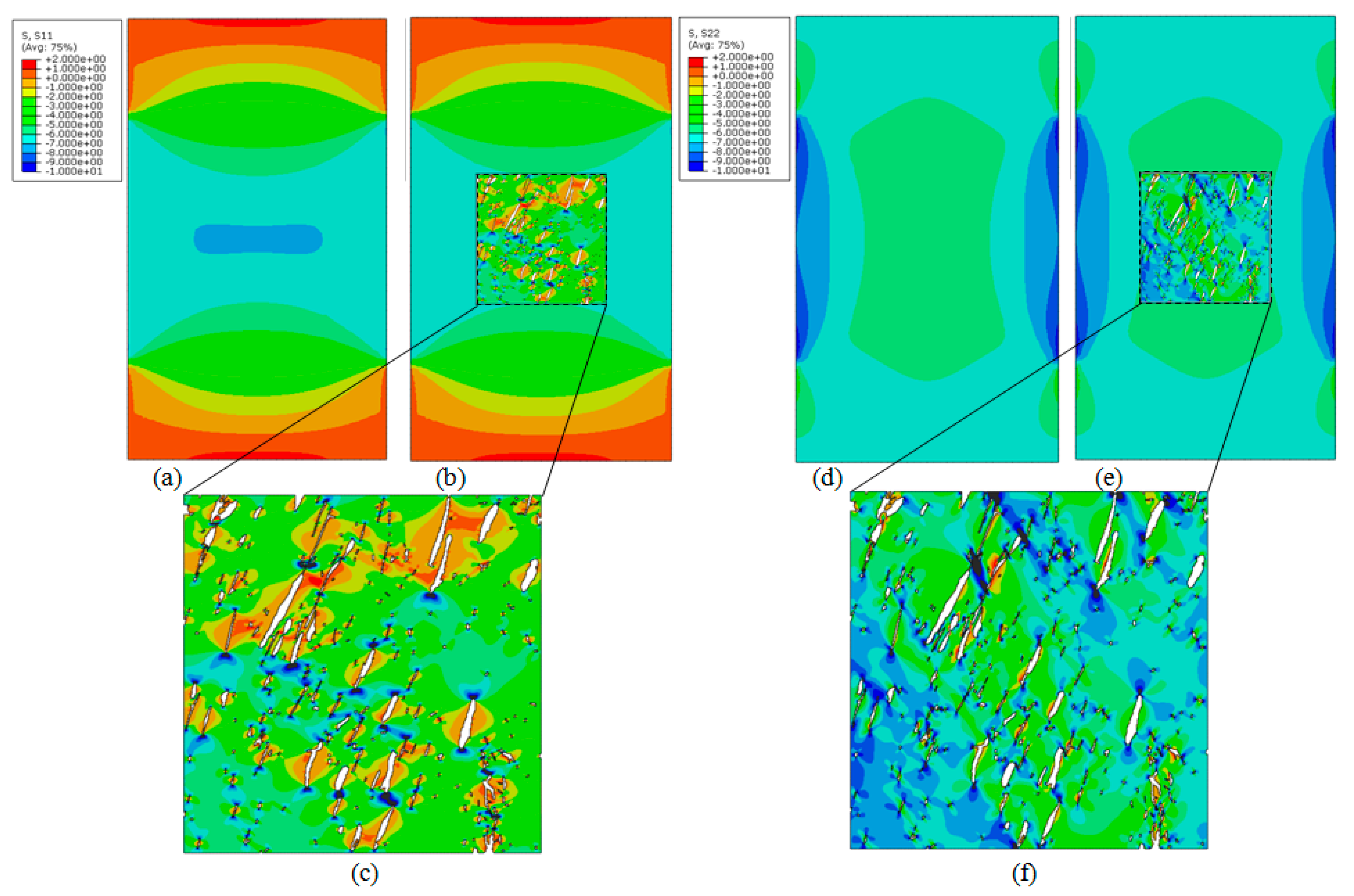
4. Results
4.1. Computational Models
4.2. Homogenisation Macrostructural Results
4.2.1. Homogenisation
4.2.2. Macrostructural Results
- (i)
- Choose values for the mechanical properties of the mineral bone tissue (assumed as orthotropic).
