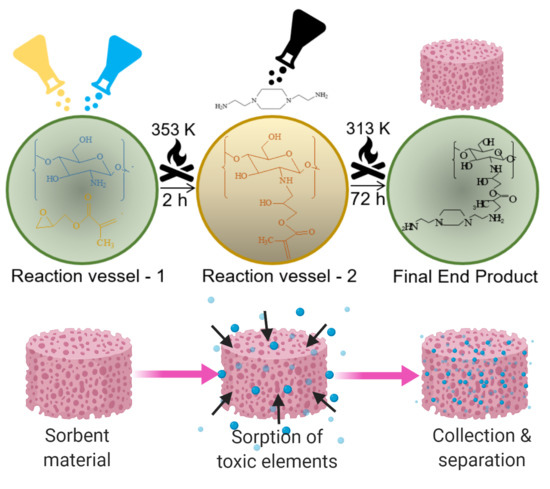
Engineering Functionalized Chitosan-Based Sorbent Material: Characterization and Sorption of Toxic Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Materials
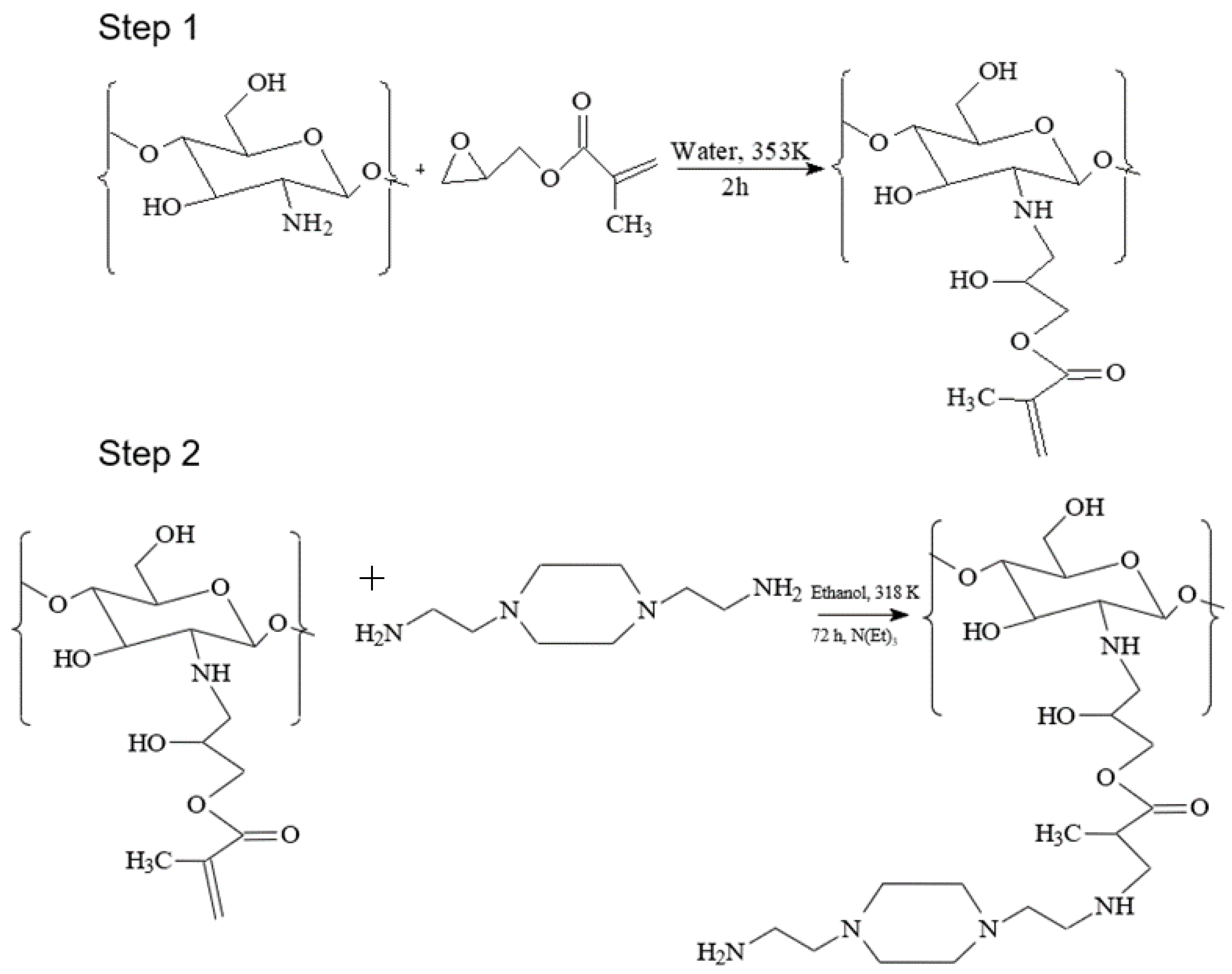
2.2. Procedure for Modification of Chitosan
2.3. Characterization Studies
2.4. Sorption Experiments
3. Results and Discussion
3.1. Elemental Analysis
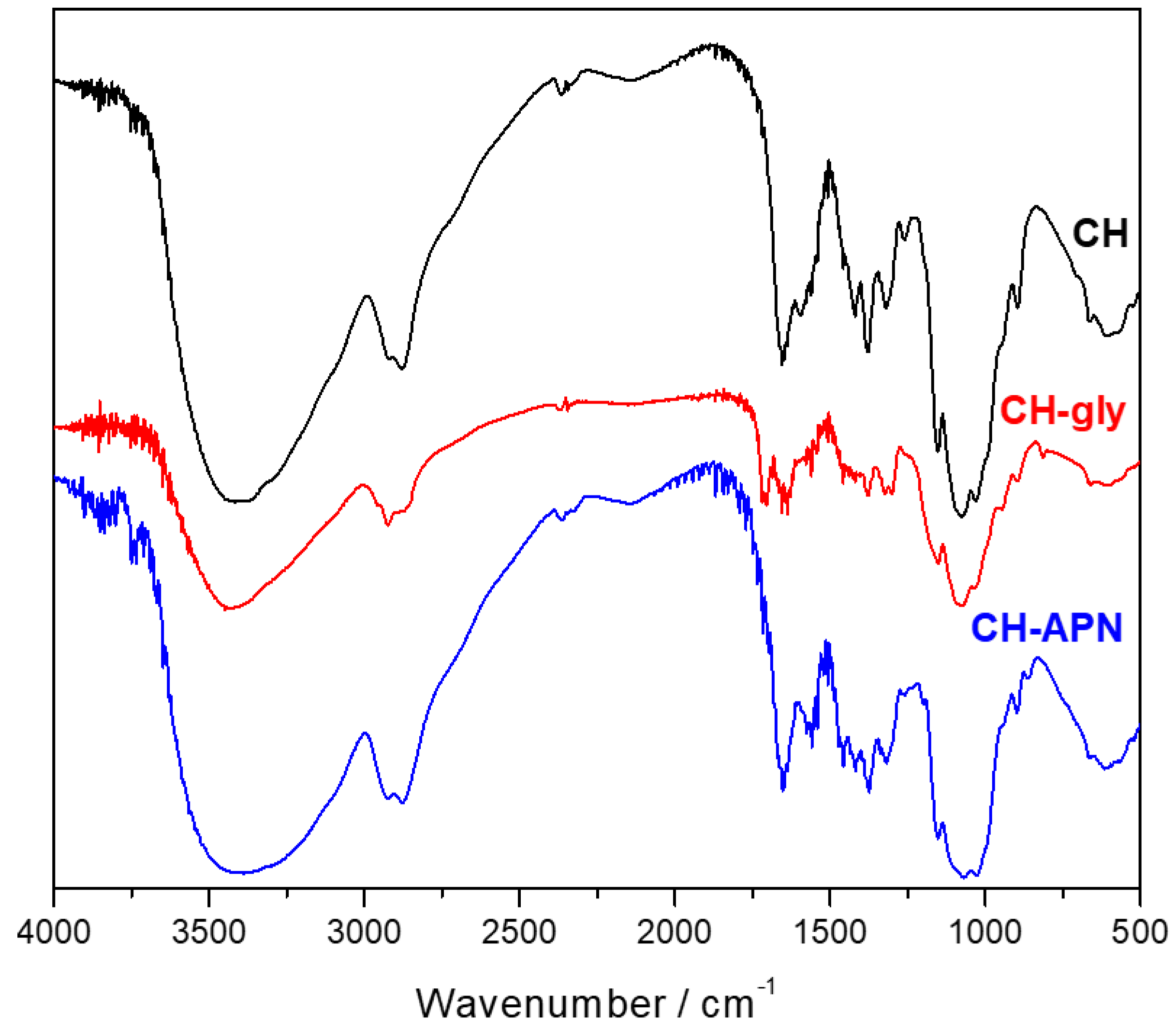
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3. The 13C NMR Spectral Analysis
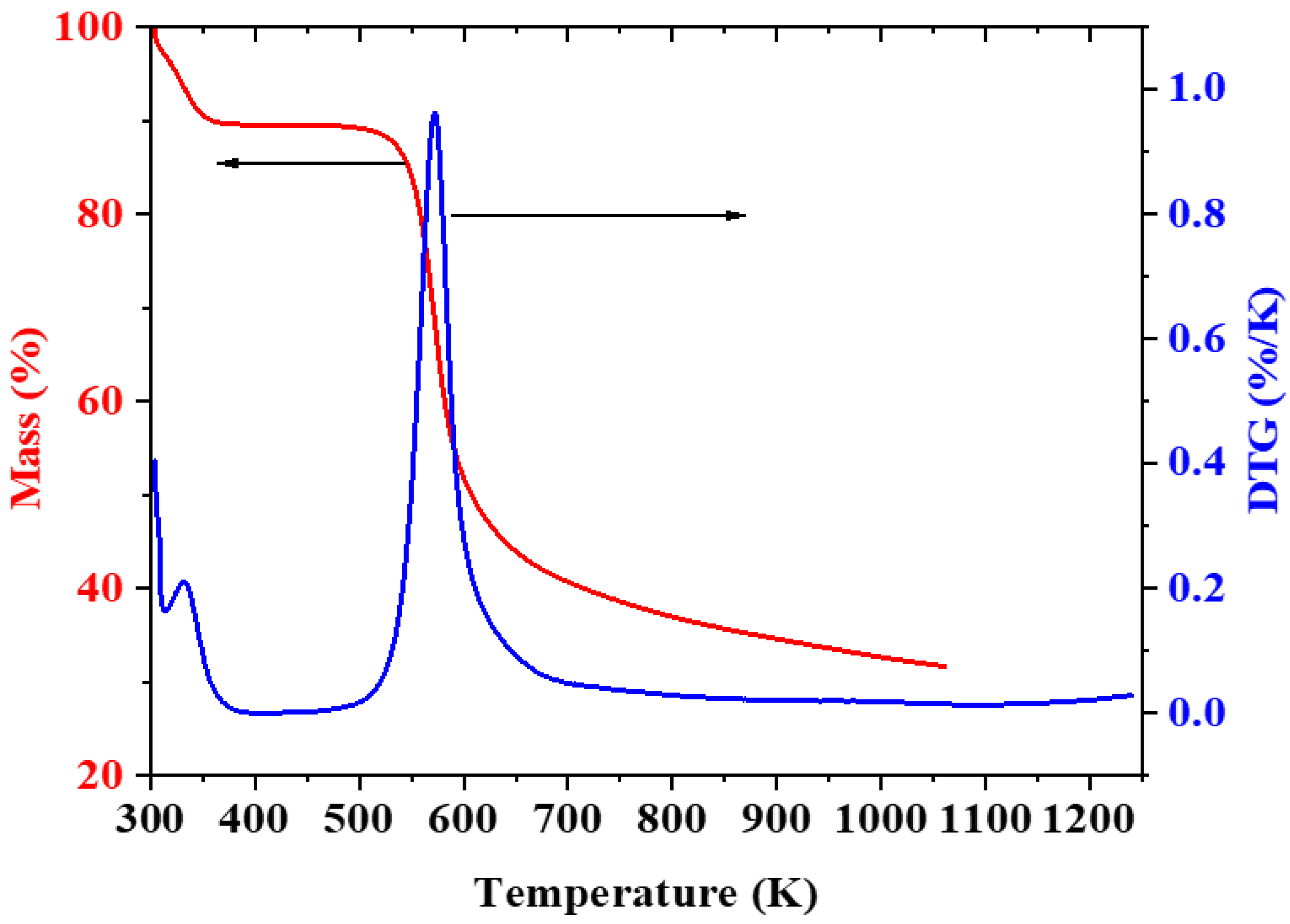
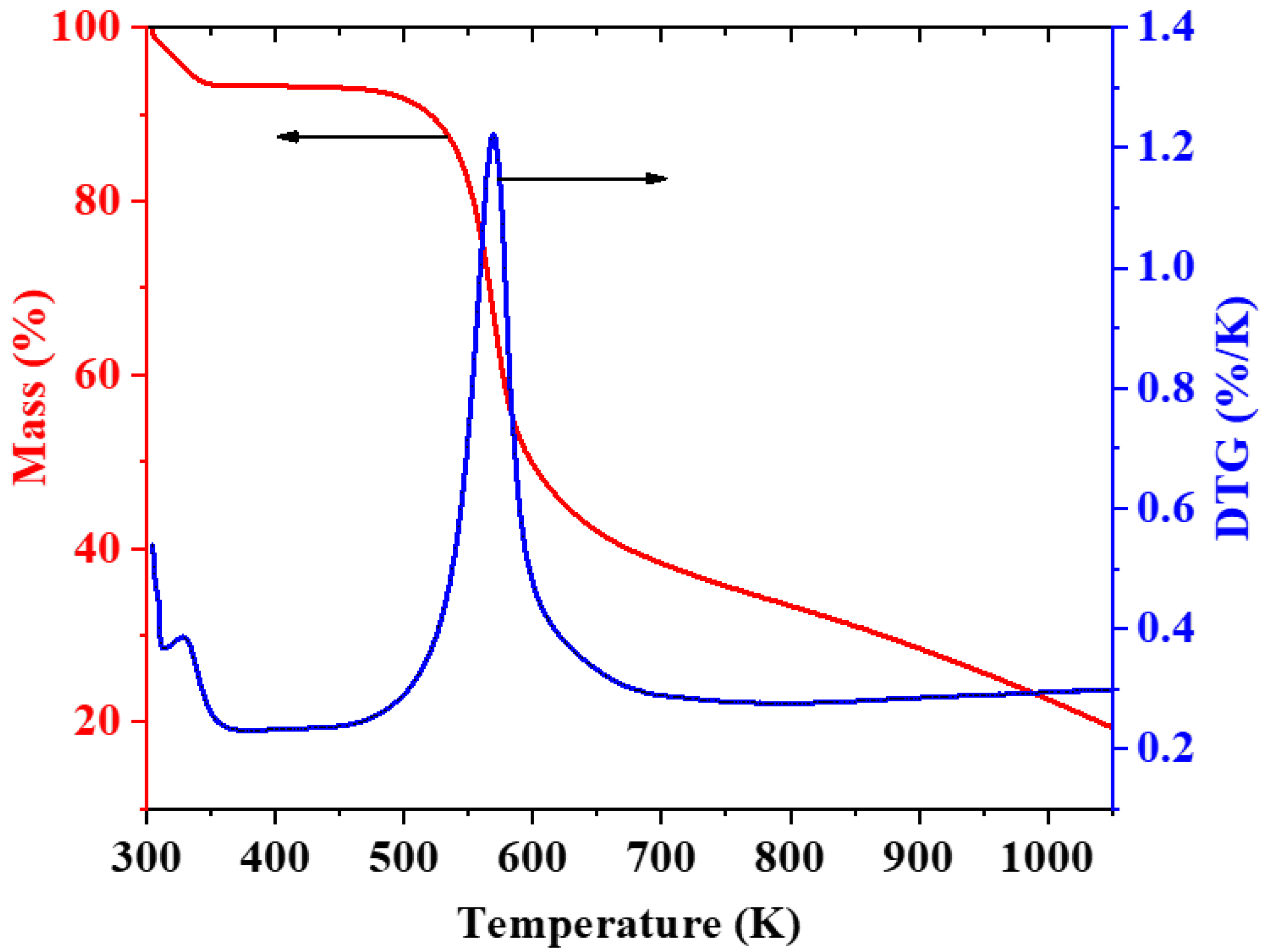
3.4. Thermal Analysis of CH-Gly and CH-ANP
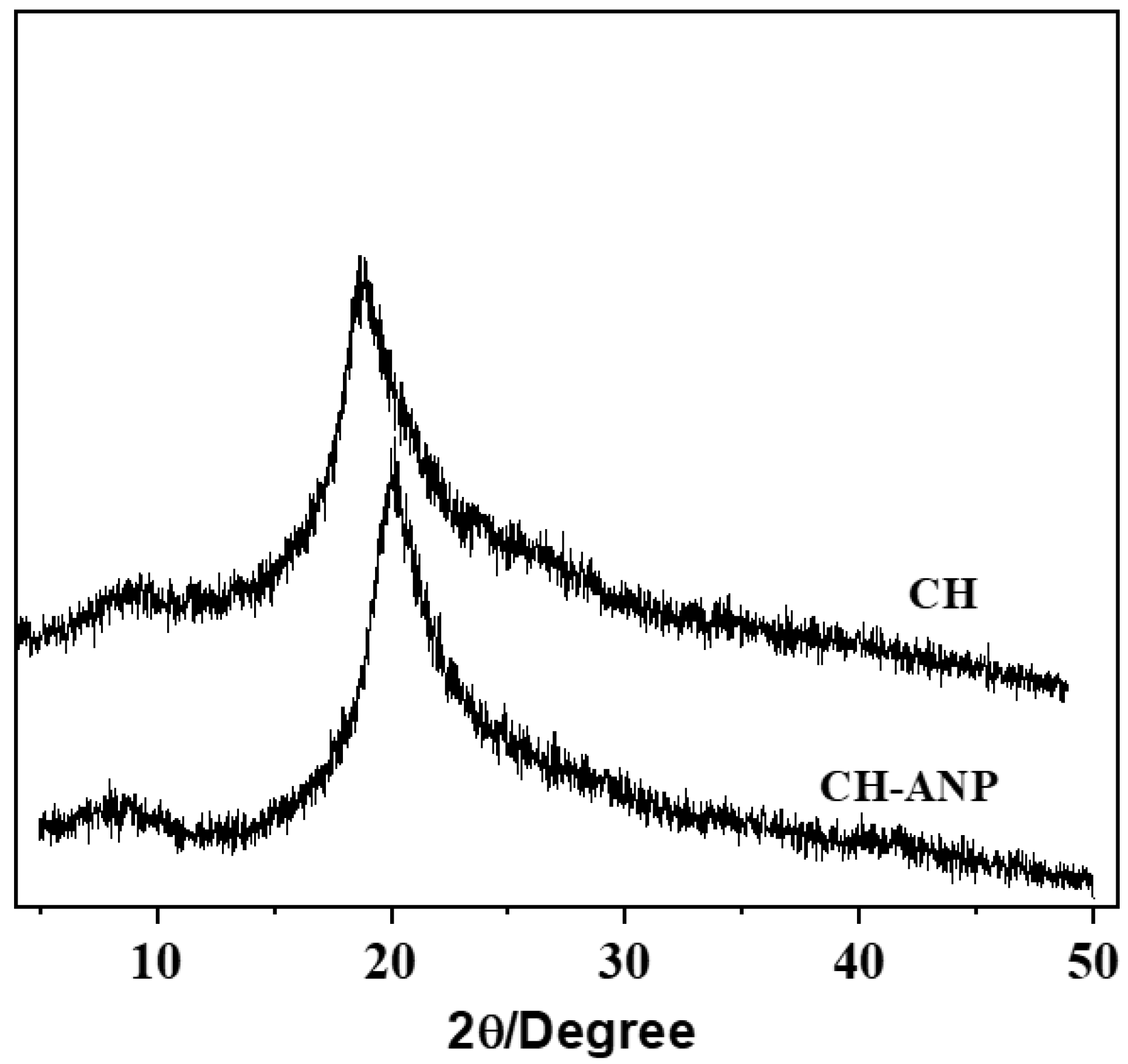
3.5. X-ray Diffraction
3.6. Sorption Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Senani, G.M.; Al-Fawzan, F.F. Adsorption study of heavy metal ions from aqueous solution by nanoparticle of wild herbs. Egypt. J. Aquat. Res. 2018, 44, 187–194. [Google Scholar] [CrossRef]
- Rahman, S.; Kathiresan, V.; Sathasivam, K.V. Heavy Metal Adsorption onto Kappaphycus sp. From Aqueous Solutions: The Use of Error Functions for Validation of Isotherm and Kinetics Models. BioMed Res. Int. 2015, 2015, 126298. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Anjum, A.R.A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Assi, M.A.; Hezmee, M.N.; Haron, A.W.; Sabri, M.Y.; Rajion, M.A. The detrimental effects of lead on human and animal health. Vet. World 2016, 9, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb Neurotoxicity: Neuropsychological Effects of Lead Toxicity. BioMed Res. Int. 2015, 2014, 840547. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Cadmium toxicity and treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Ashish, B.; Neeti, K.; Himanshu, K. Copper Toxicity: A Comprehensive Study. Res. J. Recent Sci. 2013, 2, 58–67. [Google Scholar]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An interplay between marine algae and potentially toxic elements—A review. Mar. Drugs 2018, 16, 65. [Google Scholar] [CrossRef]