- (ii)
- Solve the homogenisation problem in the microstructural domain (Figure 4) and obtain the (homogenised) macroscopic mechanical properties.
- (iii)
- Solve the macroscopic problem shown in Figure 2a. At the macroscopic level, bone is assumed as a homogeneous continuum medium with macroscopic (homogenised) mechanical properties derived from the micromechanics analysis of the microstructure (ii).
- (iv)
- Obtain the average for the solution of the macroscopic problem along the ROI and compare results with Table 1.
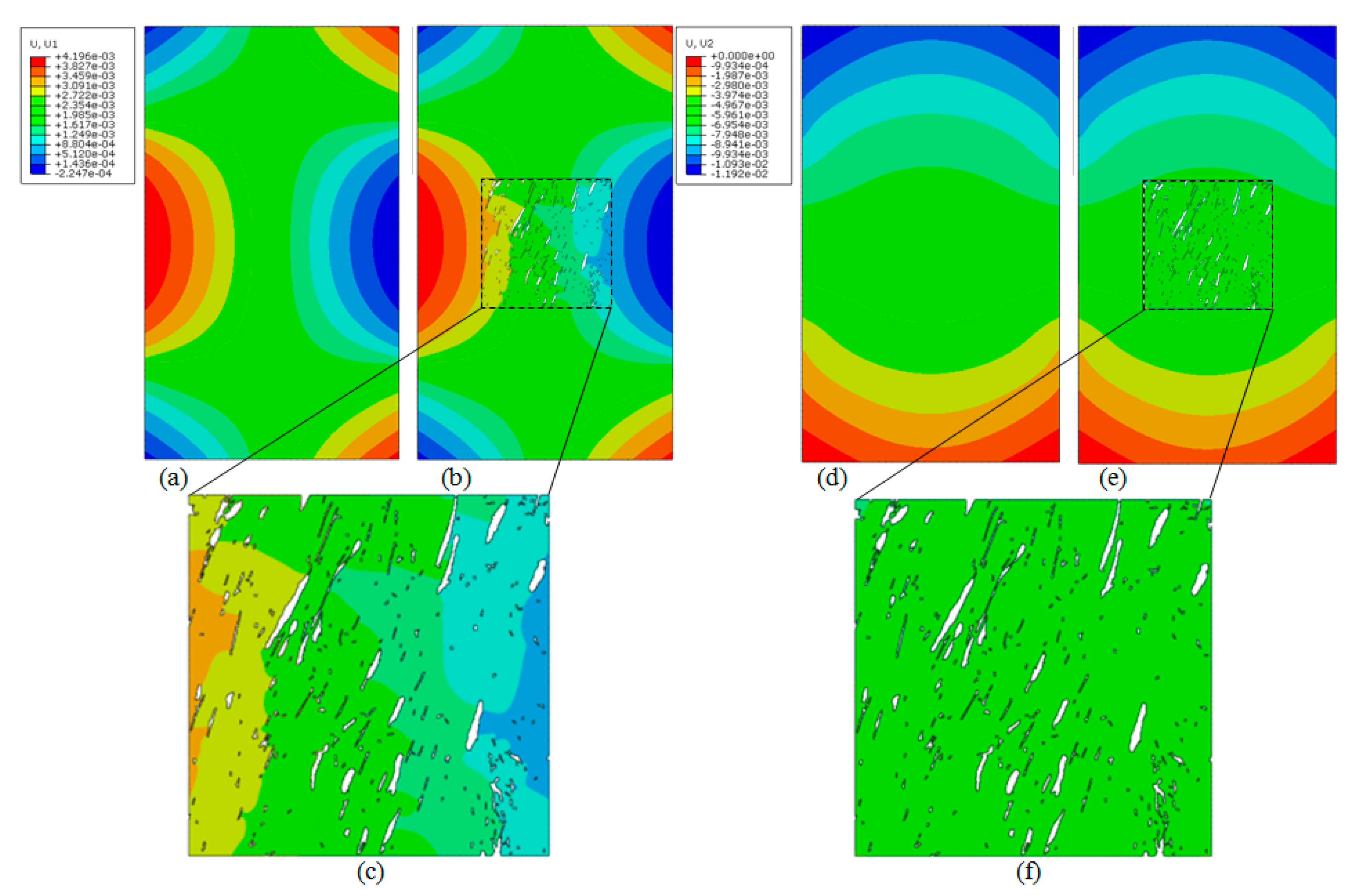
4.3. Multiscale Results
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Currey, J.D. The adaptation of bone to stress. J. Theor. Biol. 1968, 20, 91–106. [Google Scholar] [CrossRef]
- Cowin, S.C.; Moss-Salentijn, L.; Moss, M.L. Candidates for the mechanosensory system in bone. J. Biomech. Eng. 1991, 113, 191–197. [Google Scholar] [CrossRef]
- Cowin, S.C.; Sadegh, A.M.; Luo, G.M. An evolutionary Wolff’s law for trabecular architecture. J. Biomech. Eng. 1992, 114, 129–136. [Google Scholar] [CrossRef]
- Skalak, R.; Chien, S. Handbook of Bioengineering; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Currey, J.D. Bones: Structure and Mechanics; Princeton University Press Editions: Princeton, NJ, USA, 2006. [Google Scholar]
- Cowin, S.C.; Hegedus, D.H. Bone remodeling i: A theory of adaptive elasticity. J. Elast. 1976, 6, 313–326. [Google Scholar] [CrossRef]
- Huiskes, R.; Ruimerman, R.; van Lenthe, G.H.; Janssen, J.D. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 2000, 405, 704–706. [Google Scholar] [CrossRef]
- Christen, P.; Ito, K.; Ellouz, R.; Boutroy, S.; Sornay-Rendu, E.; Chapurlat, R.D.; van Rietbergen, B. Bone remodelling in humans is load-driven but not lazy. Nat. Commun. 2014, 5, 4855. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Mora-Macías, J.; Pajares, A.; Miranda, P.; Domínguez, J.; Reina-Romo, E. Mechanical characterization via nanoindentation of the woven bone developed during bone transport. J. Mech. Behav. Biomed. Mater. 2017, 74, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.; Dao, M.; Suresh, S.; Palazoglu, A.; Ortiz, C. Nanoscale heterogeneity promotes energy dissipation in bone. Nat. Mater. 2007, 6, 454–462. [Google Scholar] [CrossRef]
- Schaffler, M.B.; Burr, D.B. Stiffness of compact bone: Effects of porosity and density. J. Biomech. 1998, 21, 13–16. [Google Scholar] [CrossRef]
- Thompson, M.S.; Schell, H.; Lienau, J.; Duda, G.N. Digital image correlation: A technique for determining local mechanical conditions within early bone callus. Med. Eng. Phys. 2007, 29, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Carriero, A.; Abela, L.; Pitsillides, A.A.; Shefelbine, S.J. Ex vivo determination of bone tissue strains for an in vivo mouse tibial loading mode. J. Biomech. 2014, 47, 2490–2497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gustafsson, A.; Mathavan, N.; Turunen, M.J.; Engqvist, J.; Khayyeri, H.; Hall, S.A.; Isaksson, H. Linking multiscale deformation to microstructure in cortical bone using in situ loading, digital image correlation and synchrotron X-ray scattering. Acta Biomater. 2018, 69, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Orr, T.E.; Fyhrie, D.P. Relationships between loading history and femoral cancellous bone architecture. J. Biomech. 1989, 22, 231–244. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Beaupre, G.S.; Carter, D.R. A model of mechanobiologic and metabolic influences on bone adaptation. J. Rehabil. Res. Dev. 2000, 37, 235–244. [Google Scholar] [PubMed]
- Hazelwood, S.J.; Martin, R.B.; Rashid, M.M.; Rodrigo, J.J. A mechanistic model for internal bone remodeling exhibits different dynamic responses in disuse and overload. J. Biomech. 2001, 34, 299–308. [Google Scholar] [CrossRef]
- Garcia-Aznar, J.M.; Rueberg, T.; Doblare, M. A bone remodelling model coupling micro-damage growth and repair by 3D BMU-activity. Biomech. Model. Mechanobiol. 2005, 4, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Beaupre, G.S.; Orr, T.E.; Carter, D.R. An approach for time-dependent bone modelling and remodelling: Theoretical development. J. Orthop. Res. 1990, 8, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Beaupre, G.S.; Orr, T.E.; Carter, D.R. An approach for time-dependent bone modeling and remodeling-application: A preliminary remodeling simulation. J. Orthop. Res. 1990, 8, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.R. Numerical Simulation of Bone Adaptation to Mechanical Loading. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 1994. [Google Scholar]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 15, 4–87. [Google Scholar] [CrossRef] [PubMed]
- Cowin, S.C. Bone poroelasticity. J. Biomech. 1999, 32, 217–238. [Google Scholar] [CrossRef]
- Cowin, S.C.; Gailani, G.; Benalla, M. Hierarchical poroelasticity: Movement of interstitial fluid between porosity levels in bones. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 3401–3444. [Google Scholar] [CrossRef] [PubMed]
- Bailon-Plaza, A.; van der Meulen, C.H. A mathematical framework to study the effects of growth factor influences on fracture healing. J. Theor. Biol. 2001, 212, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, D.; Prendergast, P.J. A mechano-regulation model for tissue differentiation during fracture healing: Analysis of gap size and loading. J. Biomech. 2002, 35, 1163–1171. [Google Scholar] [CrossRef]