- Rasheed, T.; Li, C.; Bilal, M.; Yu, C.; Iqbal, H.M.N. Potentially toxic elements and environmentally-related pollutants recognition using colorimetric and ratiometric fluorescent probes. Sci. Total Environ. 2018, 640, 174–193. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Iqbal, H.M.N.; Li, C.; Zhou, Y. Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total Environ. 2018, 615, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Guo, L.; Guo, Z. Separation of metals from metal-rich particles of crushed waste printed circuit boards by low-pressure filtration. Waste Manag. 2019, 84, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ngcephe, A.M.; Sinha, M.K.; Purcell, W. Solvent extraction and separation of palladium from platinum group elements: Synthesis and characterization of 2-mercaptopyridine N-oxide-palladium (II) complex. J. Mol. Struct. 2020, 1199, 127009. [Google Scholar] [CrossRef]
- Khan-mohammadi, H.; Bayati, B.; Rahbar-Shahrouzi, J.; Babaluo, A.; Ghorbani, A. Molecular simulation of the ion exchange behavior of Cu2+, Cd2+ and Pb2+ ions on different zeolites exchanged with sodium. J. Environ. Chem. Eng. 2019, 7, 103040. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Darveau, O.; Meers, E. The fate of micronutrients and heavy metals in digestate processing using vibrating reversed osmosis as resource recovery technology. Sep. Purif. Technol. 2019, 223, 81–87. [Google Scholar] [CrossRef]
- Itam, Z.; Beddu, S.; Mohammad, D.; Kamal, N.L.M.; Hamid, Z.A.A. Extraction of Metal Oxides from Coal Bottom Ash by Carbon Reduction and Chemical Leaching. Mater. Today Proc. 2019, 17, 727–735. [Google Scholar] [CrossRef]
- Vajedi, F.S.; Dehghani, H. The characterization of TiO2-reduced graphene oxide nanocomposites and their performance in electrochemical determination for removing heavy metals ions of cadmium(II), lead(II) and copper(II). Mater. Sci. Eng. B 2019, 243, 189–198. [Google Scholar] [CrossRef]