- Wang, M.; Yang, N.; Wang, X. A review of computational models of bone fracture healing. Med. Biol. Eng. Comput. 2017, 55, 1895–1914. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.P.; Lacroix, D.; Planell, J.A.; Kelly, D.J.; Prendergast, P.J. Simulation of tissue differentiation in a scaffold as a function of porosity, Young’s modulus and dissolution rate: Application of mechanobiological models in tissue engineering. Biomaterials 2007, 28, 5544–5554. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Herrera, J.A.; Garcia-Aznar, J.M.; Doblare, M. A mathematical model for bone tissue regeneration inside a specific type of scaffold. Biomech. Model. Mechanobiol. 2008, 7, 355–366. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; Garcia-Aznar, J.M. A mathematical approach to bone tissue engineering. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 2055–2078. [Google Scholar] [CrossRef]
- Guyot, Y.; Papantoniou, I.; Chai, Y.C.; Van Bael, S.; Schrooten, J.; Geris, L. A computational model for cell/ECM growth on 3D surfaces using the level set method: A bone tissue engineering case study. Biomech. Model. Mechanobiol. 2014, 13, 1361–1371. [Google Scholar] [CrossRef]
- Claes, L.E.; Heigele, C.A. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J. Biomech. 1999, 32, 255–266. [Google Scholar] [CrossRef]
- Isaksson, H.; Comas, O.; van Donkelaar, C.C.; Mediavilla, J.; Wilson, W.; Huiskes, R.; Ito, K. Bone regeneration during distraction osteogenesis: Mechano-regulation by shear strain and fluid velocity. J. Biomech. 2007, 40, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Reina-Romo, E.; Gomez-Benito, M.J.; Garcia-Aznar, J.M.; Dominguez, J.; Doblare, M. Modeling distraction osteogenesis: Analysis of the distraction rate. Biomech. Model. Mechanobiol. 2009, 8, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Reina-Romo, E.; Gomez-Benito, M.J.; Dominguez, J.; Niemeyer, F.; Wehner, T.; Simon, U.; Claes, L.E. Effect of the fixator stiffness on the young regenerate bone after bone transport: Computational approach. J. Biomech. 2011, 44, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Tsubota, K.I.; Tomita, Y.; Hollister, S.J. Trabecular surface remodelling simulation for cancellous bone using microstructural voxel finite element models. J. Biomech. Eng. T ASME 2001, 123, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Osako, Y.; Tanaka, M.; Hojo, M.; Hollister, S.J. Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials 2006, 27, 3964–3972. [Google Scholar] [CrossRef]