- Hussain, S.; Ullah, Z.; Gul, S.; Khattak, R.; Kazmi, N.; Rehman, F.; Khan, S.; Ahmad, K.; Imad, M.; Khan, A. Adsorption Characteristics of Magnesium-Modified Bentonite Clay with Respect to Acid Blue 129 in Aqueous Media. Pol. J. Environ. Stud. 2016, 25, 1947–1953. [Google Scholar] [CrossRef]
- Alhokbany, N.; Naushad, T.A.M.; Alshehri, S.M. Feasibility of toxic metal removal from aqueous medium using Schiff-base based highly porous nanocomposite: Adsorption characteristics and post characterization. J. Mol. Liq. 2019, 294, 111598. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, S.K.; Pathak, D.D. Selective adsorption of toxic heavy metal ions using guanine-functionalized mesoporous silica [SBA-16-g] from aqueous solution. Microporous Mesoporous Mater. 2019, 288, 109577. [Google Scholar] [CrossRef]
- Khan, A.; Wahid, F.; Ali, N.; Badshah, S.; Airoldi, C. Single-step modification of chitosan for toxic cations remediation from aqueous solution. Desalin. Water Treat. 2015, 56, 1099–1109. [Google Scholar] [CrossRef]
- Khan, A.; Badshah, S.; Airoldi, C. Biosorption of some toxic metal ions by chitosan modified with glycidyl methacrylate and diethylenetriamine. Chem. Eng. J. 2011, 171, 159–166. [Google Scholar] [CrossRef]
- Khan, A.; Badshah, S.; Airoldi, C. Dithiocarbamated chitosan as a potent biopolymer for toxic cation remediation. Colloids Surf. B Biointerfaces 2011, 87, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Shah, S.J.; Mehmood, K.; Ali, N.; Khan, H. Synthesis of potent chitosan beads a suitable alternative for textile dye reduction in sunlight. J. Mater. Sci. Mater. Elect. 2018, 30, 406–414. [Google Scholar] [CrossRef]
- Cınar, S.; Kaynar, U.H.; Aydemir, T.; Kaynar, S.C.; Mehmet Ayvacıklı, M. An efficient removal of RB5 from aqueous solution by adsorption onto nano-ZnO/Chitosan composite beads. Int. J. Biol. Macromol. 2017, 96, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Norrahma, S.S.A.; Hameed, B.H.; Ismail, K. Chitosan-glyoxal film as a superior adsorbent for two structurally different reactive and acid dyes: Adsorption and mechanism study. Int. J. Biol. Macromol. 2019, 135, 569–581. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.; Hu, H.; Wang, W.; Zhang, X. Enhanced bio-catalytic performance and dye degradation potential of chitosan-encapsulated horseradish peroxidase in a packed bed reactor system. Sci. Total Environ. 2017, 575, 1352–1360. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Karim, K.J.A.; Ibrahim, W.A.W.; Jume, B.H. Equilibrium, kinetic and mechanism studies of Cu (II) and Cd (II) ions adsorption by modified chitosan beads. Int. J. Biol. Macromol. 2018, 116, 255–263. [Google Scholar] [CrossRef]
- Salih, S.S.; Ghosh, T.K. Adsorption of Zn(II) ions by chitosan coated diatomaceous earth. Int. J. Biol. Macromol. 2018, 106, 602–610. [Google Scholar] [CrossRef]
- Zhai, L.; Bai, Z.; Zhu, Y.; Wang, B.; Luo, W. Fabrication of chitosan microspheres for efficient adsorption of methyl orange. Chin. J. Chem. Eng. 2018, 26, 657–666. [Google Scholar] [CrossRef]
- Frantz, T.S.; Silveira, N., Jr.; Quadro, M.S.; Andreazza, R.; Barcelos, A.A.; Cadaval, T.R.S., Jr.; Pinto, L.A.A. Cu(II) adsorption from copper mine water by chitosan films and the matrix effects. Environ. Sci. Pollut. Res. 2017, 24, 5908–5917. [Google Scholar] [CrossRef]
- Hui, M.; Shengyan, P.; Yaqi, H.; Rongxin, Z.; Anatoly, Z.; Wei, C. A highly efficient magnetic chitosan “fluid” adsorbent with a high capacity & fast adsorption kinetics for dyeing wastewater purification. Chem. Eng. J. 2018, 345, 556–565. [Google Scholar]
- Chen, H.; Wageh, S.; Al-Ghamdi, A.A.; Wang, H.; Yu, J.; Jiang, C. Hierarchical C/NiO-ZnO nanocomposite fibers with enhanced adsorption capacity for Congo red. J. Colloid Interface Sci. 2019, 537, 736–745. [Google Scholar] [CrossRef]