- Kelly, D.J.; Prendergast, P.J. Prediction of the optimal mechanical properties for a scaffold used in osteochondral defect repair. Tissue Eng. 2006, 12, 2509–2519. [Google Scholar] [CrossRef]
- Schulte, F.A.; Ruffoni, D.; Lambers, F.M.; Christen, D.; Webster, D.J.; Kuhn, G.; Muller, R. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS ONE 2013, 8, e62172. [Google Scholar] [CrossRef]
- Terada, K.; Kikuchi, N. A class of general algorithms for multi-scale analyses of heterogeneous media. Comput. Methods Appl. Mech. Eng. 2001, 190, 5427–5464. [Google Scholar] [CrossRef]
- Kouznetsova, V.; Geers, M.G.D.; Brekelmans, W.A.M. Multi-scale constitutive modelling of heterogeneous materials with a gradient-enhanced computational homogenization scheme. Int. J. Numer. Meth. Eng. 2002, 54, 1235–1260. [Google Scholar] [CrossRef]
- Miehe, C.; Bayreuther, C.G. On multiscale FE analyses of heterogeneous structures: From homogenization to multigrid solvers. Int. J. Numer. Methods Eng. 2007, 71, 1135–1180. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Matous, K.; Geubelle, P.H. Coupled multi-scale cohesive modeling of failure in heterogeneous adhesives. Int. J. Numer. Methods Eng. 2010, 84, 916–946. [Google Scholar] [CrossRef]
- Reina-Romo, E.; Sanz-Herrera, J.A. Multiscale simulation of particle-reinforced elastic-plastic adhesives at small strains. Comput. Methods Appl. Mech. Eng. 2011, 200, 2211–2222. [Google Scholar] [CrossRef]
- Montero-Chacon, F.; Sanz-Herrera, J.A.; Doblare, M. Computational multiscale solvers for continuum approaches. Materials 2019, 12, 691. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.; Muller, R. In silico models of bone remodeling from macro to nano--from organ to cell. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 3, 241–251. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Carpentier, O.; Monchau, F.; Chai, F.; Hornez, J.C.; Hivart, P. Numerical optimization of cell colonization modelling inside scaffold for perfusion bioreactor: A multiscale model. Med. Eng. Phys. 2018, 57, 40–50. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; Garcia-Aznar, J.M.; Doblare, M. Micro–macro numerical modelling of bone regeneration in tissue engineering. Comput. Meth. Appl. Mech. Eng. 2008, 197, 3092–3107. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; Garcia-Aznar, J.M.; Doblare, M. On scaffold designing for bone regeneration: A computational multiscale approach. Acta Biomater. 2009, 5, 219–229. [Google Scholar] [CrossRef]