- Sousa, K.S.; Silva Filho, E.C.; Airoldi, C. Ethylenesulfide as a useful agent for incorporation into the biopolymer chitosan in a solvent-free reaction for use in cation removal. Carbohydr. Res. 2009, 344, 1716–1723. [Google Scholar] [CrossRef]
- Khan, A.; Badshah, S.; Airoldi, C. Single-step modification of chitosan for toxic cations remediation from aqueous solution. Polym. Bull. 2015, 72, 353–370. [Google Scholar] [CrossRef]
- Tahira, I.; Aslam, Z.; Abbas, A.; Monim-ul-Mehboob, M.; Ali, S.; Asghar, A. Adsorptive removal of acidic dye onto grafted chitosan: A plausible grafting and adsorption mechanism. Int. J. Biol. Macromol. 2019, 136, 1209–1218. [Google Scholar] [CrossRef]
- Lopes, E.C.N.; Sousa, K.S.; Airoldi, C. Chitosan–cyanuric chloride intermediary as a source to incorporate molecules—Thermodynamic data of copper/biopolymer interactions. Thermochim. Acta 2008, 483, 21–28. [Google Scholar] [CrossRef]
- Azari, A.; Noorisepehr, M.; Dehghanifard, E.; Karimyan, K.; Hashemi, S.Y.; Kalhori, E.M.; Norouzi, R.; Agarwal, S.; Gupta, V.K. Experimental design, modeling and mechanism of cationic dyes biosorption on to magnetic chitosan-glutaraldehyde composite. Int. J. Biol. Macromol. 2019, 131, 633–645. [Google Scholar] [CrossRef]
- Machado, M.O.; Lopes, E.C.N.; Sousa, K.S.; Airoldi, C. The effectiveness of the protected amino group on crosslinked chitosans for copperremoval and the thermodynamics of interaction at the solid/liquid interface. Carbohydr. Polym. 2009, 77, 760–766. [Google Scholar] [CrossRef]
- Manzoor, K.; Ahmad, M.; Ahmad, S.; Ikram, S. Removal of Pb(II) and Cd(II) from wastewater using arginine cross-linked chitosan–carboxymethyl cellulose beads as green adsorbent. RSC Adv. 2019, 9, 7890–7902. [Google Scholar] [CrossRef]
- Alakhras, F.; Al-Shahrani, H.; Al-Abbad, E.; Al-Rimawi, F.; Ouerfelli, N. Removal of Pb(II) Metal Ions from Aqueous Solutions Using Chitosan-Vanillin Derivatives of Chelating Polymers. Pol. J. Environ. Stud. 2019, 28, 1523–1534. [Google Scholar] [CrossRef]
- Anush, S.M.; Chandan, H.R.; Vishalakshi, B. Synthesis and metal ion adsorption characteristics of graphene oxide incorporated chitosan Schiff base. Int. J. Biol. Macromol. 2019, 126, 908–916. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Lu, Z.; Khawar, M.T.; Song, J.; Gong, H.; Meng, J.; Li, Z.; Li, J. Porous poly (L–lactic acid)/chitosan nanofibres for copper ion adsorption. Carbohydr. Polym. 2020, 227, 115343. [Google Scholar] [CrossRef]
- Igberase, E.; Ofomaja, A.; Osifo, P.O. Enhanced heavy metal ions adsorption by 4-aminobenzoic acid grafted on chitosan/epichlorohydrin composite: Kinetics, isotherms, thermodynamics and desorption studies. Int. J. Biol. Macromol. 2019, 123, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Liu, Y.; Zhang, J.; Zhao, M.; Zhu, K. Study on the adsorption behavior of glutaric acid modified Pb(II) imprinted chitosan-based composite membrane to Pb(II) in aqueous Solution. Mater. Lett. 2019, 251, 172–175. [Google Scholar] [CrossRef]
- Sellaoui, L.; Soetaredjo, F.E.; Ismadji, S.; Bonilla-Petriciolet, A.; Belver, C.; Bedia, J.; Lamine, A.B.; Erto, A. Insights on the statistical physics modeling of the adsorption of Cd2+ and Pb2+ ions on bentonite-chitosan composite in single and binary systems. Chem. Eng. J. 2018, 354, 569–576. [Google Scholar] [CrossRef]

| Sample | C/% | N/% | C/mmol g−1 | N/mmol g−1 | C/N |
|---|---|---|---|---|---|
| CH | 40.63 | 7.39 | 33.86 | 5.27 | 6.42 |
| CH-gly | 44.04 | 5.89 | 36.7 | 4.20 | 8.73 |
| CH-ANP | 45.65 | 5.38 | 38.04 | 5.20 | 7.31 |
| Isotherm | Nonlinear Form | Linear Form | Plot |
|---|---|---|---|
| Langmuir type 1 | |||
| Langmuir type 2 | |||
| Langmuir type 3 | |||
| Langmuir type 4 | |||
| Freundlich | |||
| Temkin | |||
| Standard error | |||