- Colloca, M.; Blanchard, R.; Hellmich, C.; Ito, K.; van Rietbergen, B. A multiscale analytical approach for bone remodeling simulations: Linking scales from collagen to trabeculae. Bone 2014, 64, 303–313. [Google Scholar] [CrossRef]
- Garcia, D.; Zysset, P.K.; Charlebois, M.; Curnier, A. A three-dimensional elastic plastic damage constitutive law for bone tissue. Biomech. Model. Mechanobiol. 2009, 8, 149–165. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Cowin, S.C. The estimated elastic constants for a single bone osteonal lamella. Biomech. Model. Mechanobiol. 2008, 7, 1–11. [Google Scholar] [CrossRef]
- Gailani, G.; Benalla, M.; Mahamud, R.; Cowin, S.C.; Cardoso, L. Experimental determination of the permeability in the lacunar-canalicular porosity of bone. J. Biomech. Eng. 2009, 131, 101007. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.; Navarro, C.; Dominguez, J. Analysis of fretting fatigue initial crack path in Al7075-T651 using cylindrical contact. Tribol. Int. 2017, 108, 87–94. [Google Scholar] [CrossRef]
- Suquet, P.M. Elements of homogenization for inelastic solid mechanics, trends and applications of pure mathematics to mechanics. In Homogenization Techniques for Composite Media, Lecture Notes in Physics; Sanchez-Palencia, E., Zaoui, A., Eds.; Springer: Berlin, Germany, 1985; Volume 272, pp. 193–278. [Google Scholar]
- Yuan, Z.; Fish, J. Toward realization of computational homogenization in practice. Int. J. Numer. Meth. Eng. 2008, 73, 361–380. [Google Scholar] [CrossRef]
- Taylor, W.R.; Roland, E.; Ploeg, H.; Hertig, D.; Klabunde, R.; Warner, M.D.; Hobatho, M.C.; Rakotomanana, L.; Clift, S.E. Determination of orthotropic bone elastic constants using FEA and modal analysis. J. Biomech. 2002, 35, 767–773. [Google Scholar] [CrossRef]
- Bernard, S.; Grimal, Q.; Laugier, P. Accurate measurement of cortical bone elasticity tensor with resonant ultrasound spectroscopy. J. Mech. Behav. Biomed. Mater. 2013, 18, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.; Burstein, A. The elastic and ultimate properties of compact bone tissue. J. Biomech. 1975, 8, 393–405. [Google Scholar] [CrossRef]
- Schryver, H.F. Bending properties of cortical bone of the horse. Am. J. Vet. Res. 1978, 39, 25–28. [Google Scholar]
- Riggs, C.M.; Vaughan, L.C.; Evans, G.P.; Lanyon, L.E.; Boyde, A. Mechanical implications of collagen fibre orientation in cortical bone of the equine radius. Anat. Embryol. 1993, 187, 239–248. [Google Scholar] [CrossRef]
- Reilly, G.C.; Currey, J.D. The development of microcracking and failure in bone depends on the loading mode to which it is adapted. J. Exp. Biol. 1999, 202, 543–552. [Google Scholar]