| Chi-square |
| Material | Isotherm | Constant | Type I | Type II | Type III | Type IV |
|---|---|---|---|---|---|---|
| CH-ANP | Nf | 2.82 | 2.82 | 2.82 | 2.82 | |
| Ns | 2.94 ± 0.01 | 2.93 ± 0.01 | 2.95 ± 0.02 | 2.95 ± 0.14 | ||
| Cu2+ | b | 2.63 ± 0.01 | 2.62 ± 0.01 | 2.59 ± 0.01 | 2.57 ± 0.06 | |
| R2 | 0.999 | 0.996 | 0.993 | 0.993 | ||
| SE | 0.034 | 0.034 | 0.031 | 0.031 | ||
| χ2 | 0.007 | 0.007 | 0.006 | 0.006 | ||
| Nf | 1.60 | 1.60 | 1.60 | 1.60 | ||
| Ns | 1.85 ± 0.03 | 1.89 ± 0.01 | 1.88 ± 0.01 | 1.88 ± 0.01 | ||
| Pb2+ | b | 0.61 ± 0.01 | 0.57 ± 0.01 | 0.58 ± 0.05 | 0.58 ± 0.08 | |
| R2 | 0.998 | 0.998 | 0.995 | 0.995 | ||
| SE | 0.016 | 0.016 | 0.016 | 0.016 | ||
| χ2 | 0.002 | 0.001 | 0.001 | 0.001 | ||
| Nf | 1.96 | 1.96 | 1.96 | 1.96 | ||
| Ns | 2.48 ± 0.03 | 2.51 ± 0.01 | 2.51 ± 0.02 | 2.52 ± 0.01 | ||
| Cd2+ | b | 0.45 ± 0.01 | 0.43 ± 0.01 | 0.43 ± 0.01 | 0.43 ± 0.01 | |
| R2 | 0.998 | 0.999 | 0.993 | 0.993 | ||
| SE | 0.025 | 0.025 | 0.025 | 0.026 | ||
| χ2 | 0.0026 | 0.003 | 0.003 | 0.003 |
| Isotherm | Constant | Cu (II) | Pb (II) | Cd (II) |
|---|---|---|---|---|
| Freundlich | Kf | 1.74 ± 0.01 | 0.71 ± 0.02 | 0.80 ± 0.02 |
| n | 3.37 ± 0.02 | 1.72 ± 0.03 | 2.46 ± 0.03 | |
| R2 | 0.916 | 0.985 | 0.951 | |
| SE | 0.59 | 0.03 | 0.35 | |
| χ2 | 5.12 | 0.30 | 0.60 | |
| Temkin | KT | 43.56 ± 0.02 | 6.24 ± 0.01 | 4.08 ± 0.02 |
| b | 0.879 ± 0.01 | 2.46 ± 0.03 | 1.39 ± 0.01 | |
| R2 | 0.970 | 0.970 | 0.991 | |
| SE | 0.08 | 0.05 | 0.04 | |
| χ2 | 0.603 | 0.010 | 0.010 |
| Isotherm | Constant | Cu (II) | Pb (II) | Cd (II) |
|---|---|---|---|---|
| Nf | 2.82 | - | - | |
| Langmuir | Ns | 2.96 ± 0.01 | 1.87 ± 0.01 | 3.50 ± 0.03 |
| b | 2.53 ± 0.07 | 0.59 ± 0.01 | 0.44 ± 0.01 | |
| R2 | 0.998 | 0.997 | 0.997 | |
| χ2 | 0.001 | 0.003 | 0.0006 | |
| Freundlich | Kf | 1.84 ± 0.08 | 0.81 ± 0.04 | 0.88 ± 0.04 |
| n | 4.12 ± 0.51 | 3.28 ± 0.32 | 2.56 ± 0.21 | |
| R2 | 0.904 | 0.945 | 0,957 | |
| χ2 | 0.062 | 0.007 | 0.01 | |
| Temkin | KT | 42.32 ± 11.40 | 6.76 ± 1.20 | 4.08 ± 0.3 |
| b | 1.94 ± 0.11 | 2.57 ± 0.31 | 1.39 ± 0.05 | |
| R2 | 0.964 | 0.980 | 0.992 | |
| χ2 | 0.023 | 0.002 | 0.001 |
| Sorbent | Cu (II) (mmol g−1) | Pb (II) (mmol g−1) | Cd (II) (mmol g−1) | References |
|---|---|---|---|---|
| CS-ag-CM | 0.88 | 1.49 | [40] | |
| Chitosan-Vanillin-Schiff base | 0.11 | [41] | ||
| Chitosan-O-Vanillin Schiff base | 0.319 | |||
| Chitosan-Schiff base | 1.74 | [42] | ||
| PLLA/CH | 1.75 | [43] | ||
| grafted crosslinked chitosan beads (G/ECH-CS) | 0.47 | 0.19 | 0.14 | [44] |
| Modified chitosan beads | 2.21 | 1.58 | [28] | |
| Glutaric acid modified Pb (II) imprinted chitosan-based composite membrane | 0.36 | [45] | ||
| bentonite-chitosan composite | 0.39 | 0.84 | [46] | |
| CH-ANP | 2.82 | 1.60 | 1.96 | Present Work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Ali, N.; Bilal, M.; Malik, S.; Badshah, S.; Iqbal, H.M.N. Engineering Functionalized Chitosan-Based Sorbent Material: Characterization and Sorption of Toxic Elements. Appl. Sci. 2019, 9, 5138. https://doi.org/10.3390/app9235138
Khan A, Ali N, Bilal M, Malik S, Badshah S, Iqbal HMN. Engineering Functionalized Chitosan-Based Sorbent Material: Characterization and Sorption of Toxic Elements. Applied Sciences. 2019; 9(23):5138. https://doi.org/10.3390/app9235138
Chicago/Turabian StyleKhan, Adnan, Nisar Ali, Muhammad Bilal, Sumeet Malik, Syed Badshah, and Hafiz M. N. Iqbal. 2019. "Engineering Functionalized Chitosan-Based Sorbent Material: Characterization and Sorption of Toxic Elements" Applied Sciences 9, no. 23: 5138. https://doi.org/10.3390/app9235138
APA StyleKhan, A., Ali, N., Bilal, M., Malik, S., Badshah, S., & Iqbal, H. M. N. (2019). Engineering Functionalized Chitosan-Based Sorbent Material: Characterization and Sorption of Toxic Elements. Applied Sciences, 9(23), 5138. https://doi.org/10.3390/app9235138