- Batson, E.L.; Reilly, G.C.; Currey, J.D.; Balderson, D.S. Post-exercise and positional variation in mechanical properties of the radius in young horses. Equine Vet. 2000, 32, 95–100. [Google Scholar] [CrossRef]
- Hellmich, C.; Barthelemy, J.; Dormieux, L. Mineral-collagen interactions in elasticity of bone ultrastructure—A continuum micromechanics approach. Eur. J. Mech. A Solids 2004, 23, 783–810. [Google Scholar] [CrossRef]
- Fritsch, A.; Hellmich, C. ‘Universal’ microstructural patterns in cortical and trabecular, extracellular and extravascular bone materials: Micromechanics-based prediction of anisotropic elasticity. J. Theor. Biol. 2007, 244, 597–620. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Blenman, P.; Beaupre, G.S. Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J. Orthop. Res. 1988, 6, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Cowin, S.C. Mechanosensation and fluid transport in living bone. J. Musculoskelet. Neuronal Interact. 2002, 2, 256–260. [Google Scholar]
- Van Rietbergen, B.; Huiskes, R.; Eckstein, F.; Ruegsegger, P. Trabecular bone tissue strains in the healthy and osteoporotic human femur. J. Bone Miner. Res. 2003, 18, 1781–1788. [Google Scholar] [CrossRef]
- Shefelbine, S.J.; Carter, D.R. Mechanobiological predictions of growth front morphology in developmental hip dysplasia. J. Orthop. Res. 2004, 22, 346–352. [Google Scholar] [CrossRef]
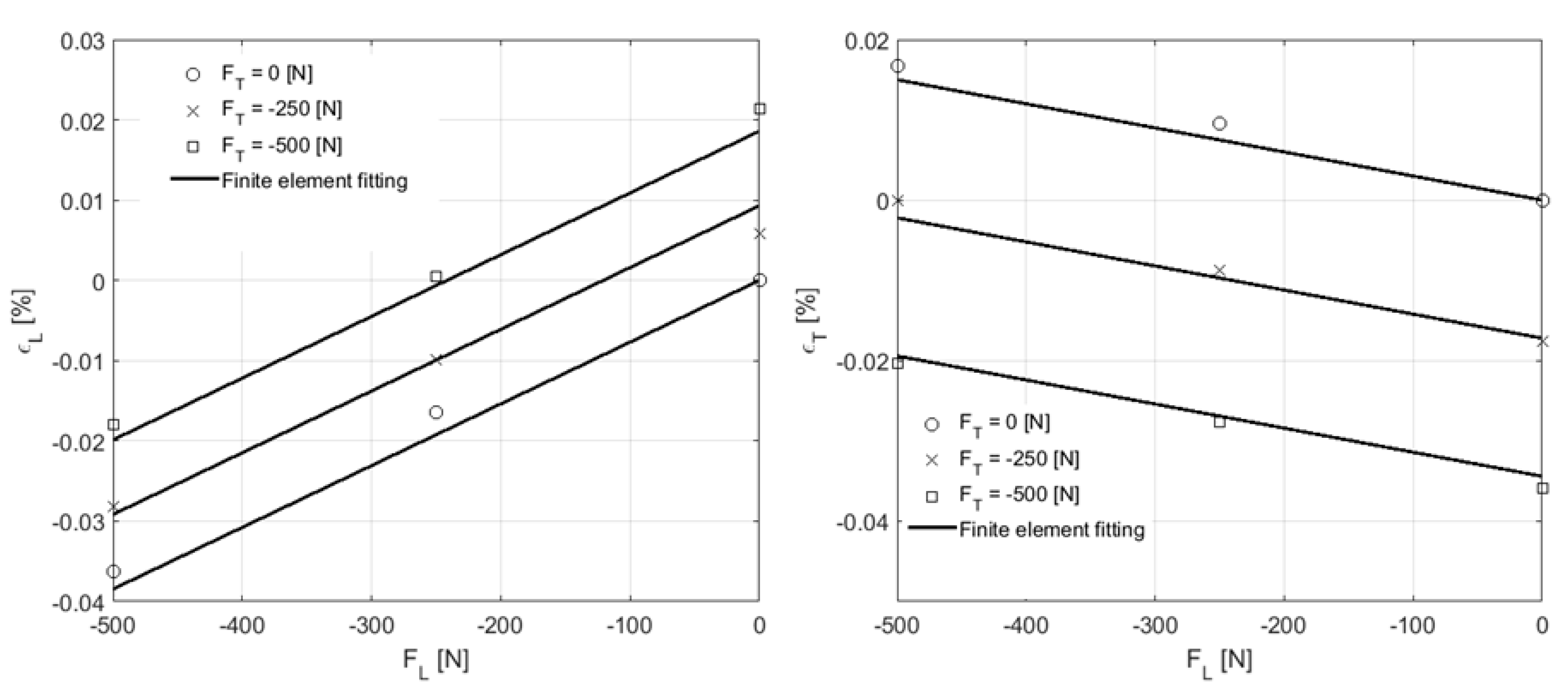
| FL [N] | FT [N] | ||
|---|---|---|---|
| 0 | −250 | 5.8 | −17.6 |
| 0 | −500 | 21.4 | −35.9 |
| −250 | 0 | −16.5 | 9.5 |
| −250 | −250 | −9.8 | −8.8 |
| −250 | −500 | 0.55 | −27.7 |
| −500 | 0 | −36.3 | 16.8 |
| −500 | −250 | −28.2 | −0.025 |
| −500 | −500 | −17.9 | −20.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz-Herrera, J.A.; Mora-Macías, J.; Reina-Romo, E.; Domínguez, J.; Doblaré, M. Multiscale Characterisation of Cortical Bone Tissue. Appl. Sci. 2019, 9, 5228. https://doi.org/10.3390/app9235228
Sanz-Herrera JA, Mora-Macías J, Reina-Romo E, Domínguez J, Doblaré M. Multiscale Characterisation of Cortical Bone Tissue. Applied Sciences. 2019; 9(23):5228. https://doi.org/10.3390/app9235228
Chicago/Turabian StyleSanz-Herrera, José A., Juan Mora-Macías, Esther Reina-Romo, Jaime Domínguez, and Manuel Doblaré. 2019. "Multiscale Characterisation of Cortical Bone Tissue" Applied Sciences 9, no. 23: 5228. https://doi.org/10.3390/app9235228
APA StyleSanz-Herrera, J. A., Mora-Macías, J., Reina-Romo, E., Domínguez, J., & Doblaré, M. (2019). Multiscale Characterisation of Cortical Bone Tissue. Applied Sciences, 9(23), 5228. https://doi.org/10.3390/app9235228







